Effects of Fly Maggot Protein Replacement of Fish Meal on Growth Performance, Immune Level, Antioxidant Level, and Fecal Flora of Blue Foxes at Weaning Stage
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Diets, Management, and Experimental Design
2.1.1. Animal Diets
2.1.2. Animal Management and Experimental Design
2.2. Sample Collection
2.3. DNA Extraction and 16S rRNA High-Throughput Amplicon Target Sequencing
2.3.1. DNA Extraction and PCR Amplification
2.3.2. Illumina MiSeq
2.3.3. Processing of Sequencing Data
2.4. Statistical Analysis
3. Results
3.1. Effects of Fly Maggot Protein Replacing Fish Meal on Growth Performance of Weaned Blue Foxes
3.2. Effects on Immune Indexes
3.3. Effects on Serum Antioxidant Indexes
3.4. Effects of Fly Maggot Protein Substitute for Fish Meal on Fecal Flora of Blue Foxes in Weaning Period
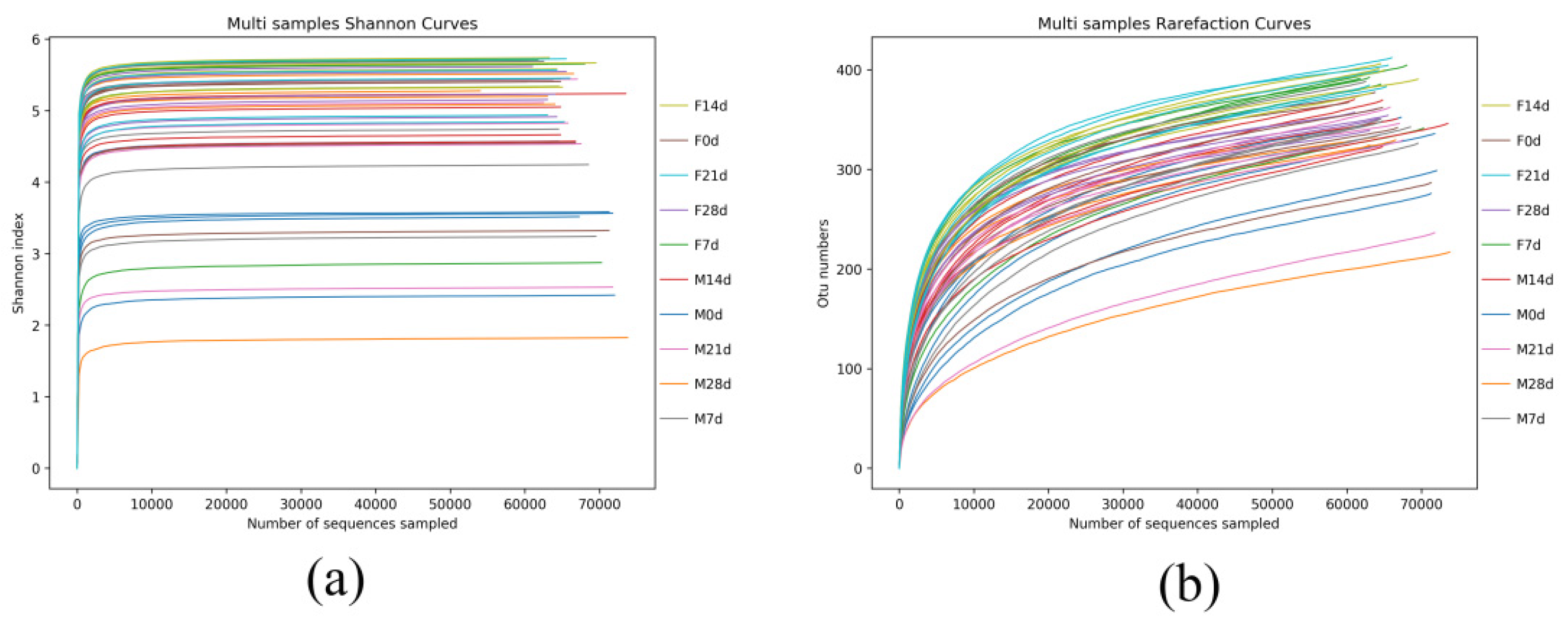
3.4.1. Sample Diversity Analysis
3.4.2. Venn Diagram Analysis Based on OTUs
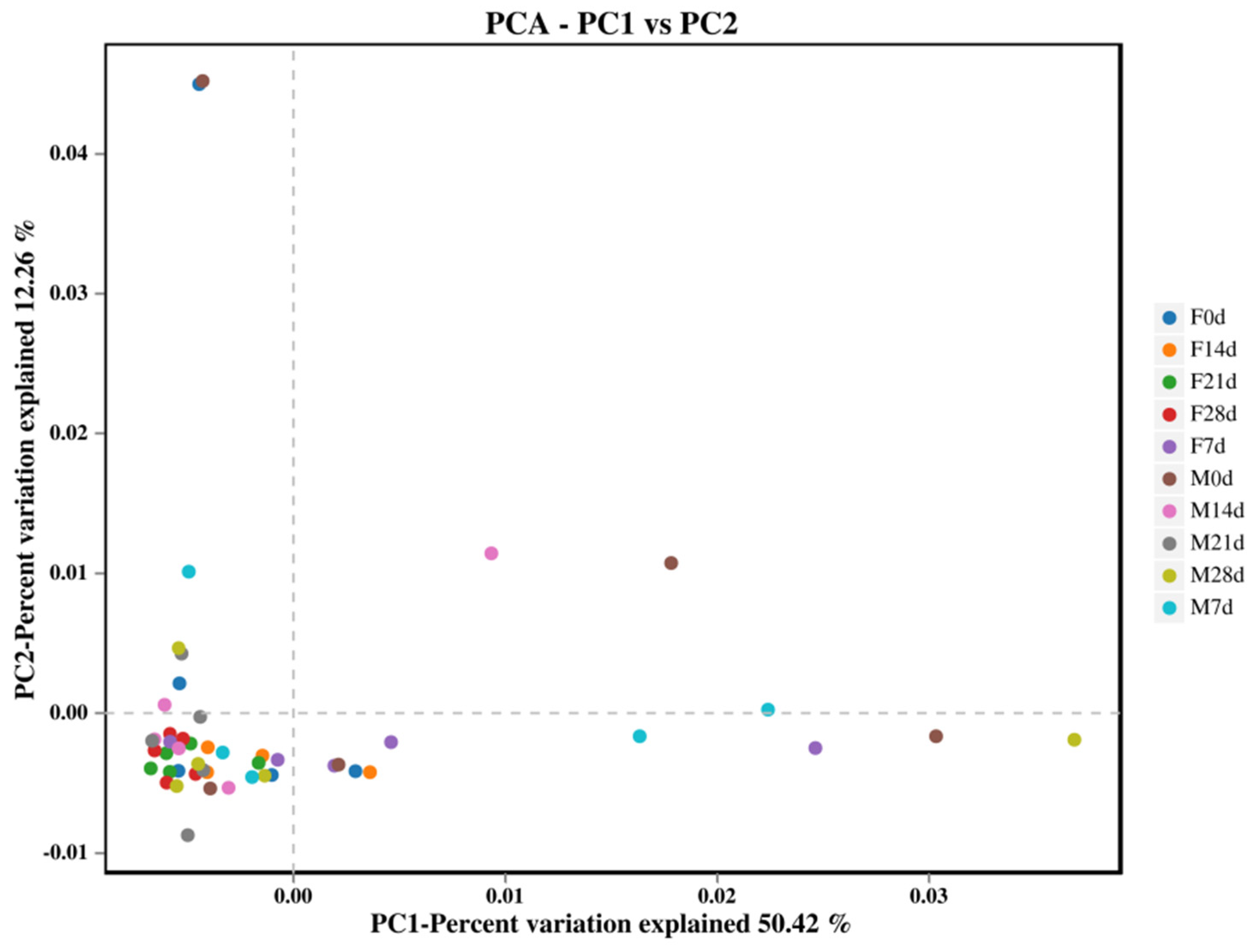
3.4.3. Multi-Sample Comparative Analysis
3.4.4. Structural Analysis of Fecal Flora
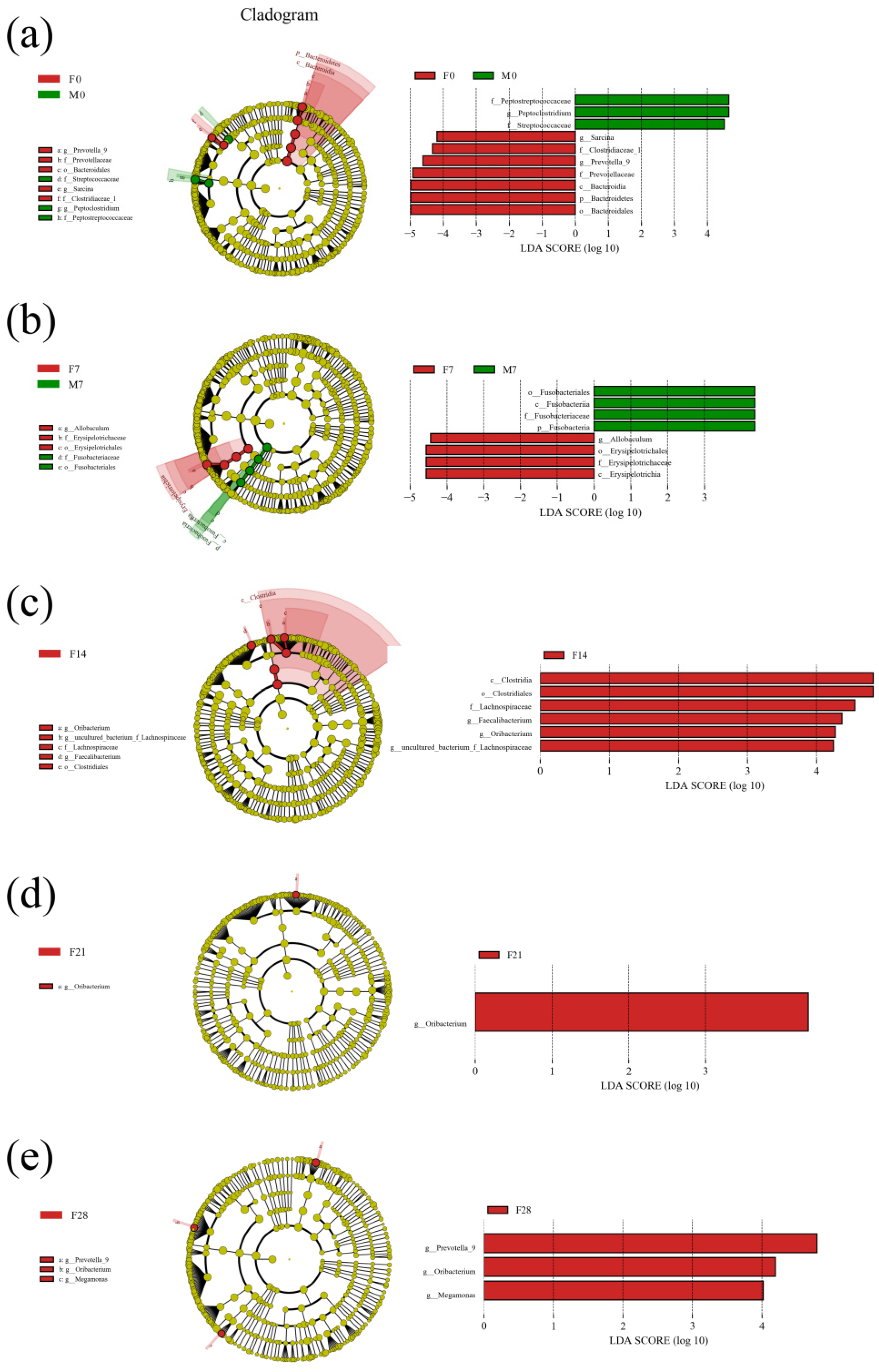
3.4.5. LEfSe Species Difference Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chia, S.Y.; Tanga, C.M.; Osuga, I.M.; Alaru, A.O.; Dicke, M. Effect of dietary replacement of fishmeal by insect meal on growth performance, blood profiles and economics of growing pigs in Kenya. Animals 2019, 9, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onsongo, V.O.; Osuga, I.M.; Gachuiri, C.K.; Wachira, A.M.; Miano, D.M. Insects for income generation through animal feed: Effect of dietary replacement of soybean and fish meal with black soldier fly meal on broiler growth and economic performance. J. Econ. Entomol. 2018, 111, 1966–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oonincx, D.G.A.B.; Van Itterbeeck, J.; Heetkamp, M.J.W.; Van Den Brand, H.; Van Loon, J.J.; Van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasco, L.B.I.; Dabbou, S.; Schiavone, A.; Gai, F. Animals fed insect-based diets: State-of-the-art on digestibility, performance and product quality. Animals 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Ogunji, J.O.; Nimptsch, J.; Wiegand, C.; Schulz, C. Evaluation of the influence of housefly maggot meal (magmeal) diets on catalase, glutathione S-transferase and glycogen concentration in the liver of Oreochromis niloticus fingerling. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 942–947. [Google Scholar] [CrossRef]
- Schiavone, A.; De Marco, M.; Martinez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Hernandez, F.; Rotolo, L.; Costa, P.; Gai, F.; et al. Nutritional value of a partially defatted and a highly defatted black soldier fly larvae (Hermetia illucens L.) meal for broiler chickens: Apparent nutrient digestibility, apparent metabolizable energy and apparent ileal amino acid digestibility. J. Anim. Sci. Biotechnol. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Iaconisi, V.; Marono, S.; Parisi, G.; Gasco, L.; Genovese, L.; Maricchiolo, G.; Bovera, F.; Piccolo, G. Dietary inclusion of Tenebrio molitor larvae meal: Effects on growth performance and final quality treats of blackspot sea bream (Pagellus bogaraveo). Aquaculture 2017, 476, 49–58. [Google Scholar] [CrossRef]
