Captive Rearing of Longfin Smelt Spirinchus thaleichthys: First Attempt of Weaning Cultured Juveniles to Dry Feed
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Broodstock Collection, Spawning, and Larviculture
2.2. Experimental Design
2.3. Size, Condition, and Tissue Analysis
2.4. Statistical Analysis
3. Results and Remarks
3.1. Weaning Success, Survival, Growth, and Condition
3.2. Fatty Acid Profile of Artemia and Dry Pellet Feeds
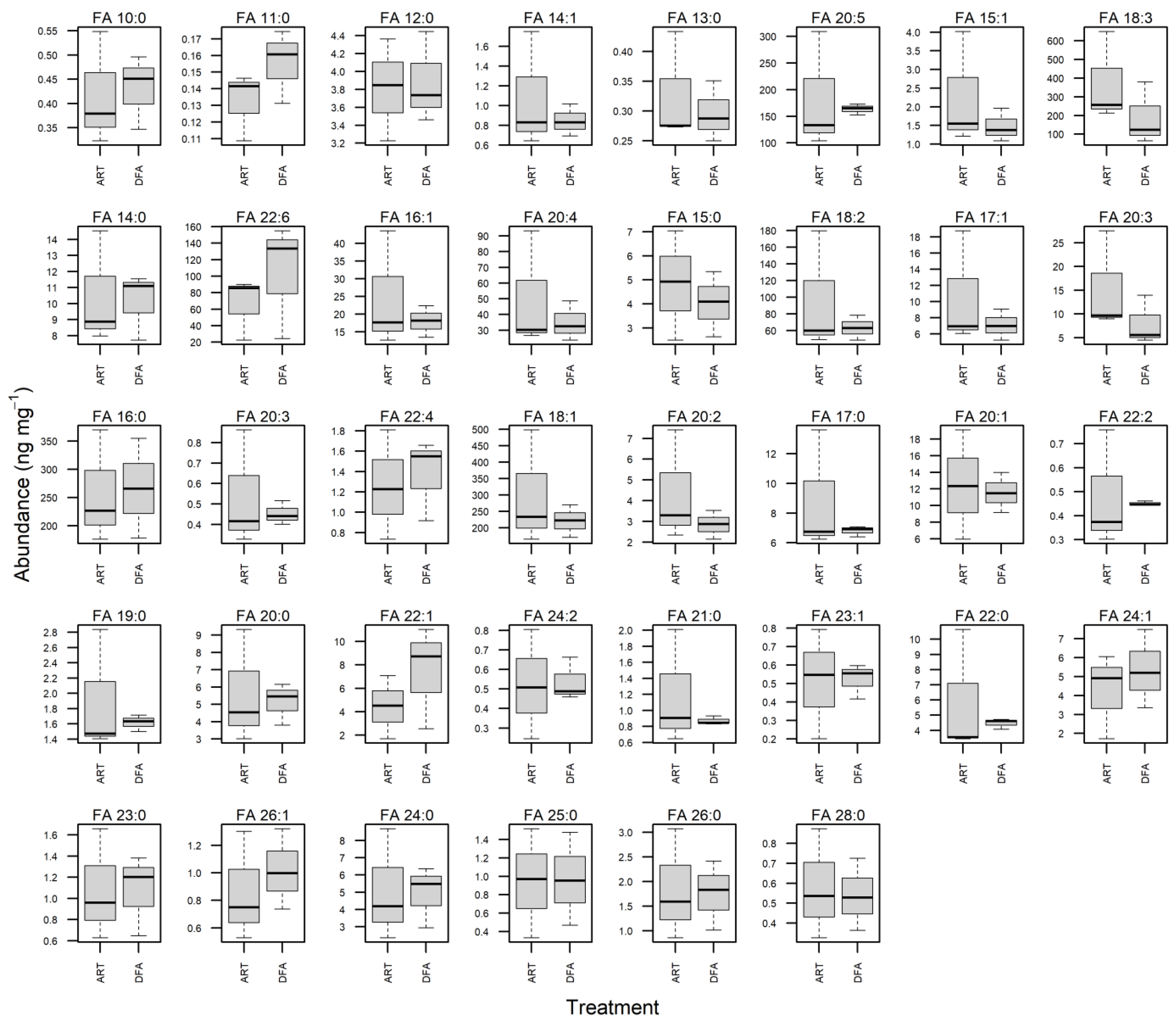
3.3. Fatty Acid Profile of Juvenile Muscle Tissues
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hobbs, J.; Moyle, P.B.; Fangue, N.; Connon, R.E. Is extinction inevitable for Delta smelt and Longfin smelt? An opinion and recommendations for recovery. San Franc. Estuary Watershed Sci. 2017, 15, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Lewis, L.S.; Willmes, M.; Barros, A.; Crain, P.K.; Hobbs, J.A. Newly discovered spawning and recruitment of threatened Longfin Smelt in restored and underexplored tidal wetlands. Ecology 2020, 101, e02868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tigan, G.; Fetherolf, S.; Hung, T.C. Longfin Smelt culture and marking study. A final report to California Department of Water Resources. Agreement 2019, #4600011161. [Google Scholar]
- Yanagitsuru, Y.R.; Main, M.A.; Lewis, L.S.; Hobbs, J.A.; Hung, T.-C.; Connon, R.E.; Fangue, N.A. Effects of temperature on hatching and growth performance of embryos and yolk-sac larvae of a threatened estuarine fish: Longfin smelt (Spirinchus thaleichthys). Aquaculture 2021, 537, 736502. [Google Scholar] [CrossRef]
- Yanagitsuru, Y.R.; Daza, I.Y.; Lewis, L.S.; Hobbs, J.A.; Hung, T.-C.; Connon, R.E.; Fangue, N.A. Growth, osmoregulation, and ionoregulation of longfin smelt (Spirinchus thaleichthys) yolk-sac larvae at different salinities. Conserv. Physiol. 2022, Accepted. [Google Scholar]
- Hung, T.-C.; Ellison, L.; Stevenson, T.; Sandford, M.; Schultz, A.A.; Eads, A.R. Early weaning in endangered Delta Smelt: Effect of weaning time on growth and survival. N. Am. J. Aquacult. 2022, 84, 249–260. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Version 4.0.2; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.r-project.org/ (accessed on 31 December 2020).
