Effect of Different Basal Culture Media and Sera Type Combinations on Primary Broiler Chicken Muscle Satellite Cell Heterogeneity during Proliferation and Differentiation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Muscle Satellite Cell Isolation
2.2. Satellite Cell Culture Conditions
2.3. Indirect Immunofluorescence Staining
2.4. Primary Satellite Cell Taxonomy
2.5. Statistical Analysis
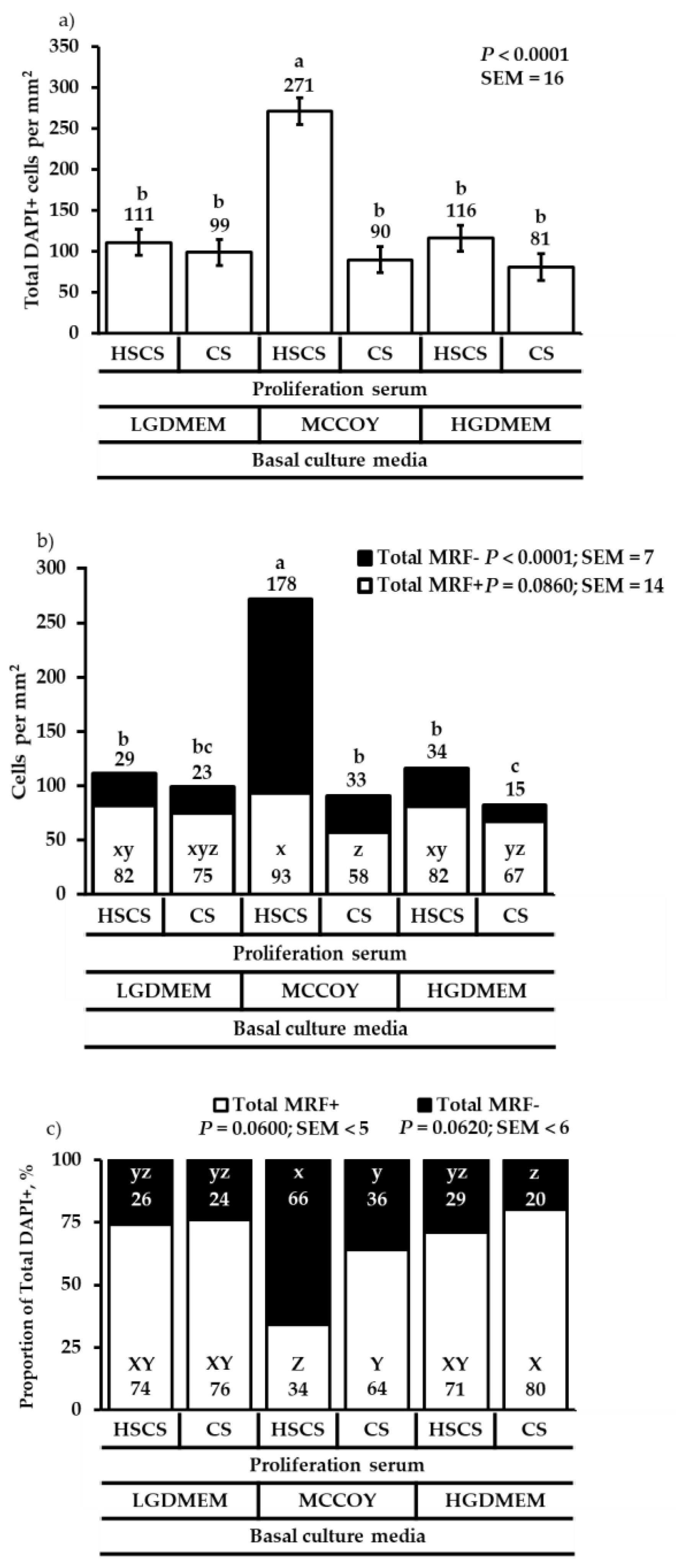
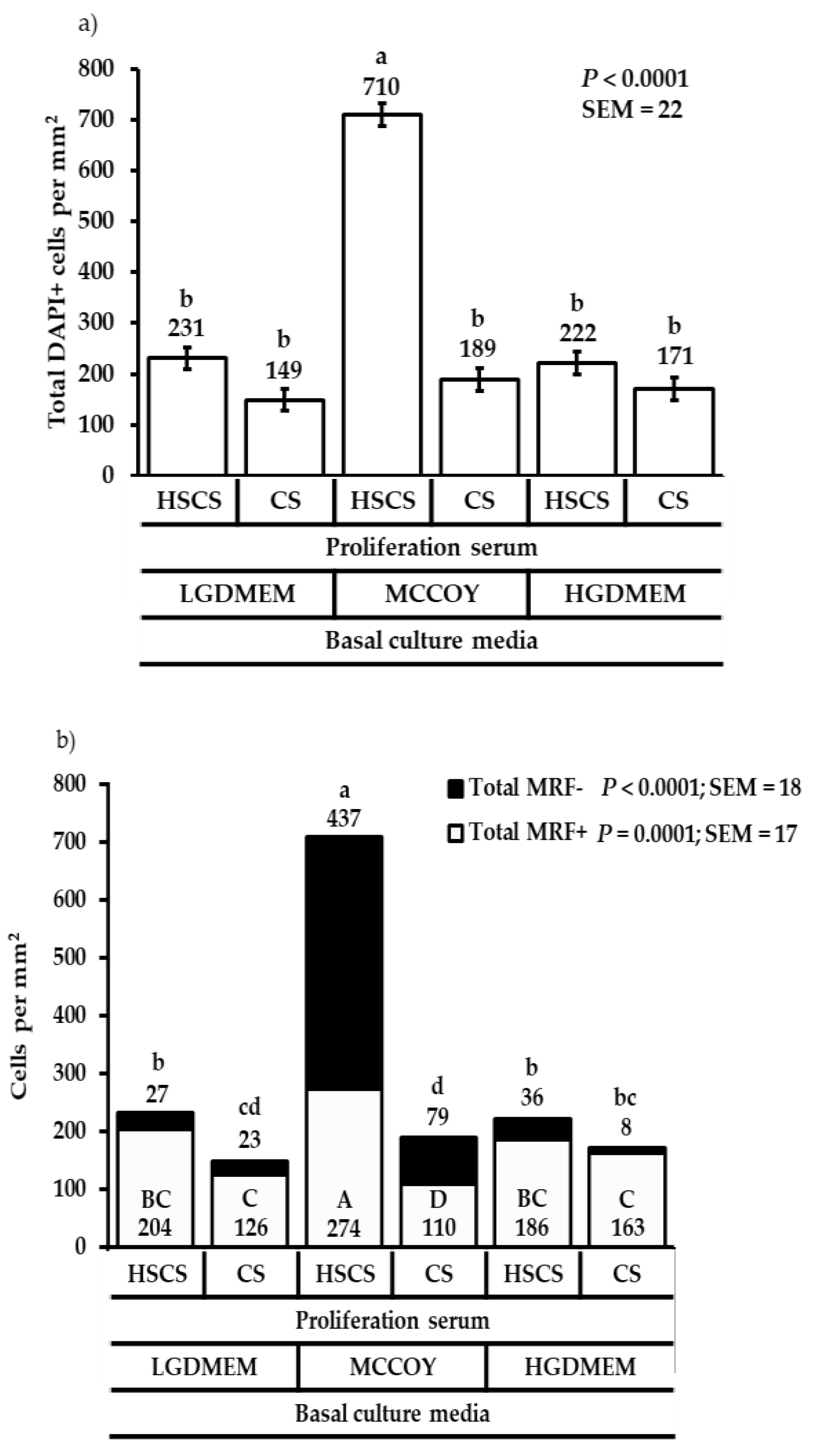
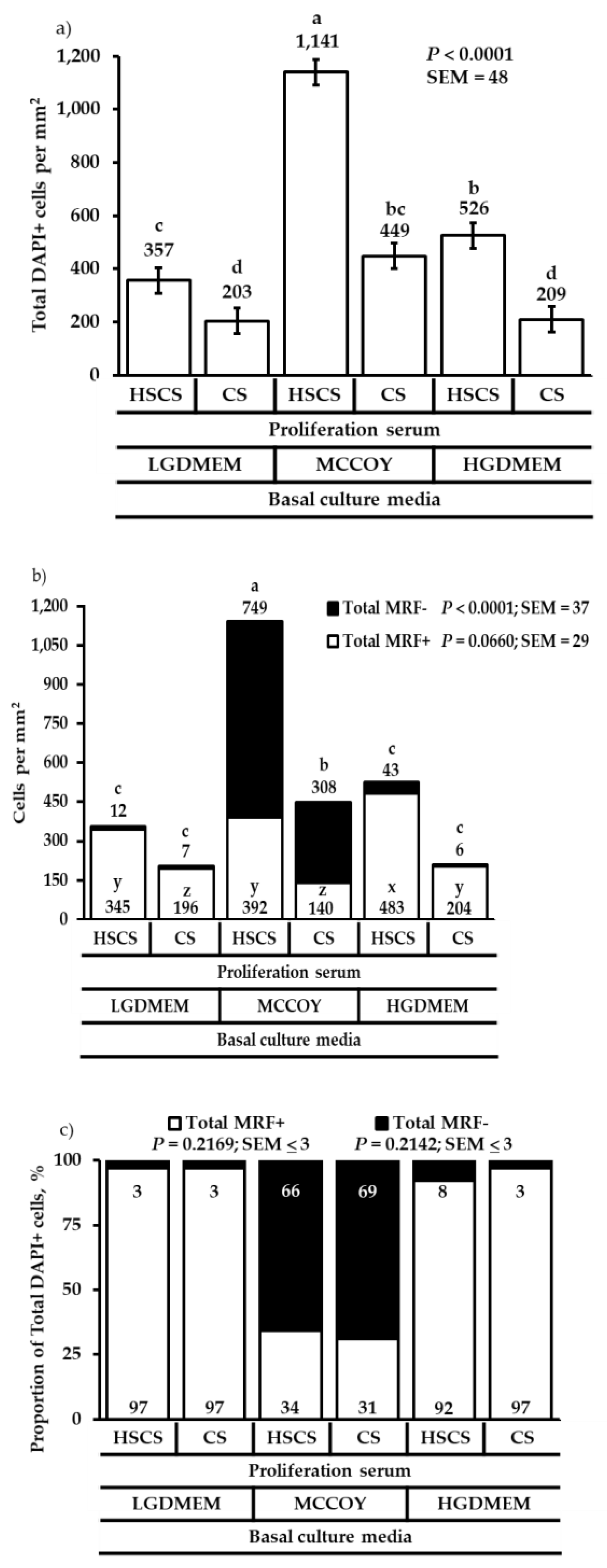
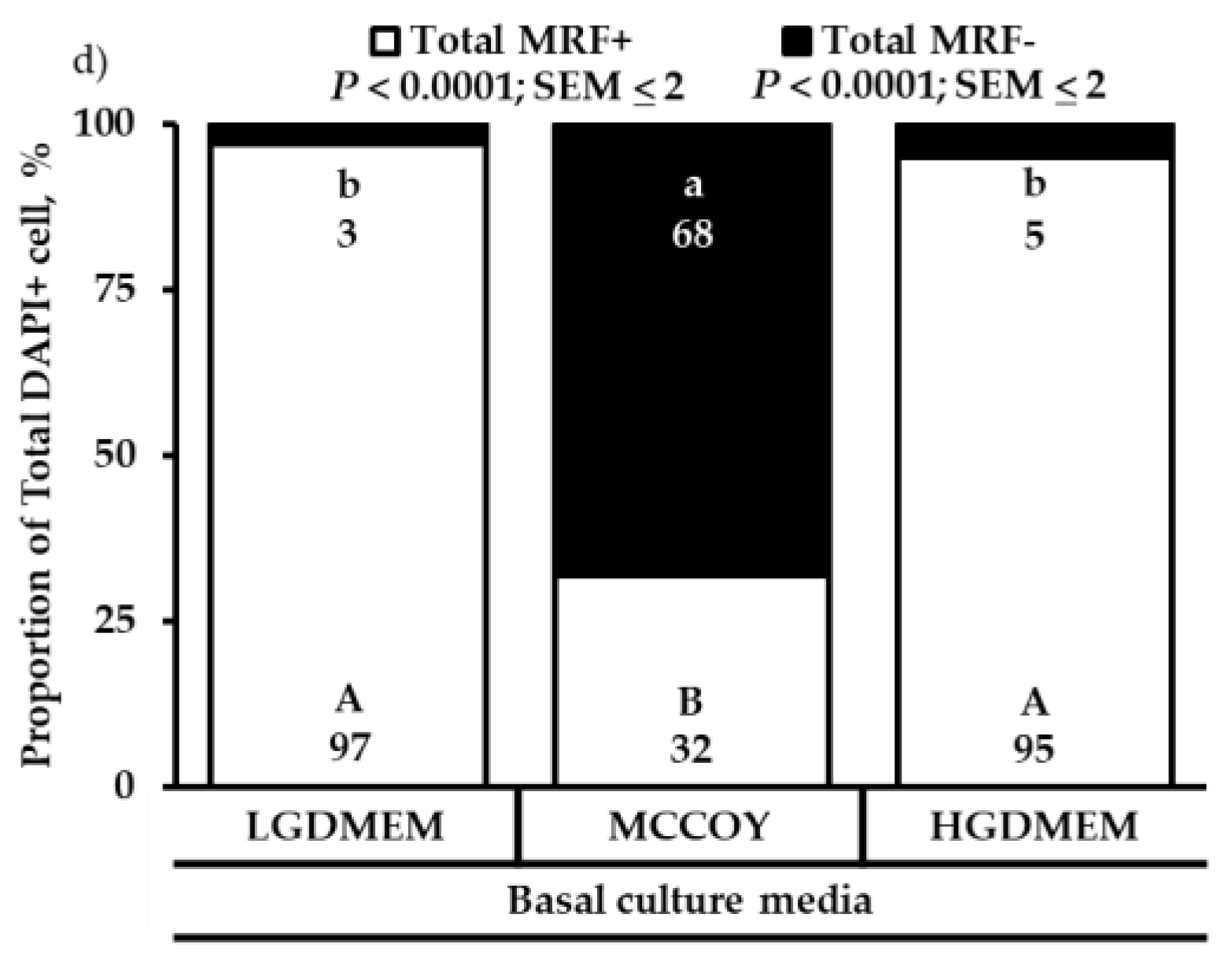
3. Results
3.1. Proliferation Heterogeneity and Population Doubling Time
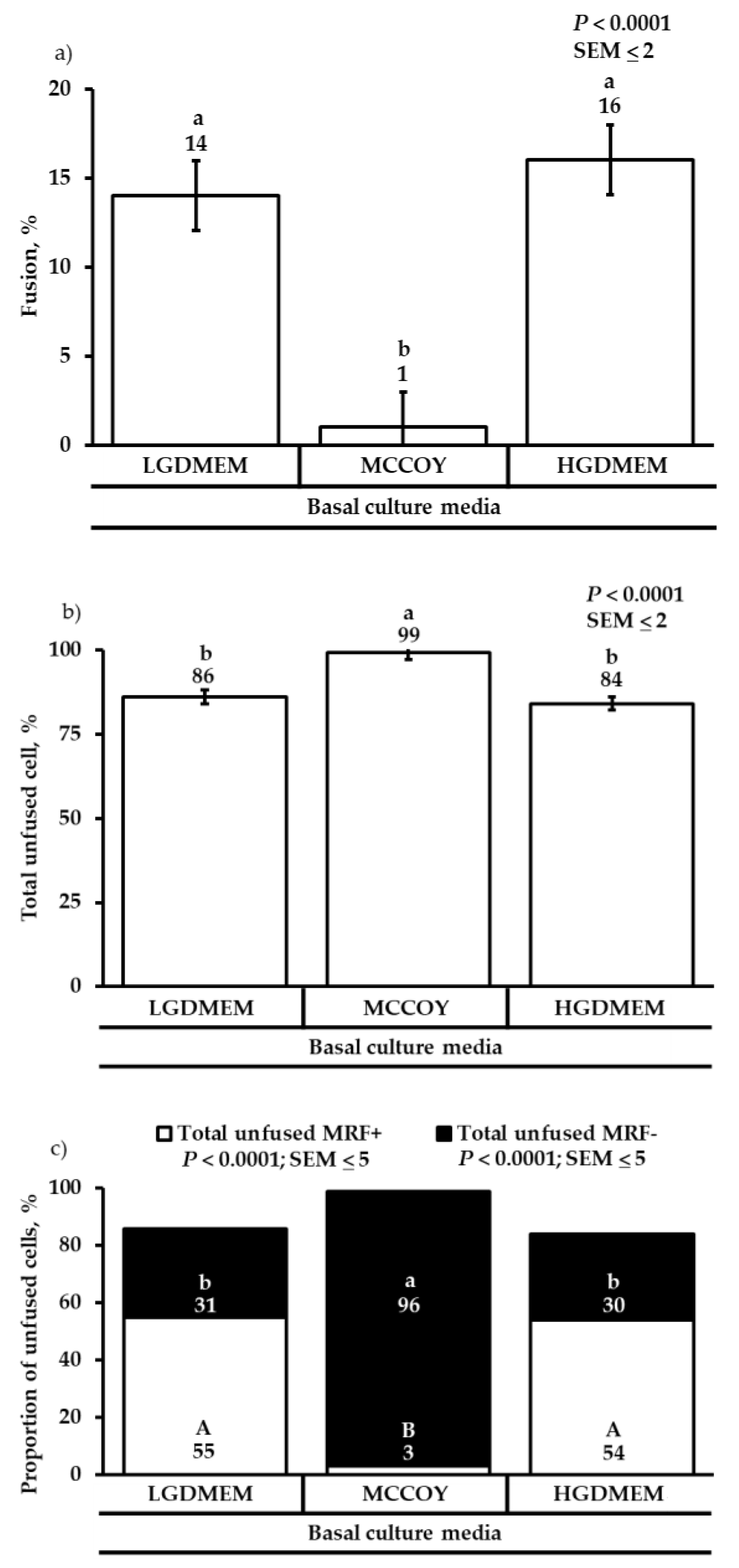
3.2. Differentiation Heterogeneity and Satellite Cell Fusion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Remignon, H.; Gardahaut, M.F.; Marche, G.; Ricard, F.H. Selection for rapid growth increases the number and the size of muscle fibres without changing their typing in chickens. J. Muscle Res. Cell Motil. 1995, 16, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev. Biol. 1986, 115, 129–139. [Google Scholar] [CrossRef]
- Moss, F.P.; Leblond, C.P. Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 1971, 170, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Yablonka-Reuveni, Z.; Quinn, L.B.S.; Nameroff, M. Isolation and Clonal Analysis of Satellite Cells from Chicken Pectoralis