- Loponte, R.; Bovera, F.; Piccolo, G.; Gasco, L.; Secci, G.; Iaconisi, V.; Parisi, G. Fatty acid profile of lipids and caeca volatile fatty acid production of broilers fed a full fat meal from Tenebrio molitor larvae. Ital. J. Anim. Sci. 2019, 18, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Allegretti, G.; Talamini, E.; Schmidt, V.; Bogorni, P.C.; Ortega, E. Insect as feed: An emergy assessment of insect meal as a sustainable protein source for the Brazilian poultry industry. J. Clean. Prod. 2018, 171, 403–412. [Google Scholar] [CrossRef]
- Odesanya, B.O.; Ajayi, S.O.; Agbaogun, B.; Okuneye, B. Comparative Evaluation of Nutritive Value of maggots. Int. J. Sci. Eng. Res. 2013, 2, 1–5. [Google Scholar]
- Mbiba, H.; Etchu, K.; Ndamukong, K. Carcass characteristics, haematology, serum chemistry and enzymes in broiler chickens fed maggot meal as a protein substitute for fishmeal. Glob. J. Med. Res. 2019, 19, 7–13. [Google Scholar]
- Hwangbo, J.; Hong, E.C.; Jang, A.; Kang, H.K.; Oh, J.S.; Kim, B.W.; Park, B.S. Utilization of house fly-maggots, a feed supplement in the production of broiler chickens. J. Environ. Biol. 2009, 30, 609–614. [Google Scholar] [PubMed]
- Song, Y.K.; Ying, S.S.; He, J.; Fan, P.P.; Zhao, Y.; Li, R. Effects of maggots on growth performance, immune function and antioxidant indexes of broilers. J. Econ. Anim. 2021, 25, 7–13. [Google Scholar]
- Wang, L.; Li, J.; Jin, J.N.; Zhu, F.; Roffeis, M.; Zhang, X.Z. A comprehensive evaluation of replacing fishmeal with housefly (Musca domestica) maggot meal in the diet of Nile tilapia (Oreochromis niloticus): Growth performance, flesh quality, innate immunity and water environment. Aquac. Nutr. 2017, 23, 983–993. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Mink and Foxes; National Academies Press: Washington, DC, USA, 1982. [Google Scholar]
- Ylinen, V.; Pylkkö, P.; Peura, J.; Tuomola, E.; Valaja, J. Performance in blue fox (Vulpes lagopus) fed low-protein diets supplemented with DL-methionine and L-histidine. Agric. Food Sci. 2018, 27, 168–178. [Google Scholar] [CrossRef]
- Areerat, S.; Chundang, P.; Lekcharoensuk, C.; Kovitvadhi, A. Possibility of using house cricket (Acheta domesticus) or mulberry silkworm (Bombyx mori) pupae meal to replace poultry meal in canine diets based on health and nutrient digestibility. Animals 2021, 11, 2680. [Google Scholar] [CrossRef]
- Józefiak, A.; Nogales-Mérida, S.; Mikołajczak, Z.; Rawski, M.; Kierończyk, B.; Mazurkiewicz, J. The utilization of full-fat insect meal in rainbow trout (Oncorhynchus mykiss) nutrition: The effects on growth performance, intestinal microbiota and gastrointestinal tract histomorphology. Ann. Anim. Sci. 2019, 19, 747–765. [Google Scholar] [CrossRef] [Green Version]