- Mejri, S.C.; Tremblay, R.; Audet, C.; Wills, P.S.; Riche, M. Essential fatty acid requirements in tropical and cold-water marine fish larvae and juveniles. Front. Mar. Sci. 2021, 8, 680003. [Google Scholar] [CrossRef]
- Glencross, B.D. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev. Aquac. 2009, 1, 71–124. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 717–732. [Google Scholar] [CrossRef]
- Furuita, H.; Konishi, K.; Takeuchi, T. Effect of different levels of eicosapentaenoic acid and docosahexaenoic acid in Artemia nauplii on growth, survival and salinity tolerance of larvae of the Japanese flounder, Paralichthys olivaceus. Aquaculture 1999, 170, 59–69. [Google Scholar] [CrossRef]
- Gawlicka, A.; Herold, M.A.; Barrows, F.T.; de la Noue, J.; Hung, S.S.O. Effects of dietary lipids on growth, fatty acid composition, intestinal absorption and hepatic storage in white sturgeon (Acipenser transmontanus R.) larvae. J. Appl. Ichthyol. 2002, 18, 673–681. [Google Scholar] [CrossRef]
- Watanabe, T. Importance of docosahexaenoic acid in marine larval fish. J. World Aquac. Soc. 1993, 24, 152–161. [Google Scholar] [CrossRef]
- Khan, M.A.; Jafri, A.K.; Chadha, N.K.; Usmani, N. Growth and body composition of rohu (Labeo rohita) fed diets containing oilseed meals: Partial or total replacement of fish meal with soybean meal. Aquac. Nutr. 2003, 9, 391–396. [Google Scholar] [CrossRef]
- Ljubobratovic, U.; Kosanovic, D.; Demény, F.Z.; Krajcsovics, A.; Vukotic, G.; Stanisavljevic, N.; Golic, N.; Jeney, G.; Lukic, J. The effect of live and inert feed treatment with lactobacilli on weaning success in intensively reared pike-perch larvae. Aquaculture 2020, 516, 734608. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Wahab, M.A.; Phillips, M.; Rahman, A.; Padiyar, A.; Puvanendran, V.; Bangera, R.; Belton, B.; De, D.K.; Meena, D.K.; et al. Breeding and culture status of Hilsa (Tenualosa ilisha, Ham. 1822) in South Asia: A review. Rev. Aquac. 2018, 10, 96–110. [Google Scholar] [CrossRef]
- Hamidoghli, A.; Bae, J.; Won, S.; Lee, S.; Kim, D.-J.; Bai, S.C. A review on Japanese eel (Anguilla japonica) aquaculture, with special emphasis on nutrition. Rev. Fish. Sci. Aquac. 2019, 27, 226–241. [Google Scholar] [CrossRef]
- Hamidoghli, A.; Won, S.; Lee, S.; Lee, S.; Farris, N.W.; Bai, S.C. Nutrition and feeding of olive flounder Paralichthys olivaceus: A review. Rev. Fish. Sci. Aquac. 2020, 28, 340–357. [Google Scholar] [CrossRef]


| Feed Treatment | Nfish | Feed Quantity | Frequency |
|---|---|---|---|
| 1. Artemiaa (ART) | 114 | 500 mL | 100 mL; 5 × daily |
| 2. Dry feed b + Artemia (DFA) | 114 | 1.0 g (dry feed) + 100 mL (Artemia) | 0.25 g; 4 × daily 100 mL 1 × daily |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulvaney, W.; Rahman, M.M.; Lewis, L.S.; Cheng, J.; Hung, T.-C. Captive Rearing of Longfin Smelt Spirinchus thaleichthys: First Attempt of Weaning Cultured Juveniles to Dry Feed. Animals 2022, 12, 1478. https://doi.org/10.3390/ani12121478
Mulvaney W, Rahman MM, Lewis LS, Cheng J, Hung T-C. Captive Rearing of Longfin Smelt Spirinchus thaleichthys: First Attempt of Weaning Cultured Juveniles to Dry Feed. Animals. 2022; 12(12):1478. https://doi.org/10.3390/ani12121478
Chicago/Turabian StyleMulvaney, William, Md Moshiur Rahman, Levi S. Lewis, Jiayi Cheng, and Tien-Chieh Hung. 2022. "Captive Rearing of Longfin Smelt Spirinchus thaleichthys: First Attempt of Weaning Cultured Juveniles to Dry Feed" Animals 12, no. 12: 1478. https://doi.org/10.3390/ani12121478
APA StyleMulvaney, W., Rahman, M. M., Lewis, L. S., Cheng, J., & Hung, T.-C. (2022). Captive Rearing of Longfin Smelt Spirinchus thaleichthys: First Attempt of Weaning Cultured Juveniles to Dry Feed. Animals, 12(12), 1478. https://doi.org/10.3390/ani12121478