Muscle. Dev. Biol. 1987, 119, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Dodson, M.V.; Martin, E.L.; Brannon, M.A.; Mathison, B.A.; McFarland, D.C. Optimization of bovine satellite cell-derived myotube formation in vitro. Tissue Cell 1987, 19, 159–166. [Google Scholar] [CrossRef]
- Will, K.; Schering, L.; Albrecht, E.; Kalbe, C.; Maak, S. Differentiation of bovine satellite cell-derived myoblasts under different culture conditions. In Vitro Cell Dev. Biol. Anim. 2015, 51, 885–889. [Google Scholar] [CrossRef]
- Woods, T.L.; Smith, C.W.; Zeece, M.G.; Jones, S.J. Conditions for the culture of bovine embryonic myogenic cells. Tissue Cell 1997, 29, 207–215. [Google Scholar] [CrossRef]
- Dodson, M.V.; Mathison, B.A.; Mathison, B.D. Effects of medium and substratum on ovine satellite cell attachment, proliferation and differentiation in vitro. Cell Differ. Dev. 1990, 29, 59–66. [Google Scholar] [CrossRef]
- Doumit, M.E.; Merkel, R.A. Conditions for isolation and culture of porcine myogenic satellite cells. Tissue Cell 1992, 24, 253–262. [Google Scholar] [CrossRef]
- McFarland, D.C.; Doumit, M.E.; Minshall, R.D. The turkey myogenic satellite cell: Optimization of in vitro proliferation and differentiation. Tissue Cell 1988, 20, 899–908. [Google Scholar] [CrossRef]