- Gasco, L.; Finke, M.; Van Huis, A. Can diets containing insects promote animal health? J. Insects Food Feed. 2018, 4, 1–4. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Song, Y.K.; He, J. Nutritional characteristics of four animal protein feeds and their application in livestock and poultry breeding. Chin. J. Anim. Nutr. 2019, 31, 109–118. [Google Scholar]
- Shen, H. Study on Nutrition Value Assessment and Exploitation of Utilization of Tenebrio molitor L. Master’s Thesis, Shihezi University, Shihezi, Xinjiang, China, 2005. [Google Scholar]
- Guo, R.P.; Li, C.; Cheng, X.J.; Li, C.; Tian, H.L.; Yang, J.F.; Zhang, X.G. Effects of fishmeal replaced by house-fly-larvace-meal on growth performance, immunity and economic benefit in weaned piglets. China Feed 2014, 7, 22–24, 28. [Google Scholar]
- Cheng, Y.M.; Hang, Q.C.; Wang, F.H.; Li, G.H. Effects of dietary housefly maggot protein on serum biochemical indexes and nonspecific immunity in Soft Shelled Turtle Trionyx sinensis. Fish. Sci. 2017, 36, 768–772. [Google Scholar]
- Cheng, Y.M.; Hang, Q.C.; Wang, F.H.; Li, G.H. Effects of dietary housefly protein on growthperformance and nutritional quality of Soft Shelled Turtle Trionyx sinensis. Fish. Sci. 2018, 37, 51–58. [Google Scholar]
- Zhang, H.Q.; Zhou, F.; Wang, W.P.; Xu, X.J.; Zhang, J.R.; He, Z.Y. Effects of housefly maggot meal instead of fish meal on growth performance, textural mechanical properties, serum parameters in Pelodiscus sinensis Japanese strain. Acta Agric. Zhejiangensis 2013, 25, 225–229. [Google Scholar]
- Kurniawan, D.R.; Arief, M.; Agustono; Lamid, M. Effect of maggot (Hermetia illucens) flour in commercial feed on protein retention, energy retention, protein content, and fat content in tilapia (Oreochromis niloticus). IOP Conf. Ser. Earth Environ. Sci. 2018, 137, 012030. [Google Scholar] [CrossRef]
- Sanchez-Muros, M.J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Zhang, H.T.; Gao, F.; Li, Y.L.; Gu, K.L. Effects of fish meal replaced by maggot meal in dietary supplementing chitinase on growth performance and non-speicfic immunity of loach (Misgurnus anguillicaudatus). China Feed 2017, 11, 39–43. [Google Scholar]
- Wu, D.; Liu, H.S.; Long, S.F.; Pu, X.S. Sources, Nutrients and Special Functions of Insect Protein and Its Application Effects in Pig and Chicken Diets. Chin. J. Anim. Nutr. 2020, 32, 2491–2499. [Google Scholar]
- Wang, C.; Wang, M.Q.; Ye, S.S.; Tao, W.J.; Du, Y.J. Effects of copper-loaded chitosan nanoparticles on growth and immunity in broilers. Poult. Sci. 2011, 90, 2223–2228. [Google Scholar] [CrossRef]
- Horton, R.E.; Vidarsson, G. Antibodies and their receptors: Different potential roles mucosal defense. Front. Immunol. 2013, 4, 200. [Google Scholar] [CrossRef] [Green Version]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Li, Y.W.; He, M.R.; Pang, Y. Effect of housefly maggot proteins on the growth, meat quality and disease resistance of Qingyuan partridge chickens. China Poult. 2012, 34, 35–39. [Google Scholar]
