Plasma-Ionized Magnesium in Hospitalized Horses with Gastrointestinal Disorders and Systemic Inflammatory Response Syndrome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Sampling
2.3. Data Analysis
3. Results
3.1. Patient Characteristics and Diagnostic Classification
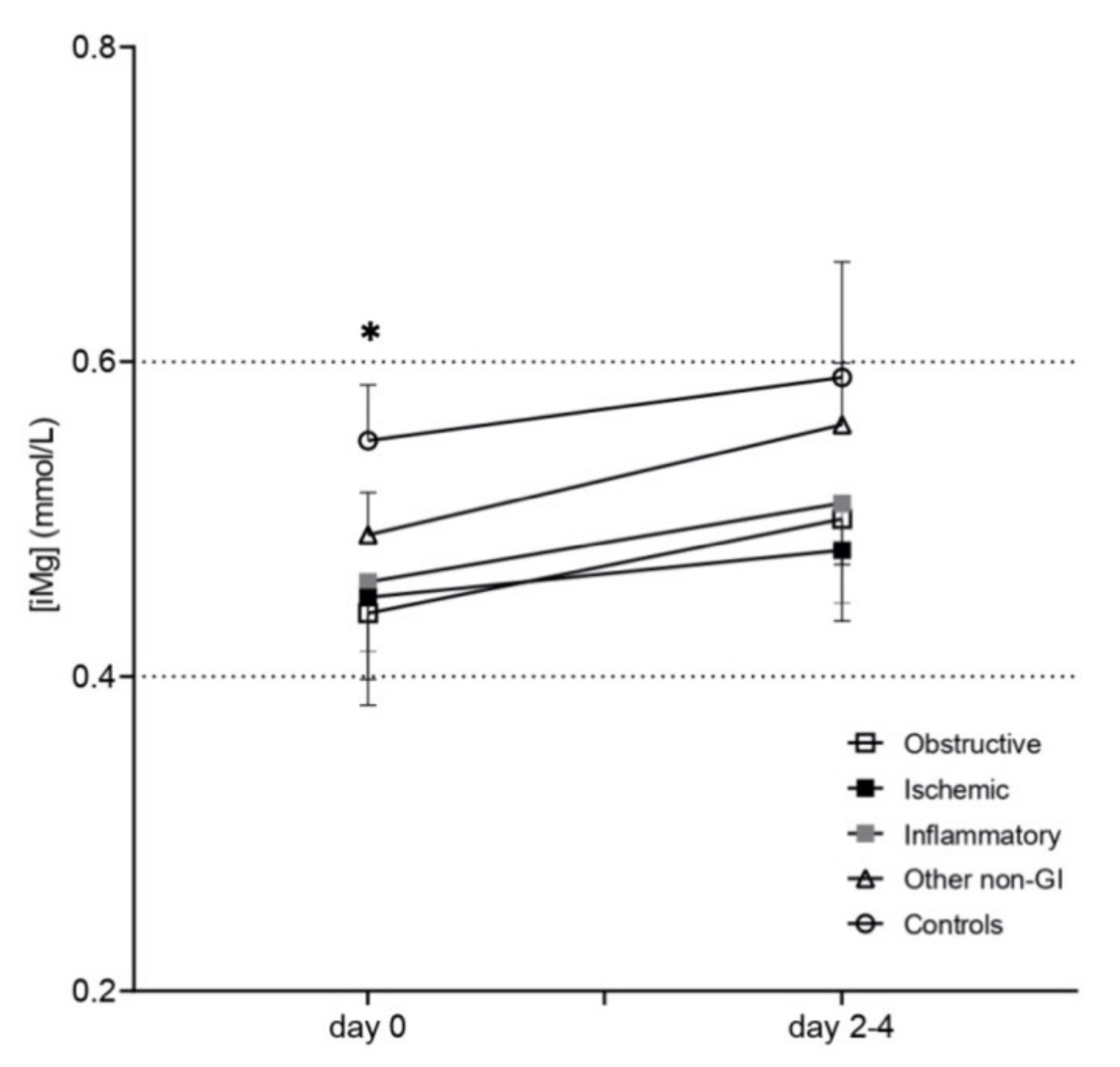
3.2. Plasma-Ionized Magnesium Concentrations
3.3. Plasma-Ionized Calcium Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toribio, R.E.; Kohn, C.W.; Rourke, K.M.; Levine, A.L.; Rosol, T.J. Effects of Hypercalcemia on Serum Concentrations of Magnesium, Potassium, and Phosphate and Urinary Excretion of Electrolytes in Horses. Am. J. Vet. Res. 2007, 68, 543–554. [Google Scholar] [CrossRef]
- Toribio, R.E.; Kohn, C.W.; Hardy, J.; Rosol, T.J. Alterations in Serum Parathyroid Hormone and Electrolyte Concentrations and Urinary Excretion of Electrolytes in Horses with Induced Endotoxemia. J. Vet. Intern. Med. 2005, 19, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Lopez, I.; Estepa, J.C.; Mendoza, F.J.; Rodriguez, M.; Aguilera-Tejero, E. Serum Concentrations of Calcium, Phosphorus, Magnesium and Calciotropic Hormones in Donkeys. Am. J. Vet. Res. 2006, 67, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Lopez, I.; Estepa, J.C.; Mendoza, F.J.; Mayer-Valor, R.; Aguilera-Tejero, E. Fractionation of Calcium and Magnesium in Equine Serum. Am. J. Vet. Res. 2006, 67, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.; Aroch, I. Concentrations of Ionized and Total Magnesium and Calcium in Healthy Horses: Effects of Age, Pregnancy, Lactation, PH and Sample Type. Vet. J. 2009, 181, 305–311. [Google Scholar] [CrossRef]
- Stewart, A.J.; Hardy, J.; Kohn, C.W.; Toribio, R.E.; Hinchcliff, K.W.; Silver, B. Validation of Diagnostic Tests for Determination of Magnesium Status in Horses with Reduced Magnesium Intake. Am. J. Vet. Res. 2004, 65, 422–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, M.; Monreal, L.; Segura, D.; Armengou, L.; Añor, S. A Comparison of Traditional and Quantitative Analysis of Acid-Base and Electrolyte Imbalances in Horses with Gastrointestinal Disorders. J. Vet. Intern. Med. 2005, 19, 871–877. [Google Scholar] [CrossRef]
- Altura, B.M.; Altura, B.T. Role of Magnesium in Patho-Physiological Processes and the Clinical Utility of Magnesium Ion Selective Electrodes. Scand. J. Clin. Lab. Investig. 1996, 224, 211–234. [Google Scholar] [CrossRef]