- Allen, R.E.; Dodson, M.V.; Luiten, L.S.; Boxhorn, L.K. A serum-free medium that supports the growth of cultured skeletal muscle satellite cells. In Vitro Cell Dev. Biol. 1985, 21, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Daughtry, M.R.; Berio, E.; Shen, Z.; Suess, E.J.R.; Shah, N.; Geiger, A.E.; Berguson, E.R.; Dalloul, R.A.; Persia, M.E.; Shi, H.; et al. Satellite cell-mediated breast muscle regeneration decreases with broiler size. Poult. Sci. 2017, 96, 3457–3464. [Google Scholar] [CrossRef] [PubMed]
- Halevy, O.; Piestun, Y.; Allouh, M.Z.; Rosser, B.W.; Rinkevich, Y.; Reshef, R.; Rozenboim, I.; Wleklinski-Lee, M.; Yablonka-Reuveni, Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev. Dyn. 2004, 231, 489–502. [Google Scholar] [CrossRef]
- Harding, R.L.; Clark, D.L.; Halevy, O.; Coy, C.S.; Yahav, S.; Velleman, S.G. The effect of temperature on apoptosis and adipogenesis on skeletal muscle satellite cells derived from different muscle types. Physiol. Rep. 2015, 3, e12539. [Google Scholar] [CrossRef]
- Hines, E.A.; Coffey, J.D.; Starkey, C.W.; Chung, T.K.; Starkey, J.D. Improvement of maternal vitamin D status with 25-hydroxycholecalciferol positively impacts porcine fetal skeletal muscle development and myoblast activity. J. Anim. Sci. 2013, 91, 4116–4122. [Google Scholar] [CrossRef]
- Li, J.; Gonzalez, J.M.; Walker, D.K.; Hersom, M.J.; Ealy, A.D.; Johnson, S.E. Evidence of heterogeneity within bovine satellite cells isolated from young and adult animals. J. Anim. Sci. 2011, 89, 1751–1757. [Google Scholar] [CrossRef] [Green Version]