- Zhao, H.Y.; Mao, X.B.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Luo, J.Q.; Wang, Q.Y.; Chen, D.W. Excess of dietary montmorillonite impairs growth performance, liver function, and antioxidant capacity in starter pigs. J. Anim. Sci. 2017, 95, 2943–2951. [Google Scholar] [CrossRef]
- Wei, R. Effects of Changes in Salinity on the Nonspecific Immune Factors of Japanese Flounder and Antioxidant Enzymes Activities in Amphioxus. Master’s Thesis, Ocean University of China, Qingdao, Shandong, China, 2004. [Google Scholar]
- Ogunji, J.O.; Kloas, W.; Wirth, M.; Neumann, N.; Pietsch, C. Effect of housefly maggot meal (magmeal) diets on the performance, concentration of plasma glucose, cortisol and blood characteristics of Oreochromis niloticus fingerlings. J. Anim. Physiol. Anim. Nutr. 2008, 92, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.J.; Gomez, A.; White, B.; Loften, J.R.; Drackley, J.K. Changes in the intestinal bacterial community, short-chain fatty acid profile, and intestinal development of preweaned Holstein calves. 2. Effects of gastrointestinal site and age. J. Dairy Sci. 2016, 99, 9703–9715. [Google Scholar] [CrossRef] [PubMed]
- Edrington, T.S.; Dowd, S.E.; Farrow, R.F.; Hagevoort, G.R.; Callaway, T.R.; Anderson, R.C.; Nisbet, D.J. Development of colonic microflora as assessed by pyrosequencing in dairy calves fed waste milk. J. Dairy Sci. 2012, 95, 4519–4525. [Google Scholar] [CrossRef]
- Kamra, D.N. Rumen microbial ecosystem. Curr. Sci. 2005, 89, 124–135. [Google Scholar]
- Purushe, J.; Fouts, D.E.; Morrison, M.; White, B.A.; Mackie, R.I.; Coutinho, P.M.; Henrissat, B.; Nelson, K.E. Comparative genome analysis of Prevotella ruminicola and Prevotella bryantii: Insights into their environmental niche. Microb. Ecol. 2010, 60, 721–729. [Google Scholar] [CrossRef]
- Bekele, A.Z.; Koike, S.; Kobayashi, Y. Genetic diversity and diet specificity of ruminal Prevotella revealed by 16S rRNA gene-based analysis. FEMS Microbiol. Lett. 2010, 305, 49–57. [Google Scholar] [CrossRef]
- Schofield, B.J.; Lachner, N.; Le, O.T.; McNeill, D.N.; Dart, P.; Ouwerkerk, D.; Hugenholtz, P.; Klieve, A.V. Beneficial changes in rumen bacterial community profile in sheep and dairy calves as a result of feeding the probiotic Bacillus amyloliquefaciens H57. J. Appl. Microbiol. 2018, 124, 855–866. [Google Scholar] [CrossRef]
- Mcgregor, N.; Morar, M.; Fenger, T.H.; Stogios, P.; Lenfant, N.; Yin, V.; Xu, X.H.; Evdokimova, E.; Cui, H.; Henrissat, B.; et al. Structure-function analysis of a mixed-linkage β-Glucanase/Xyloglucanase from the key ruminal bacteroidetes Prevotella bryantii B14. J. Biol. Chem. 2016, 291, 1175–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, H.; Ogata, K.; Tajima, K.; Nakamura, M.; Nagamine, T.; Aminov, R.I.; Benno, Y. Phenotypic characterization of polysaccharidases produced by four Prevotella type strains. Curr. Microbiol. 2000, 41, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.L. Assessment of Fiber Digestibility and Energy Metabolizability of Boer Goats’ Diet and Research on the Relationships among Structure of the Rumen Microorganism from Boer Goats and Them. Master’s Thesis, Sichuan Agricultural, Yaan, Sichuan, China, 2016. [Google Scholar]