- Velissaris, D.; Karamouzos, V.; Pierrakos, C.; Aretha, D.; Karanikolas, M. Hypomagnesemia in Critically Ill Sepsis Patients. J. Clin. Med. Res. 2015, 7, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Toribio, R.E.; Kohn, C.W.; Chew, D.J.; Sams, R.A.; Rosol, T.J. Comparison of Serum Parathyroid Hormone and Ionized Calcium and Magnesium Concentrations and Fractional Urinary Clearance of Calcium and Phosphorus in Healthy Horses and Horses with Enterocolitis. Am. J. Vet. Res. 2001, 62, 938–947. [Google Scholar] [CrossRef]
- Stewart, A.J. Magnesium Disorders in Horses. Vet. Clin. N. Am. Equine Pract. 2011, 27, 149–163. [Google Scholar] [CrossRef]
- Kolte, D.; Vijayaraghavan, K.; Khera, S.; Sica, D.A.; Frishman, W.H. Role of Magnesium in Cardiovascular Diseases. Cardiol. Rev. 2014, 22, 182–192. [Google Scholar] [CrossRef] [Green Version]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Johansson, A.M.; Gardner, S.Y.; Jones, S.L.; Fuquay, L.R.; Reagan, V.H.; Levine, J.F. Hypomagnesemia in Hospitalized Horses. J. Vet. Intern. Med. 2003, 17, 860–867. [Google Scholar] [CrossRef]
- Garcia-Lopez, J.M.; Provost, P.J.; Rush, J.E.; Zicker, S.C.; Burmaster, H.; Freeman, L.M. Prevalence and Prognostic Importance of Hypomagnesemia and Hypocalcemia in Horses That Have Colic Surgery. Am. J. Vet. Res. 2001, 62, 7–12. [Google Scholar] [CrossRef]
- Hurcombe, S.D.A.; Toribio, R.E.; Slovis, N.M.; Saville, W.J.; Mudge, M.C.; Macgillivray, K.; Frazer, M.L. Calcium Regulating Hormones and Serum Calcium and Magnesium Concentrations in Septic and Critically Ill Foals and Their Association with Survival. J. Vet. Intern. Med. 2009, 23, 335–343. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.-F.; Kwong, G.P.S.; Lambert, J.; Massie, S.; Lockhart, S. Prognostic Value and Development of a Scoring System in Horses with Systemic Inflammatory Response Syndrome. J. Vet. Intern. Med. 2017, 31, 582–592. [Google Scholar] [CrossRef] [Green Version]
- Robles-Guirado, J.A.; Sanmartí, J.; Jose-Cunilleras, E.; Bassols, A. Sample Stability and Heparin Interference Using a Stat Profile Prime plus Vet Critical Care Analyzer to Quantify Ionized Calcium (ICa) and Ionized Magnesium (IMg). In Proceedings of the ACVP, ASVCP, ISACP Virtual Annual Meeting, Virtual Meeting, 30 October–1 November 2020; p. 40. [Google Scholar]
- Toribio, R.E. Disorders of Calcium and Phosphate Metabolism in Horses. Vet. Clin. N. Am. Equine Pract. 2011, 27, 129–147. [Google Scholar] [CrossRef]
- Hardin, J.W.; James, W.; Hilbe, J.M. Generalized Estimating Equations, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-0-4291-1103-7. [Google Scholar]
- Lohmann, K.L.; Barton, M.H. Systemic Inflammatory Response Syndrome. In Equine Infectious Diseases, 2nd ed.; Sellon, D.C., Long, M.T., Eds.; W.B. Saunders: St. Louis, MI, USA, 2014; pp. 119–131.e6. ISBN 978-1-4557-0891-8. [Google Scholar]