- Café, M.B.; Rinaldi, F.P.; Morais, H.R.; Nascimento, M.; Mundim, A.V.; Marchini, C.F.P. Biochemical blood parameters of broilers at different ages under thermoneutral environment. Worlds Poult. Sci. J. 2012, 5, 143–146. [Google Scholar]
- Aguiari, P.; Leo, S.; Zavan, B.; Vindigni, V.; Rimessi, A.; Bianchi, K.; Franzin, C.; Cortivo, R.; Rossato, M.; Vettor, R.; et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc. Natl. Acad Sci. USA 2008, 105, 1226–1231. [Google Scholar] [CrossRef] [Green Version]
- Guillet-Deniau, I.; Pichard, A.L.; Kone, A.; Esnous, C.; Nieruchalski, M.; Girard, J.; Prip-Buus, C. Glucose induces de novo lipogenesis in rat muscle satellite cells through a sterol-regulatory-element-binding-protein-1c-dependent pathway. J. Cell Sci. 2004, 117, 1937–1944. [Google Scholar] [CrossRef] [Green Version]
- Furuichi, Y.; Kawabata, Y.; Aoki, M.; Mita, Y.; Fujii, N.L.; Manabe, Y. Excess Glucose Impedes the Proliferation of Skeletal Muscle Satellite Cells Under Adherent Culture Conditions. Front. Cell Dev. Biol. 2021, 9, 640399. [Google Scholar] [CrossRef] [PubMed]
- Zuidhof, M.J.; Schneider, B.L.; Carney, V.L.; Korver, D.R.; Robinson, F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014, 93, 2970–2982. [Google Scholar] [CrossRef] [PubMed]