- Rosenberg, E. The Family Prevotellaceae; Springer: Berlin/Heidelberg, Germany, 2014; pp. 825–827. [Google Scholar]
- Guo, J.G.; Zhang, T.T.; Wu, X.S.; Liu, Z.; Gao, X.H.; Yang, F.H.; Xing, X.M.; Yue, Z.G. Effects of dietary methionine level on reproductive performance of blue foxes (Alopex lagopus) in reproductive period. Chin. J. Anim. Nutr. 2015, 27, 165–170. [Google Scholar]
- Makras, L.; Triantafyllou, V.; Fayol-Messaoudi, D.; Adriany, T.; Zoumpopoulou, G.; Tsakalidou, E.; Servin, A.; De Vuyst, L. Kinetic analysis of the antibacterial activity of probiotic lactobacilli towards Salmonella enterica serovar Typhimurium reveals a role for lactic acid and other inhibitory compounds. Res. Microbiol. 2006, 157, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Huang, Y. Evaluation of bacterial diversity during fermentation process: A comparison between handmade and machine-made high-temperature Daqu of Maotai-flavor liquor. Ann. Microbiol. 2020, 70, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Wang, X.; Li, Q.; Gao, X.; Yi, X.; Ma, Y. High-throughput sequencing method to study the dynamic changes of microbial communities in second-generation fermented lettuce. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Harbin, China, 2021; Volume 680, p. 012064. [Google Scholar]
- Wang, L.; Zhang, H.; Tong, X.L.; Li, J.K. Antibacterial activity of Weissella cibaria strains isolated from Tibetan chickens. Anim. Husb. Vet. Med. 2017, 49, 55–59. [Google Scholar]
- Nam, H.; Ha, M.; Bae, O.; Lee, Y. Effect of Weissella confusa Strain PL9001 on the adherence and growth of Helicobacter pylori. Appl. Environ. Microbiol. 2002, 68, 4642–4645. [Google Scholar] [CrossRef] [Green Version]
- Kavitake, D.; Devi, P.B.; Singh, S.P.; Shetty, P.H. Characterization of a novel galactan produced by Weissella confusa KR780676 from an acidic fermented food. Int. J. Biol. Macromol. 2016, 86, 681–689. [Google Scholar] [CrossRef]
- Maina, N.H.; Tenkanen, M.; Maaheimo, H.; Juvonen, R.; Virkki, L. NMR spectroscopic analysis of exopolysaccharides produced by Leuconostoc citreum and Weissella confusa. Carbohydr. Res. 2008, 343, 1446–1455. [Google Scholar] [CrossRef]
- Devi, P.B.; Kavitake, D.; Shetty, P.H. Physico-chemical characterization of galactan exopolysaccharide produced by Weissella confusa KR780676. J. Biol. Macromol. 2016, 93, 822–828. [Google Scholar] [CrossRef]
- Falck, P.; Linares-Pastén, J.A.; Adlercreutz, P.; Karlsson, E.N. Characterization of a family 43 beta-xylosidase from the xylooligosaccharide utilizing putative probiotic Weissella sp. strain 92. Glycobiology 2016, 26, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Wang, L.Y.; Zhao, D.B.; Liu, X.J. Optimization of fermentation conditions for producing antioxidant ingredient of Weissella hellenica isolated from dry cure beef. Meat Ind. 2016, 1, 19–23. [Google Scholar]
