Improved Joint Health Following Oral Administration of Glycosaminoglycans with Native Type II Collagen in a Rabbit Model of Osteoarthritis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Test Compounds
2.2. Histological Evaluation
- Macroscopic evaluation
- b.
- Microscopic evaluation
2.3. Synovial Fluid Hyaluronic Acid Measurements
2.4. MRI Quantitative Biomarkers Analysis
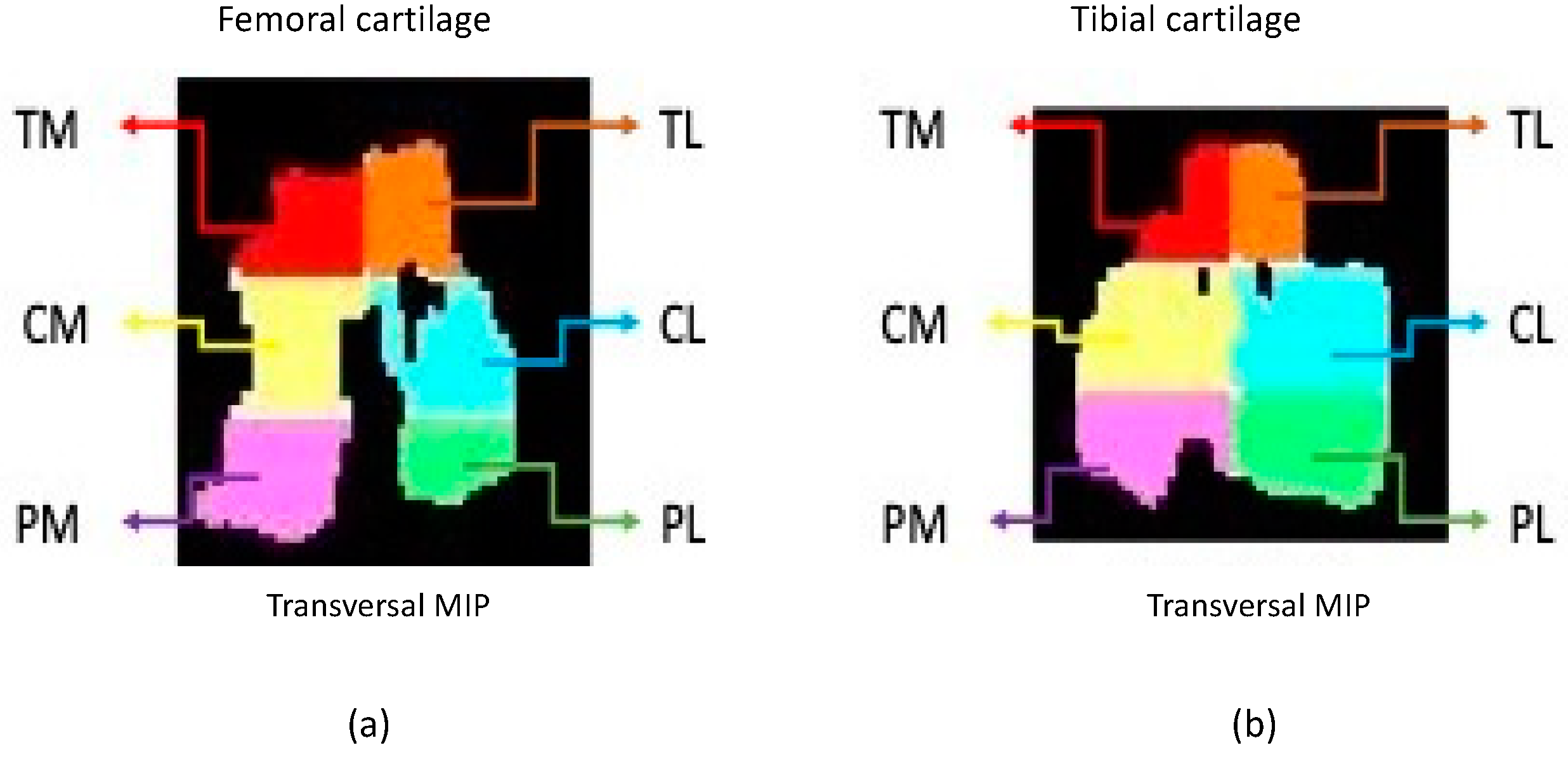
- Acquisition, preparation and processing of the imagesA 3 Tesla clinical scanner (Philips Achieva 3.0 TX, Madrid, Spain) with a 16-channel coil was used to perform the study. Cartilage imaging was performed with 3 different sequences on the sagittal plane for each individual stifle. A scan of the subchondral bone trabecula was performed with 3D high-resolution T1-weighted balanced Fast Field Echo (T1-FFE-3D) acquired on the transversal plane.Cartilage and subchondral bone images were transformed to NIfTI (Neuroimaging Informatics Technology Initiative) format to allow evaluation using the free distribution ITK-Snap software [38,39]. Femoral and tibial cartilage parcellation was done following the 6 segment scheme: medial anterior region (TM), lateral anterior region (TL), medial central region (CM), lateral central region (CL), medial posterior region (PM) and lateral posterior region (PL) [32,40] (Figure 1). Subchondral bone parcellation followed the 2-parcel scheme labeling both as medial and lateral [40].Prior to the cartilage image processing, open code Elastix toolbox [41]. was used for the spatial recording of the different eco times and flip angles into a common geometric space corresponding to a high-resolution Turbo Spin Echo T1 weighted sequence with fat suppression (T1 TSE SPIR). The imaging biomarkers were extracted using an ad-hoc program written in MATLAB (R2016b, Mathworks, Natick, MA, USA) for both cartilage and subchondral bone.