- Havenstein, G.B.; Ferket, P.R.; Qureshi, M.A. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003, 82, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Havenstein, G.B.; Ferket, P.R.; Qureshi, M.A. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003, 82, 1500–1508. [Google Scholar] [CrossRef]
- Havenstein, G.B.; Ferket, P.R.; Scheideler, S.E.; Larson, B.T. Growth, livability, and feed conversion of 1957 vs 1991 broilers when fed “typical” 1957 and 1991 broiler diets. Poult. Sci. 1994, 73, 1785–1794. [Google Scholar] [CrossRef]
- Havenstein, G.B.; Ferket, P.R.; Scheideler, S.E.; Rives, D.V. Carcass composition and yield of 1991 vs 1957 broilers when fed “typical” 1957 and 1991 broiler diets. Poult. Sci. 1994, 73, 1795–1804. [Google Scholar] [CrossRef]


| Cell Populations 3 | Basal Culture Media 1 | SEM 4 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| LGDMEM | MCCOY | HGDMEM | ||||||
| Proliferation Serum 2 | ||||||||
| HSCS | CS | HSCS | CS | HSCS | CS | |||
| Myf-5+ | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0.8210 |
| MyoD+ | - | - | - | - | - | - | - | - |
| Pax7+ | 18 | 27 | 17 | 10 | 24 | 12 | 5 | 0.1442 |
| Myf-5+:MyoD+ | 1 | 1 | 4 | 1 | 0 | 1 | 1 | 0.1162 |
| Myf-5+:Pax7+ | 39 | 28 | 26 | 21 | 35 | 38 | 4 | 0.2069 |
| MyoD+:Pax7+ | 0 | 0 | 1 | 0 | 1 | 0 | 0.3 | 0.1004 |
| Myf-5+:MyoD+:Pax7+ | 23 yz | 19 yz | 45 x | 25 y | 23 yz | 16 z | 4 | 0.0911 |