- Liu, C.J.; Liu, Q.; Jiang, B.; Yan, J.F.; Qi, X.H. Antioxidative and cholesterol-rducing Ability of Weissella viridescens ORC4 from dry fermented sausages. J. Food Sci. Biotechnol. 2015, 34, 512–516. [Google Scholar]
- Li, Q.Y.; Chen, J.; Zeng, W.Z.; Fang, F. Isolation and characterization of Weissella strains from soy sauce moromi mash. Microbiol. China 2018, 45, 2449–2462. [Google Scholar]
- Chen, S.S. Analysis of Gut Microbiota Diversity in Blue Fox and Comparative Study on Its Enrichment Methods In Vitro. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. [Google Scholar]


| Ingredient (DM%) | Groups | |
|---|---|---|
| Fish Meal | Fly Maggot Protein | |
| Expanded corn | 36.98 | 36.97 |
| Rice bran meal | 2.70 | 0.50 |
| Soybean meal | 19.99 | 19.98 |
| Expanded soybean | 3.00 | 3.00 |
| Corn gluten meal | 5.00 | 6.50 |
| Distillers Dried Grains with Solubles | 10.00 | 9.99 |
| Fish meal | 6.00 | - |
| Fly maggot complex protein | - | 6.00 |
| Meat and bone meal | 6.00 | 6.00 |
| Feather meal | 5.00 | 5.00 |
| Poultry fat | 4.00 | 4.00 |
| Limestone | 0.00 | 0.40 |
| Sodium chloride | 0.05 | 0.16 |
| Lysine | 0.00 | 0.16 |
| Methionine | 0.30 | 0.35 |
| Premix 1 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 |
| Nutrient levels 2 (DM%) | ||
| Crude protein | 29.90 | 29.92 |
| Crude fat | 8.47 | 8.36 |
| Ash | 6.15 | 5.99 |
| Total phosphorus | 0.89 | 0.72 |
| Available phosphorous | 0.64 | 0.51 |
| Ca | 1.07 | 0.99 |
| Lysine | 1.42 | 1.40 |
| Methionine | 0.79 | 0.79 |
| Salt | 0.43 | 0.45 |
| Score | Diarrhea | Fecal State |
|---|---|---|
| 0 | Normal | Stools come in streaks or pellets |
| 1 | Mild | Forming, soft stools |
| 2 | Moderate | No separation of feces and water, thick and loose stools |
| 3 | Severe | Feces are liquid, formless, the feces are separated from water |
| Items | Groups | t-Value | p-Value | |
|---|---|---|---|---|
| Fish Meal | Fly Maggot Protein | |||
| Day 0 BW/kg | 1.96 ± 0.24 | 2.04 ± 0.28 | −0.730 | 0.475 |
| Day 7 BW/kg | 2.47 ± 0.20 | 2.33 ± 0.22 | 1.488 | 0.154 |
| Day 14 BW/kg | 2.92 ± 0.17 | 2.74 ± 0.29 | 1.788 | 0.091 |
| Day 21 BW/kg | 3.42 ± 0.22 | 3.45 ± 0.30 | −0.254 | 0.802 |
| Day 28 BW/kg | 4.05 ± 0.20 | 4.08 ± 0.34 | −0.199 | 0.845 |
| Days 1 to 7 | ||||
| Average daily gain/g | 74.29 ± 11.27 A | 42.14 ± 22.20 B | 4.082 | 0.001 |
| Diarrhea% | 10.71 ± 7.72 B | 20.00 ± 10.54 A | −2.248 | 0.037 |
| Diarrhea index | 0.63 ± 0.18 | 0.81 ± 0.21 | −2.051 | 0.055 |
| Days 8 to 14 | ||||
| Average daily gain/g | 64.29 ± 17.82 | 57.14 ± 18.75 | 0.873 | 0.394 |
| Diarrhea% | 14.29 ± 13.47 | 20.71 ± 10.88 | −1.174 | 0.256 |
| Diarrhea index | 0.64 ± 0.34 | 0.74 ± 0.12 | −0.940 | 0.367 |
| Days 15 to 21 | ||||
| Average daily gain/g | 70.71 ± 18.58 B | 102.14 ± 19.65 A | −3.675 | 0.002 |