- Articular cartilage biomarkersCartilage longitudinal T1 relaxation time analysis was computed with the flip angles (2, 5, 10, 15, 25 and 45°) in a voxel-wise approach. The calculation for the longitudinal relaxation time was performed using the method described by Fram et al. (1987) [42,43].The cartilage transversal T2 relaxation time analysis used all the echo times (2.7, 4.1, 5.5, 6.9, 8.3, 9.7, 11.1, 12.5, 13.9, 15.3, 16.7, 18.1, 19.5, 20.9, 22.3, 23.7) and the method described by Li et al. (1996) [44].Cartilage volume and thickness analysis for each cartilage segments were obtained as described by Alberich-Bayarri et al. (2008) [33].
- Subchondral bone biomarkersTrabecular bone volume analysis used an algorithm based on local Laplacian to reduce heterogenicity and partial volume effect presents on the region of interest to obtain the bone volume fraction [45].Bone volume to total volume (BVTV) was calculated using the ratio between the number of voxels in the trabeculae and the total number of voxels of the volume of interest (VOI), Tb.Th and Tb.Sp were calculated based on the distance transformation of the skeleton on the contour as described by Alberich-Bayarri et al. (2008) [33]. TbN can be calculated as the ratio between BVTV and Tb.Th. The spatial distribution of the trabeculae was also evaluated by calculating the D2D and D3D Fractal Dimensions as described by Alberich-Bayarri et al. (2010) [34]. QTS was calculated, this biomarker provides a single score that reflects the quality of the bone trabecula (patent filing ID: 201931050)
2.5. Statistical Analysis
3. Results
3.1. Histological Study
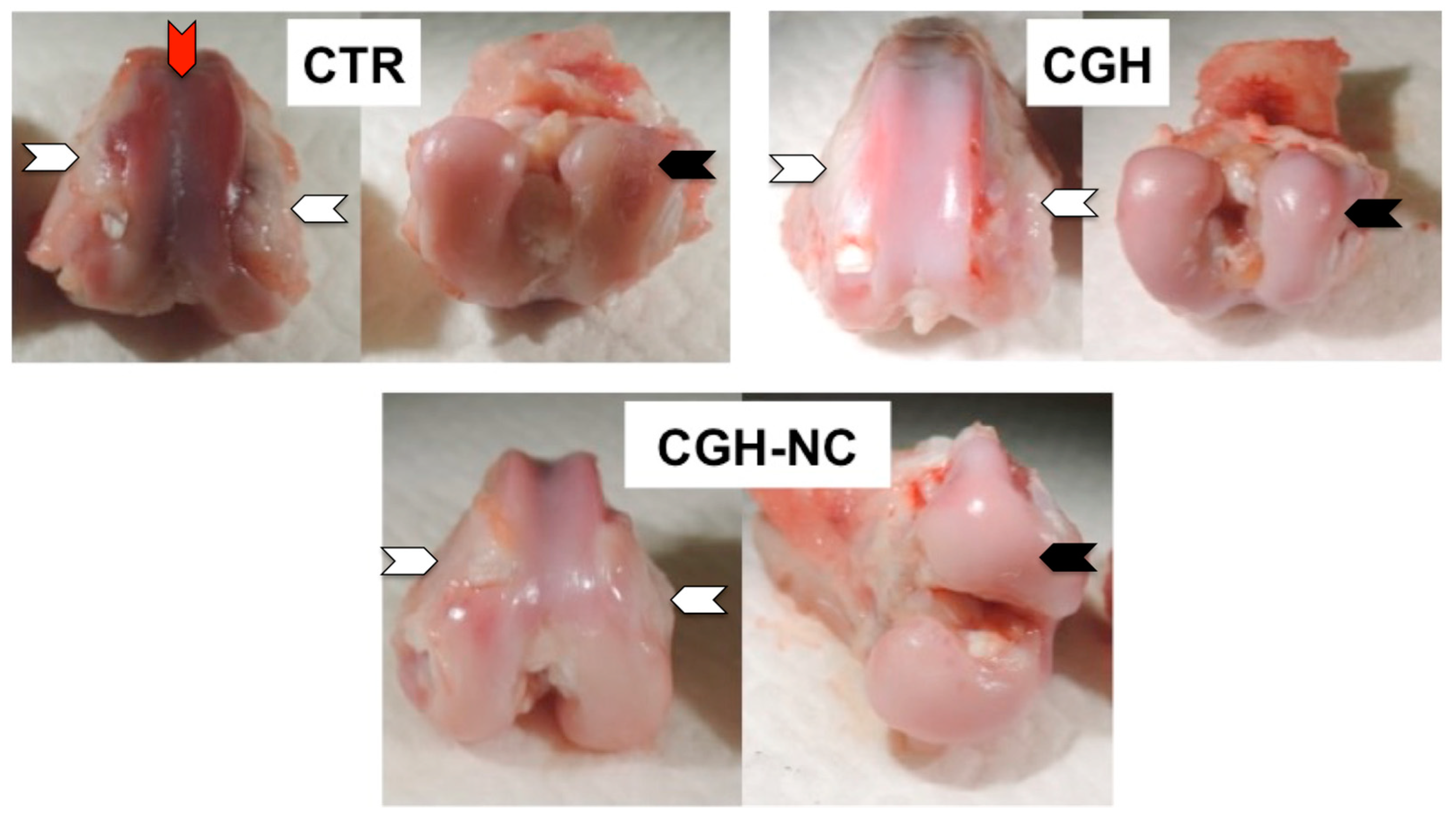
3.1.1. Macroscopic Evaluation
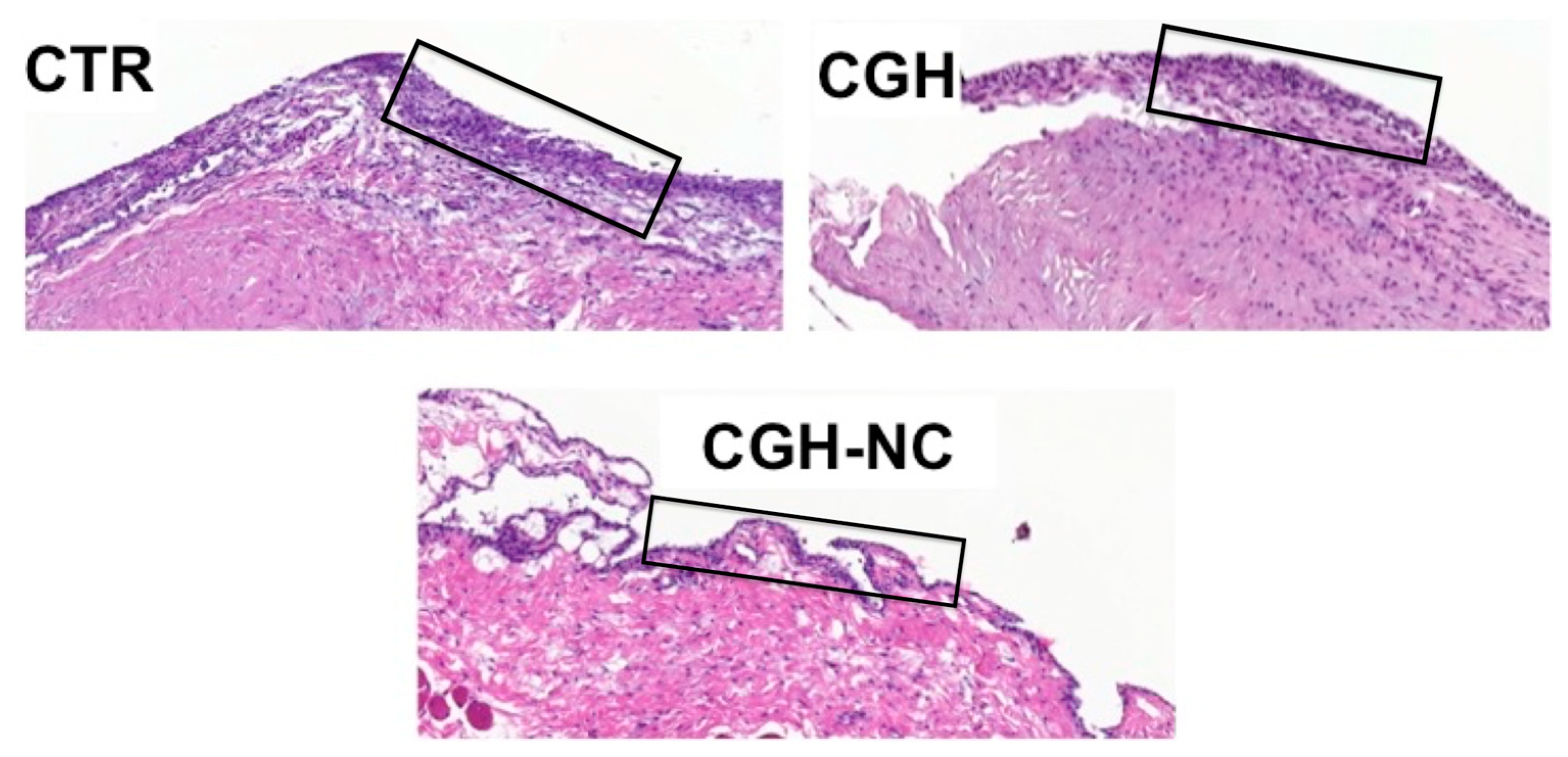
3.1.2. Microscopic Evaluation
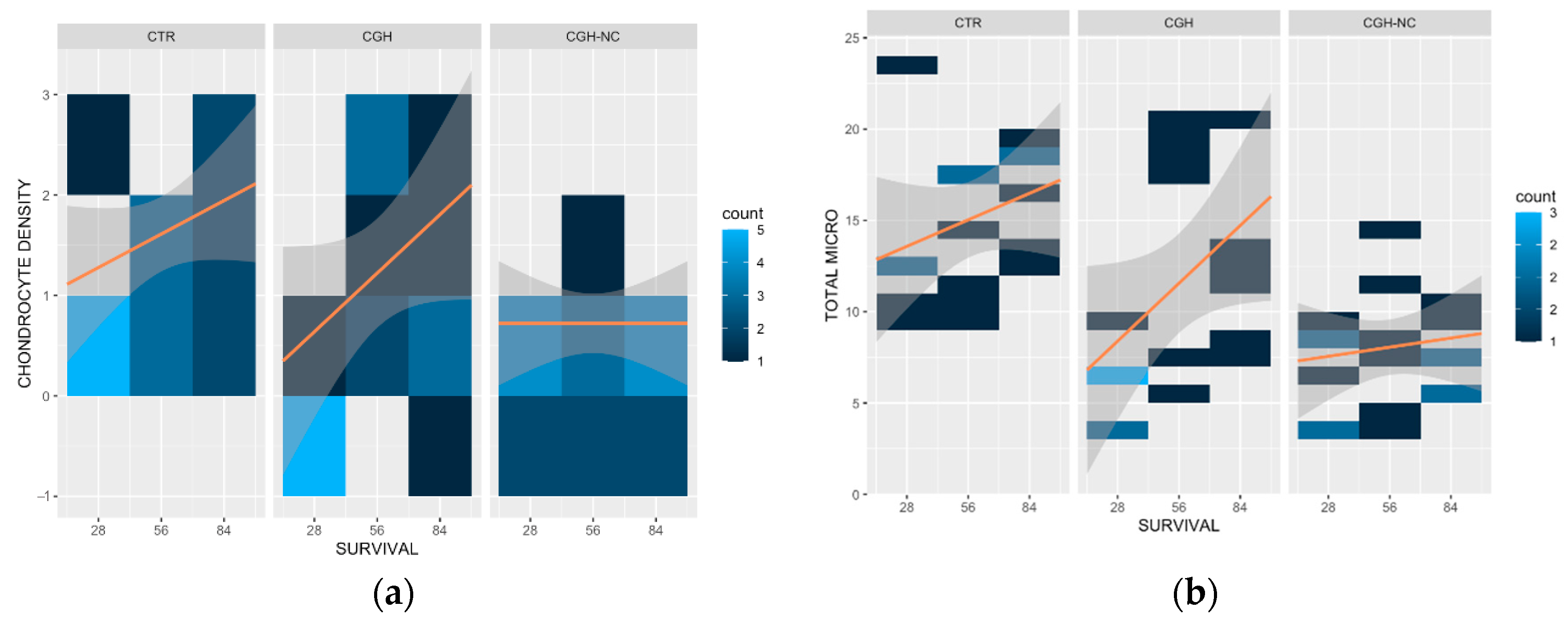
- Articular Cartilage
- b.
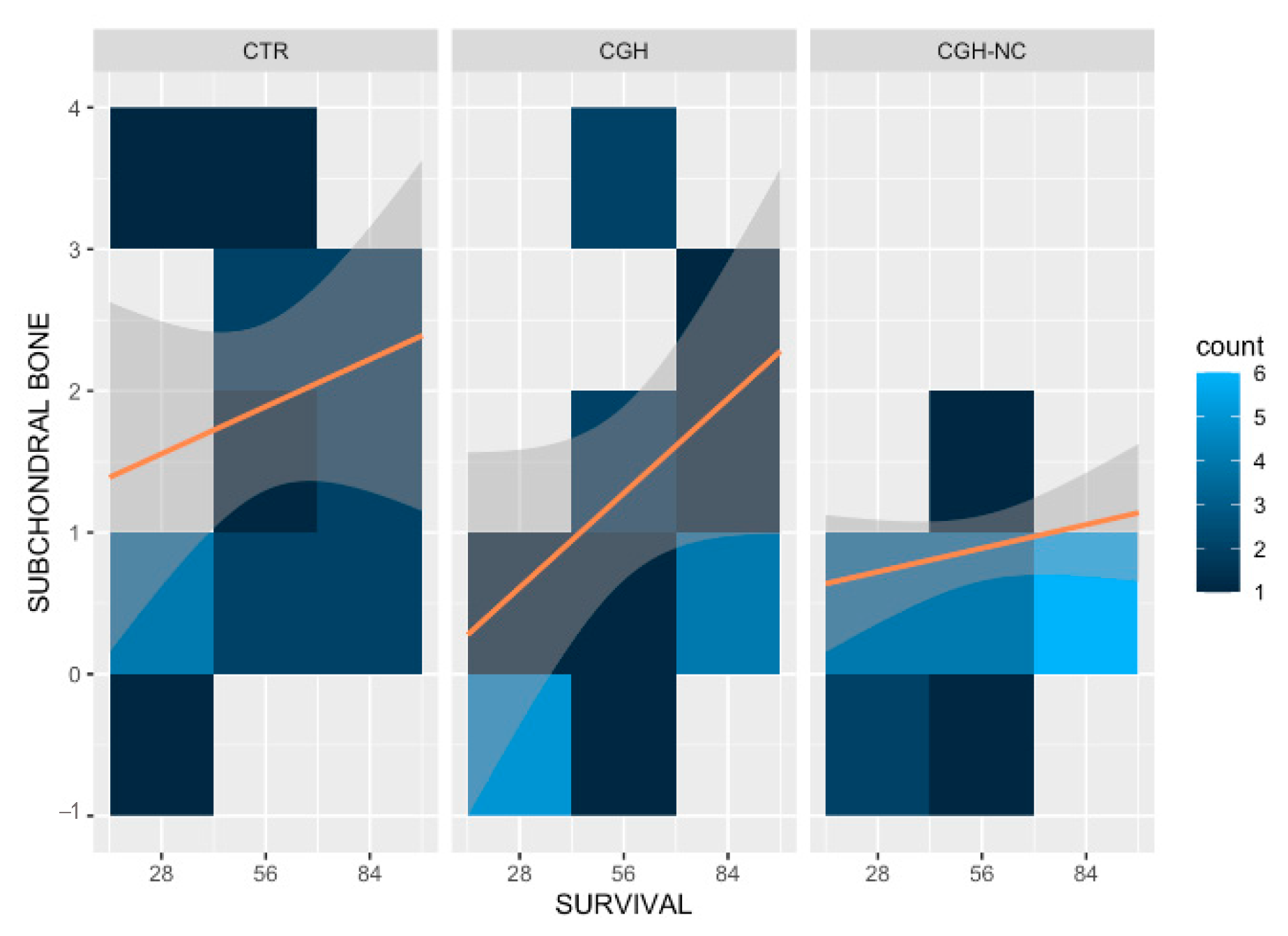
- Subchondral Bone
- c.