- Woods, G.A.; Oikonomidis, I.L.; Gow, A.G.; Tørnqvist-Johnsen, C.; Boyé, P.; Chng, Y.; Mellanby, R.J. Investigation of Hypomagnesaemia Prevalence and Underlying Aetiology in a Hospitalised Cohort of Dogs with Ionised Hypocalcaemia. Vet. Rec. 2021, 189, e301. [Google Scholar] [CrossRef]
- Schweigel, M.; Martens, H. Magnesium Transport in the Gastrointestinal Tract. Front. Biosci. 2000, 5, D666–D677. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.J.; Ritchie, G.; Kerstan, D.; Kang, H.S.; Cole, D.E.; Quamme, G.A. Magnesium Transport in the Renal Distal Convoluted Tubule. Physiol. Rev. 2001, 81, 51–84. [Google Scholar] [CrossRef]
- Quamme, G.A.; de Rouffignac, C. Epithelial Magnesium Transport and Regulation by the Kidney. Front. Biosci. 2000, 5, D694–D711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.N.; Vandenplas, M.L. Is It the Systemic Inflammatory Response Syndrome or Endotoxemia in Horses with Colic? Vet. Clin. N. Am. Equine Pract. 2014, 30, 337–351. [Google Scholar] [CrossRef]
- Jiang, P.; Lv, Q.; Lai, T.; Xu, F. Does Hypomagnesemia Impact on the Outcome of Patients Admitted to the Intensive Care Unit? A Systematic Review and Meta-Analysis. Shock 2017, 47, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.G.; Matteson, V.L.; Wingfield, W.E.; Van Pelt, D.R.; Hackett, T.B. Abnormalities of Serum Magnesium in Critically III Dogs: Incidence and Implications. J. Vet. Emerg. Crit. Care 1994, 4, 15–20. [Google Scholar] [CrossRef]
- Toll, J.; Erb, H.; Birnbaum, N.; Schermerhorn, T. Prevalence and Incidence of Serum Magnesium Abnormalities in Hospitalized Cats. J. Vet. Intern. Med. 2002, 16, 217–221. [Google Scholar] [CrossRef]
- Murphy, E. Mysteries of Magnesium Homeostasis. Circ. Res. 2000, 86, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Romani, A.M.P. Cellular Magnesium Homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroll, M.H.; Elin, R.J. Relationships between Magnesium and Protein Concentrations in Serum. Clin. Chem. 1985, 31, 244–246. [Google Scholar] [CrossRef]
- Schumacher, S.A.; Yardley, J.; Bertone, A.L. Ionized Magnesium and Calcium Concentration and Their Ratio in Equine Plasma Samples as Determined by a Regulatory Laboratory Compared to a Clinical Reference Laboratory. Drug Test. Anal. 2019, 11, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; McDonnell, E.H.; Sedor, F.A.; Toffaletti, J.G. PH Effects on Measurements of Ionized Calcium and Ionized Magnesium in Blood. Arch. Pathol. Lab. Med. 2002, 126, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, C.V.; Horney, B.S.; Burton, S.A.; MacKenzie, A.L. Evaluation of Ionized and Total Serum Magnesium Concentrations in Hyperthyroid Cats. Can. J. Vet. Res. 2006, 70, 137–142. [Google Scholar] [PubMed]