| Diarrhea% | 15.00 ± 10.35 | 15.71 ± 7.38 | −0.178 | 0.861 |
| Diarrhea index | 0.66 ± 0.22 | 0.62 ± 0.17 | 0.404 | 0.691 |
| Days 22 to 28 | ||||
| Average daily gain/g | 90.00 ± 13.55 | 89.29 ± 9.67 | 0.136 | 0.894 |
| Diarrhea% | 11.43 ± 9.04 | 13.57 ± 5.27 | −0.648 | 0.525 |
| Diarrhea index | 0.65 ± 0.27 | 0.83 ± 0.37 | −1.238 | 0.232 |
| Days 0 to 28 | ||||
| Average daily gain/g | 74.82 ± 4.94 | 72.68 ± 7.81 | 0.733 | 0.473 |
| Diarrhea% | 12.86 ± 5.24 b | 17.50 ± 4.44 a | −2.137 | 0.047 |
| Diarrhea index | 0.64 ± 0.17 | 0.75 ± 0.11 | −1.687 | 0.112 |
| Items | Groups | t-Value | p-Value | |
|---|---|---|---|---|
| Fish Meal | Fly Maggot Protein | |||
| IL-1β (ng/L) | ||||
| 0 d | 173.06 ± 21.31 | 183.55 ± 6.67 | −0.183 | 0.462 |
| 7 d | 233.96 ± 13.09 | 223.88 ± 17.84 | 0.789 | 0.474 |
| 14 d | 204.12 ± 10.91 | 210.97 ± 13.09 | −0.697 | 0.524 |
| 21 d | 182.74 ± 12.59 b | 227.10 ± 12.94 a | −4.256 | 0.013 |
| 28 d | 186.37 ± 17.71 | 211.78 ± 4.58 | −2.405 | 0.074 |
| IL-2 (pg/mL) | ||||
| 0 d | 563.02 ± 38.41 B | 800.10 ± 41.94 A | −7.220 | 0.002 |
| 7 d | 681.56 ± 53.80 B | 877.93 ± 28.06 A | −5.605 | 0.005 |
| 14 d | 719.88 ± 40.48 | 733.05 ± 16.20 | −0.523 | 0.628 |
| 21 d | 606.13 ± 30.97 b | 736.64 ± 54.04 a | −3.629 | 0.022 |
| 28 d | 698.32 ± 12.95 | 739.03 ± 45.63 | −1.487 | 0.211 |
| IL-6 (ng/L) | ||||
| 0 d | 51.80 ± 18.51 | 76.42 ± 37.43 | −1.021 | 0.365 |
| 7 d | 58.00 ± 20.60 | 63.26 ± 7.88 | −0.413 | 0.701 |
| 14 d | 63.55 ± 25.12 | 63.80 ± 11.32 | −0.016 | 0.988 |
| 21 d | 45.19 ± 24.46 b | 93.12 ± 16.85 a | −2.795 | 0.049 |
| 28 d | 40.26 ± 7.60 B | 93.15 ± 13.09 A | −6.054 | 0.004 |
| SIgA (µg/mL) | ||||
| 0 d | 29.44 ± 1.08 | 29.94 ± 1.60 | −0.454 | 0.673 |
| 7 d | 28.56 ± 1.85 | 30.64 ± 0.58 | −1.862 | 0.136 |
| 14 d | 27.17 ± 0.49 | 29.67 ± 1.64 | −2.523 | 0.065 |
| 21 d | 31.15 ± 1.06 | 29.95 ± 1.00 | 2.052 | 0.227 |
| 28 d | 32.90 ± 1.60 | 30.05 ± 1.40 | 6.389 | 0.233 |
| IgG (µg/mL) | ||||
| 0 d | 713.46 ± 32.37 b | 796.70 ± 36.09 a | −2.974 | 0.041 |
| 7 d | 715.84 ± 21.70 | 730.11 ± 29.20 | −0.679 | 0.534 |
| 14 d | 720.59 ± 13.51 B | 739.57 ± 20.90 A | −4.800 | 0.009 |
| 21 d | 677.78 ± 44.32 B | 721.13 ± 44.32 A | −5.619 | 0.005 |
| 28 d | 665.89 ± 46.38 b | 739.35 ± 21.41 a | −3.508 | 0.025 |
| IgM (µg/mL) | ||||
| 0 d | 29.35 ± 2.37 | 33.50 ± 0.31 | −3.010 | 0.091 |
| 7 d | 32.90 ± 1.03 B | 38.59 ± 0.74 A | −7.787 | 0.001 |
| 14 d | 30.06 ± 2.42 | 33.50 ± 0.99 | −2.277 | 0.085 |
| 21 d | 33.73 ± 2.52 | 35.27 ± 1.58 | −0.897 | 0.421 |
| 28 d | 33.91 ± 2.08 | 33.02 ± 1.04 | 0.661 | 0.545 |
| TNF-α (ng/L) | ||||
| 0 d | 174.74 ± 4.19 | 155.13 ± 33.23 | 1.014 | 0.415 |
| 7 d | 167.83 ± 31.07 | 145.64 ± 84.19 | 0.428 | 0.690 |
| 14 d | 175.74 ± 23.98 | 123.28 ± 14.01 | −1.406 | 0.233 |
| 21 d | 181.11 ± 34.10 | 151.22 ± 7.88 | 1.479 | 0.213 |
| 28 d | 192.54 ± 24.15 | 201.22 ± 17.79 | −0.502 | 0.642 |
| Items | Groups | t-Value | p-Value | |
|---|---|---|---|---|
| Fish Meal | Fly Maggot Protein | |||