- Synovial membrane
3.2. Hyaluronic Acid
3.3. MRI Imaging Biomarkers
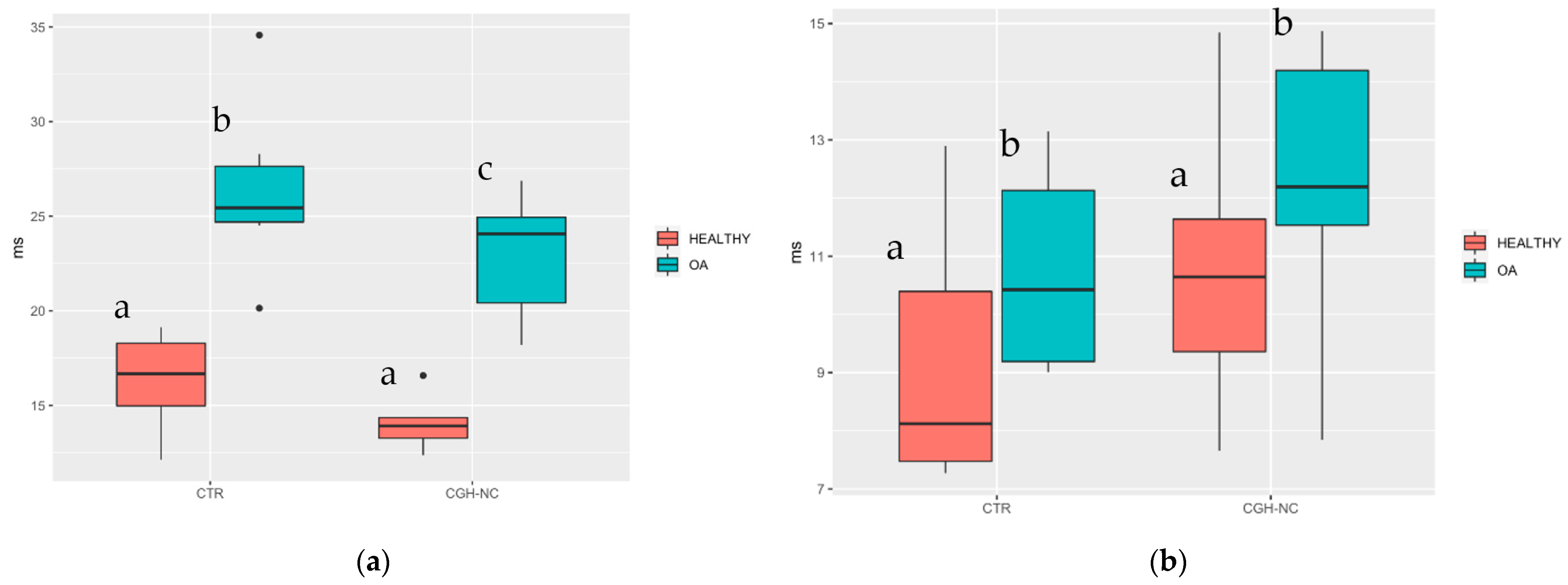
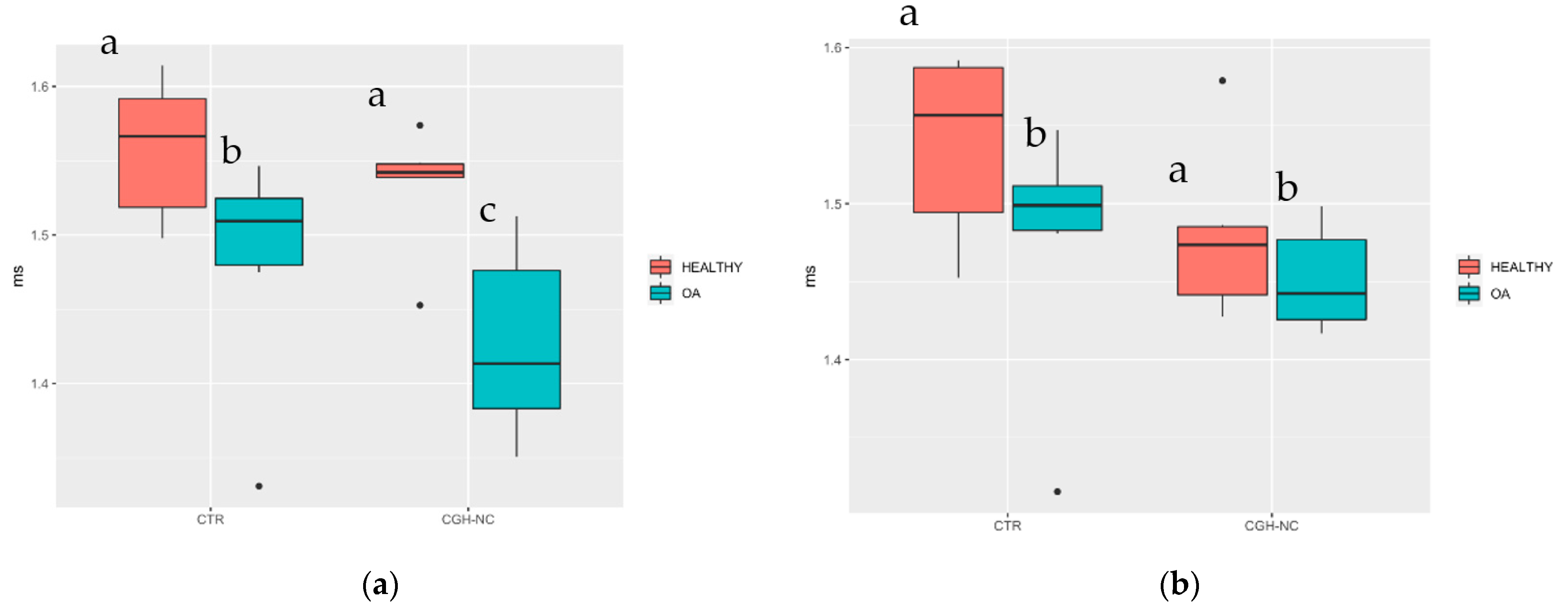
3.3.1. Articular Cartilage Biomarkers
3.3.2. Subchondral Bone Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal Models of Osteoarthritis: Classification, Update, and Measurement of Outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Permuy, M.; Guede, D.; López-Peña, M.; Muñoz, F.; Caeiro, J.-R.; González-Cantalapiedra, A. Comparison of Various SYSADOA for the Osteoarthritis Treatment: An Experimental Study in Rabbits. BMC Musculoskelet. Disord. 2015, 16, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, M.; Cibere, J.; Sayre, E.C.; Kopec, J.A. Efficacy of Commonly Prescribed Analgesics in the Management of Osteoarthritis: A Systematic Review and Meta-Analysis. Rheumatol. Int. 2018, 38, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- April, K.T.; Bisaillon, J.; Welch, V.; Maxwell, L.J.; Jüni, P.; Rutjes, A.W.; Husni, M.E.; Vincent, J.; Hindi, T.E.; Wells, G.A.; et al. Tramadol for Osteoarthritis. Cochrane Database Syst. Rev. 2019, 5, CD005522. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Martel-Pelletier, J.; Monfort, J.; Möller, I.; Castillo, J.R.; Arden, N.; Berenbaum, F.; Blanco, F.J.; Conaghan, P.G.; Doménech, G.; et al. Combined Chondroitin Sulfate and Glucosamine for Painful Knee Osteoarthritis: A Multicentre, Randomised, Double-Blind, Non-Inferiority Trial versus Celecoxib. Ann. Rheum. Dis. 2016, 75, 37–44. [Google Scholar] [CrossRef]
- Pereira, H.; Sousa, D.A.; Cunha, A.; Andrade, R.; Espregueira-Mendes, J.; Oliveira, J.M.; Reis, R.L. Hyaluronic Acid. Adv. Exp. Med. Biol. 2018, 1059, 137–153. [Google Scholar] [CrossRef]
- Gallagher, B.; Tjoumakaris, F.P.; Harwood, M.I.; Good, R.P.; Ciccotti, M.G.; Freedman, K.B. Chondroprotection and the Prevention of Osteoarthritis Progression of the Knee: A Systematic Review of Treatment Agents. Am. J. Sports Med. 2015, 43, 734–744. [Google Scholar] [CrossRef]
- Reginster, J.-Y.; Veronese, N. Highly Purified Chondroitin Sulfate: A Literature Review on Clinical Efficacy and Pharmacoeconomic Aspects in Osteoarthritis Treatment. Aging Clin. Exp. Res. 2021, 33, 37–47. [Google Scholar] [CrossRef]
- Monfort, J.; Martel-Pelletier, J.; Pelletier, J.-P. Chondroitin Sulphate for Symptomatic Osteoarthritis: Critical Appraisal of Meta-Analyses. Curr. Med. Res. Opin. 2008, 24, 1303–1308. [Google Scholar] [CrossRef]
- Chiusaroli, R.; Piepoli, T.; Zanelli, T.; Ballanti, P.; Lanza, M.; Rovati, L.C.; Caselli, G. Experimental Pharmacology of Glucosamine Sulfate. Int. J. Rheumatol. 2011, 2011, 939265. [Google Scholar] [CrossRef] [Green Version]