| Cell Populations 3 | Basal Culture Media 1 | SEM 4 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| LGDMEM | MCCOY | HGDMEM | ||||||
| Proliferation Serum 2 | ||||||||
| HSCS | CS | HSCS | CS | HSCS | CS | |||
| Myf-5+ | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1.000 |
| MyoD+ | - | - | - | - | - | - | - | - |
| Pax7+ | 18 b | 22 b | 43 a | 11 b | 16 b | 18 b | 6 | 0.004 |
| Myf-5+:MyoD+ | 26 a | 8 b | 8 b | 3 b | 27 a | 8 b | 3 | 0.015 |
| Myf-5+:Pax7+ | 32 | 16 | 15 | 4 | 22 | 19 | 3 | 0.1191 |
| MyoD+:Pax7+ | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 0.3200 |
| Myf-5+:MyoD+:Pax7+ | 126 b | 79 d | 206 a | 92 cd | 119 bc | 117 bc | 12 | <0.0001 |
| Cell Populations 3 | Basal Culture Media 1 | SEM 4 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| LGDMEM | MCCOY | HGDMEM | ||||||
| Proliferation Serum 2 | ||||||||
| HSCS | CS | HSCS | CS | HSCS | CS | |||
| MYF5+ | 2 | 2 | 1 | 1 | 4 | 1 | 1 | 0.0986 |
| MYOD+ | - | - | - | - | - | - | - | - |
| PAX7+ | 28 | 16 | 66 | 21 | 75 | 23 | 11 | 0.1640 |
| MYF5+:MYOD+ | 34 b | 9 c | 21 bc | 6 c | 78 a | 9 c | 6 | 0.0001 |
| MYF5+:PAX7+ | 56 | 33 | 17 | 6 | 65 | 29 | 7 | 0.2297 |
| MYOD+:PAX7+ | 2 | 7 | 1 | 2 | 0 | 1 | 3 | 0.6778 |
| MYF5+:MYOD+:PAX7+ | 224 | 130 | 287 | 105 | 261 | 140 | 19 | 0.0572 |
| Cell Populations 4 | Basal Culture Media 1 | SEM 5 | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LGDMDM | MCCOY | HGDMEM | ||||||||||||