- Hollis, A.R.; Wilkins, P.A.; Palmer, J.E.; Boston, R.C. Bacteremia in Equine Neonatal Diarrhea: A Retrospective Study (1990–2007). J. Vet. Intern. Med. 2008, 22, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.J.; Bindels, R.J.M. Epithelial Ca2+ and Mg2+ Channels in Health and Disease. J. Am. Soc. Nephrol. 2005, 16, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Control (n = 26) | Obstructive (n = 17) | Inflammatory (n = 10) | Ischemic (n = 12) | Non-GI (n = 36) | SIRS (n = 26) | Non-SIRS (n = 75) | |
|---|---|---|---|---|---|---|---|
| ↓(iMg) n (%) | 1 (3.9%) | 8 (47.1%) * | 3 (30%) * | 4 (33.3%) * | 5 (13.9%) * | 7 (26.9%) | 14 (18.7%) |
| ↑(iMg) n (%) | 9 (34.6%) | 2 (11.8%) | 0 * | 0 * | 2 (5.6%) * | 2 (7.7%) | 11 (14.7%) |
| ↓(iCa) + ↓(iMg) n (%) | 1 (3.9%) | 5 (29.4%) * | 2 (20%) | 3 (25%) | 2 (5.6%) $ | 4 (15.4%) | 9 (12%) |
| Age median (95%CI) (range) | 7 (6–11) (2–26) | 14 (11–19) (1.5–23) | 9.5 (6–15) (4–32) | 9 (4–11) (2–23) | 12 (10–15) (2–26) | 10 (10–12) (2–26) | 11 (9–17) (1.5–21) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmartí, J.; Armengou, L.; Troya-Portillo, L.; Robles-Guirado, J.Á.; Bassols, A.; Ríos, J.; Jose-Cunilleras, E. Plasma-Ionized Magnesium in Hospitalized Horses with Gastrointestinal Disorders and Systemic Inflammatory Response Syndrome. Animals 2022, 12, 1479. https://doi.org/10.3390/ani12121479
Sanmartí J, Armengou L, Troya-Portillo L, Robles-Guirado JÁ, Bassols A, Ríos J, Jose-Cunilleras E. Plasma-Ionized Magnesium in Hospitalized Horses with Gastrointestinal Disorders and Systemic Inflammatory Response Syndrome. Animals. 2022; 12(12):1479. https://doi.org/10.3390/ani12121479
Chicago/Turabian StyleSanmartí, Julia, Lara Armengou, Lucas Troya-Portillo, José Ángel Robles-Guirado, Anna Bassols, José Ríos, and Eduard Jose-Cunilleras. 2022. "Plasma-Ionized Magnesium in Hospitalized Horses with Gastrointestinal Disorders and Systemic Inflammatory Response Syndrome" Animals 12, no. 12: 1479. https://doi.org/10.3390/ani12121479
APA StyleSanmartí, J., Armengou, L., Troya-Portillo, L., Robles-Guirado, J. Á., Bassols, A., Ríos, J., & Jose-Cunilleras, E. (2022). Plasma-Ionized Magnesium in Hospitalized Horses with Gastrointestinal Disorders and Systemic Inflammatory Response Syndrome. Animals, 12(12), 1479. https://doi.org/10.3390/ani12121479