- Martí-Bonmatí, L.; Sanz-Requena, R.; Rodrigo, J.L.; Alberich-Bayarri, Á.; Carot, J.M. Glucosamine Sulfate Effect on the Degenerated Patellar Cartilage: Preliminary Findings by Pharmacokinetic Magnetic Resonance Modeling. Eur. Radiol. 2009, 19, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L.; Polyak, A.; Mathe, D.; Kiraly, R.; Thuroczy, J.; Terez, M.; Janoki, G.; Ting, Y.; Bucci, L.R.; Schauss, A.G. Absorption, Uptake and Tissue Affinity of High-Molecular-Weight Hyaluronan after Oral Administration in Rats and Dogs. J. Agric. Food Chem. 2008, 56, 10582–10593. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, C.I.; Ramos-Plá, J.J.; Soler, C.; Segarra, S.; Moratalla, V.; Redondo, J.I. Effects of Oral Hyaluronic Acid Administration in Dogs Following Tibial Tuberosity Advancement Surgery for Cranial Cruciate Ligament Injury. Animals 2021, 11, 1264. [Google Scholar] [CrossRef] [PubMed]
- Crowley, D.C.; Lau, F.C.; Sharma, P.; Evans, M.; Guthrie, N.; Bagchi, M.; Bagchi, D.; Dey, D.K.; Raychaudhuri, S.P. Safety and Efficacy of Undenatured Type II Collagen in the Treatment of Osteoarthritis of the Knee: A Clinical Trial. Int. J. Med. Sci. 2009, 6, 312. [Google Scholar] [CrossRef] [Green Version]
- Martí-Angulo, S.; García-López, N.; Díaz-Ramos, A. Efficacy of an Oral Hyaluronate and Collagen Supplement as a Preventive Treatment of Elbow Dysplasia. J. Vet. Sci. 2014, 15, 569–574. [Google Scholar] [CrossRef] [Green Version]
- Mannelli, L.D.C.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Low Dose Native Type II Collagen Prevents Pain in a Rat Osteoarthritis Model. BMC Musculoskelet. Disord. 2013, 14, 228–229. [Google Scholar] [CrossRef] [Green Version]
- Bakilan, F.; Armagan, O.; Ozgen, M.; Tascioglu, F.; Bolluk, O.; Alatas, O. Effects of Native Type II Collagen Treatment on Knee Osteoarthritis: A Randomized Controlled Trial. Eurasian J. Med. 2016, 48, 95–101. [Google Scholar] [CrossRef]
- Gupta, R.C.; Canerdy, T.D.; Skaggs, P.; Stocker, A.; Zyrkowski, G.; Burke, R.; Wegford, K.; Goad, J.T.; Rohde, K.; Barnett, D.; et al. Therapeutic Efficacy of Undenatured Type-II Collagen (UC-II) in Comparison to Glucosamine and Chondroitin in Arthritic Horses1. J. Vet. Pharmacol. Ther. 2009, 32, 577–584. [Google Scholar] [CrossRef]
- Oo, W.M.; Little, C.; Duong, V.; Hunter, D.J. The Development of Disease-Modifying Therapies for Osteoarthritis (DMOADs): The Evidence to Date. Drug Des. Dev. Ther. 2021, 15, 2921–2945. [Google Scholar] [CrossRef]