| Proliferation Serum 2 | ||||||||||||||
| HSCS | CS | HSCS | CS | HSCS | CS | |||||||||
| Differentiation Serum 3 | ||||||||||||||
| HS | CS | HS | CS | HS | CS | HS | CS | HS | CS | HS | CS | |||
| Fused myonuclei | ||||||||||||||
| Myonuclei: MRF- | 10 d | 81 b | 16 cd | 18 cd | 2 d | 1 d | 1 d | 1 d | 16 dc | 130 a | 8 cd | 35 c | 8 | 0.0002 |
| MYOD+ | 0 b | 3 b | 0 b | 0 b | 0 b | 0 b | 0 b | 0 b | 0 b | 10 a | 3 b | 0 b | 2 | 0.0108 |
| PAX7+ | 10 | 27 | 6 | 27 | 1 | 1 | 0 | 0 | 5 | 29 | 4 | 39 | 6 | 0.8192 |
| MYOD+:PAX7+ | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 1 | 1 | 0.2266 |
| Total myonuclei | 20 de | 112 b | 23 de | 45 d | 3 e | 2 e | 1 e | 1 e | 21 de | 175 a | 15 e | 75 c | 9 | 0.0007 |
| Unfused cell populations | ||||||||||||||
| MYOD+ | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| PAX7+ | 78 bc | 380 a | 57 cd | 139 b | 27 cd | 49 cd | 9 d | 9 d | 79 bc | 441 a | 61 cd | 133 b | 22 | <0.0001 |
| MYOD+:PAX7+ | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Total unfused MRF+ | 78 cd | 380 b | 57 de | 139 c | 27 de | 10 de | 9 e | 9 e | 79 cd | 443 a | 61 de | 133 c | 22 | <0.0001 |
| Total unfused MRF- | 29 | 436 | 13 | 396 | 684 | 965 | 374 | 478 | 20 | 508 | 12 | 257 | 71 | 0.5308 |
| Total unfused cells | 107 | 815 | 70 | 535 | 711 | 1015 | 383 | 487 | 99 | 952 | 73 | 389 | 61 | 0.1081 |