| MDA (nmol/mL) | ||||
| 0 d | 103.14 ± 1.86 | 100.40 ± 2.80 | 1.413 | 0.230 |
| 7 d | 103.05 ± 0.59 | 102.20 ± 2.80 | 0.515 | 0.654 |
| 14 d | 107.86 ± 2.09 | 104.65 ± 2.16 | 1.849 | 0.138 |
| 21 d | 106.64 ± 1.71 | 104.75 ± 2.80 | 0.999 | 0.374 |
| 28 d | 111.55 ± 3.61 | 104.56 ± 0.75 | 3.283 | 0.073 |
| SOD (U/mL) | ||||
| 0 d | 932.31 ± 17.40 A | 668.37 ± 14.37 B | 20.257 | <0.001 |
| 7 d | 876.39 ± 4.73 A | 724.30 ± 2.05 B | 51.160 | <0.001 |
| 14 d | 879.11 ± 23.54 A | 775.45 ± 23.06 B | 5.449 | 0.006 |
| 21 d | 927.54 ± 21.88 A | 737.25 ± 27.25 B | 9.432 | 0.001 |
| 28 d | 886.62 ± 16.41 A | 741.38 ± 27.78 B | 10.105 | 0.001 |
| T-AOC (U/mL) | ||||
| 0 d | 171.02 ± 5.27 a | 154.58 ± 5.54 b | 3.721 | 0.020 |
| 7 d | 169.24 ± 3.53 A | 150.12 ± 4.78 B | 5.571 | 0.005 |
| 14 d | 166.44 ± 3.91 a | 152.04 ± 4.05 b | 4.432 | 0.011 |
| 21 d | 165.42 ± 3.34 A | 152.99 ± 2.13 B | 9.808 | 0.001 |
| 28 d | 165.29 ± 2.49 | 160.57 ± 4.65 | 1.548 | 0.196 |
| Items | ACE | Chao1 | Simpson | Shannon |
|---|---|---|---|---|
| 0 d | ||||
| F1 | 386.14 ± 23.59 | 397.32 ± 35.01 | 0.90 ± 0.08 | 4.82 ± 0.92 a |
| M1 | 374.10 ± 20.09 | 376.29 ± 18.38 | 0.76 ± 0.14 | 3.53 ± 0.76 b |
| 7 d | ||||
| F7 | 430.59 ± 23.38 | 427.58 ± 21.40 | 0.88 ± 0.13 | 5.04 ± 1.22 |
| M7 | 412.34 ± 13.68 | 413.90 ± 15.85 | 0.88 ± 0.11 | 4.67 ± 0.98 |
| 14 d | ||||
| F14 | 436.65 ± 10.72 | 437.48 ± 9.97 | 0.95 ± 0.01 | 5.53 ± 0.19 A |
| M14 | 427.35 ± 36.86 | 419.26 ± 21.12 | 0.92 ± 0.02 | 4.95 ± 0.31 B |
| 21 d | ||||
| F21 | 436.19 ± 11.90 | 436.76 ± 10.33 | 0.94 ± 0.02 | 5.31 ± 0.39 |
| M21 | 391.90 ± 46.89 | 387.47 ± 59.51 | 0.87 ± 0.12 | 4.49 ± 1.14 |
| 28 d | ||||
| F28 | 392.65 ± 11.82 | 406.49 ± 23.06 | 0.93 ± 0.02 | 5.29 ± 0.29 |
| M28 | 359.04 ± 45.55 | 363.32 ± 48.99 | 0.83 ± 0.24 | 4.58 ± 1.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Su, H.; Li, T.; Lv, J.; Liu, J.; Bai, X. Effects of Fly Maggot Protein Replacement of Fish Meal on Growth Performance, Immune Level, Antioxidant Level, and Fecal Flora of Blue Foxes at Weaning Stage. Animals 2022, 12, 1480. https://doi.org/10.3390/ani12121480
Xu Y, Su H, Li T, Lv J, Liu J, Bai X. Effects of Fly Maggot Protein Replacement of Fish Meal on Growth Performance, Immune Level, Antioxidant Level, and Fecal Flora of Blue Foxes at Weaning Stage. Animals. 2022; 12(12):1480. https://doi.org/10.3390/ani12121480
Chicago/Turabian StyleXu, Yuan, Hang Su, Ting Li, Jing Lv, Jiayu Liu, and Xiujuan Bai. 2022. "Effects of Fly Maggot Protein Replacement of Fish Meal on Growth Performance, Immune Level, Antioxidant Level, and Fecal Flora of Blue Foxes at Weaning Stage" Animals 12, no. 12: 1480. https://doi.org/10.3390/ani12121480
APA StyleXu, Y., Su, H., Li, T., Lv, J., Liu, J., & Bai, X. (2022). Effects of Fly Maggot Protein Replacement of Fish Meal on Growth Performance, Immune Level, Antioxidant Level, and Fecal Flora of Blue Foxes at Weaning Stage. Animals, 12(12), 1480. https://doi.org/10.3390/ani12121480