- Pritzker, K.P.H.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.-P.; Revell, P.A.; Salter, D.; Van den Berg, W.B. Osteoarthritis Cartilage Histopathology: Grading and Staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laverty, S.; Girard, C.A.; Williams, J.M.; Hunziker, E.B.; Pritzker, K.P.H. The OARSI Histopathology Initiative—Recommendations for Histological Assessments of Osteoarthritis in the Rabbit. Osteoarthr. Cartil. 2010, 18, S53–S65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsurumoto, T.; Saiki, K.; Okamoto, K.; Imamura, T.; Maeda, J.; Manabe, Y.; Wakebe, T. Periarticular Osteophytes as an Appendicular Joint Stress Marker (JSM): Analysis in a Contemporary Japanese Skeletal Collection. PLoS ONE 2013, 8, e57049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, J.L.; Kuroki, K.; Visco, D.; Pelletier, J.-P.; Schulz, L.; Lafeber, F.P.J.G. The OARSI Histopathology Initiative—Recommendations for Histological Assessments of Osteoarthritis in the Dog. Osteoarthr. Cartil. 2010, 18, S66–S79. [Google Scholar] [CrossRef] [Green Version]
- Bray, R.C.; Shrive, N.G.; Frank, C.B.; Chimich, D.D. The Early Effects of Joint Immobilization on Medial Collateral Ligament Healing in an ACL-Deficient Knee: A Gross Anatomic and Biomechanical Investigation in the Adult Rabbit Model. J. Orthop. Res. 1992, 10, 157–166. [Google Scholar] [CrossRef]
- Gushue, D.L.; Houck, J.; Lerner, A.L. Rabbit Knee Joint Biomechanics: Motion Analysis and Modeling of Forces during Hopping. J. Orthop. Res. 2005, 23, 735–742. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI Histopathology Initiative—Recommendations for Histological Assessments of Osteoarthritis in the Rat. Osteoarthr. Cartil. 2010, 18, S24–S34. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.T.; Shao, C.J.; Wu, K.C.; Wu, T.T.; Chern, T.C.; Kuo, L.C.; Jou, I.M. Pain in Patients with Equal Radiographic Grades of Osteoarthritis in Both Knees: The Value of Gray Scale Ultrasound. Osteoarthr. Cartil. 2012, 20, 1507–1513. [Google Scholar] [CrossRef] [Green Version]
- Duvvuri, U.; Kudchodkar, S.; Reddy, R.; Leigh, J.S. T(1rho) Relaxation Can Assess Longitudinal Proteoglycan Loss from Articular Cartilage in Vitro. Osteoarthr. Cartil. 2002, 10, 838–844. [Google Scholar] [CrossRef] [Green Version]
- Nieminen, M.T.; Rieppo, J.; Töyräs, J.; Hakumäki, J.M.; Silvennoinen, J.; Hyttinen, M.M.; Helminen, H.J.; Jurvelin, J.S. T2 Relaxation Reveals Spatial Collagen Architecture in Articular Cartilage: A Comparative Quantitative MRI and Polarized Light Microscopic Study. Magn. Reson. Med. 2001, 46, 487–493. [Google Scholar] [CrossRef]
- Liess, C.; Lüsse, S.; Karger, N.; Heller, M.; Glüer, C.C. Detection of Changes in Cartilage Water Content Using MRI T2-Mapping in Vivo. Osteoarthr. Cartil. 2002, 10, 907–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florea, C.; Malo, M.K.H.; Rautiainen, J.; Mäkelä, J.T.A.; Fick, J.M.; Nieminen, M.T.; Jurvelin, J.S.; Davidescu, A.; Korhonen, R.K. Alterations in Subchondral Bone Plate, Trabecular Bone and Articular Cartilage Properties of Rabbit Femoral Condyles at 4 Weeks after Anterior Cruciate Ligament Transection. Osteoarthr. Cartil. 