| Total DAPI+ cells | 127 f | 927 b | 93 f | 580 cd | 714 c | 1017 ab | 384 e | 488 de | 119 f | 1126 a | 87 f | 463 de | 62 | 0.041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flees, J.J.; Starkey, C.W.; Starkey, J.D. Effect of Different Basal Culture Media and Sera Type Combinations on Primary Broiler Chicken Muscle Satellite Cell Heterogeneity during Proliferation and Differentiation. Animals 2022, 12, 1425. https://doi.org/10.3390/ani12111425
Flees JJ, Starkey CW, Starkey JD. Effect of Different Basal Culture Media and Sera Type Combinations on Primary Broiler Chicken Muscle Satellite Cell Heterogeneity during Proliferation and Differentiation. Animals. 2022; 12(11):1425. https://doi.org/10.3390/ani12111425
Chicago/Turabian StyleFlees, Joshua J., Charles W. Starkey, and Jessica D. Starkey. 2022. "Effect of Different Basal Culture Media and Sera Type Combinations on Primary Broiler Chicken Muscle Satellite Cell Heterogeneity during Proliferation and Differentiation" Animals 12, no. 11: 1425. https://doi.org/10.3390/ani12111425
APA StyleFlees, J. J., Starkey, C. W., & Starkey, J. D. (2022). Effect of Different Basal Culture Media and Sera Type Combinations on Primary Broiler Chicken Muscle Satellite Cell Heterogeneity during Proliferation and Differentiation. Animals, 12(11), 1425. https://doi.org/10.3390/ani12111425