2015, 23, 414–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberich-Bayarri, Á.; Martí-Bonmatí, L.; Sanz-Requena, R.; Belloch, E.; Moratal, D. In Vivo Trabecular Bone Morphologic and Mechanical Relationship Using High-Resolution 3-T MRI. Am. J. Roentgenol. 2008, 191, 721–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberich-Bayarri, Á.; Martí-Bonmatí, L.; Pérez, M.A.; Sanz-Requena, R.; Lerma-Garrido, J.J.; García-Martí, G.; Moratal, D. Assessment of 2D and 3D Fractal Dimension Measurements of Trabecular Bone from High-Spatial Resolution Magnetic Resonance Images at 3 T. Med. Phys. 2010, 37, 4930–4937. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.I.; Soler, C. Animal Models of Osteoarthritis in Small Mammals. Vet. Clin. N. Am. Exot. Anim. Pract. 2019, 22, 211–221. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.M. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet. Pathol. 2015, 52, 803–818. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, X.; Man, Z.; Zhang, J.; Jiang, Y.; Ao, Y. A Novel Rabbit Model of Early Osteoarthritis Exhibits Gradual Cartilage Degeneration after Medial Collateral Ligament Transection Outside the Joint Capsule. Sci. Rep. 2016, 6, 34423. [Google Scholar] [CrossRef] [Green Version]
- Matsui, H.; Shimizu, M.; Tsuji, H. Cartilage and Subchondral Bone Interaction in Osteoarthrosis of Human Knee Joint: A Histological and Histomorphometric Study. Microsc. Res. Tech. 1997, 37, 333–342. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-Guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [Green Version]
- Bobinac, D.; Spanjol, J.; Zoricic, S.; Maric, I. Changes in Articular Cartilage and Subchondral Bone Histomorphometry in Osteoarthritic Knee Joints in Humans. Bone 2003, 32, 284–290. [Google Scholar] [CrossRef]
- Klein, S.; Staring, M.; Murphy, K.; Viergever, M.A.; Pluim, J.P.W. Elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Trans. Med Imaging 2010, 29, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Requena, R.; Martí-Bonmatí, L.; Hervás, V.; Vega, M.; Alberich-Bayarri, Á.; García-Martí, G.; Carot, J.M. Modification of Longitudinal Relaxation Time (T1) as a Biomarker of Patellar Cartilage Degeneration. Radiología 2010, 52, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Fram, E.K.; Herfkens, R.J.; Johnson, G.A.; Glover, G.H.; Karis, J.P.; Shimakawa, A.; Perkins, T.G.; Pelc, N.J. Rapid Calculation of T1 Using Variable Flip Angle Gradient Refocused Imaging. Magn. Reson. Imaging 1987, 5, 201–208. [Google Scholar] [CrossRef]
- Li, D.; Dhawale, P.; Rubin, P.J.; Haacke, E.M.; Gropler, R.J. Myocardial Signal Response to Dipyridamole and Dobutamine: Demonstration of the BOLD Effect Using a Double-echo Gradient-echo Sequence. Magn. Reson. Med. 1996, 36, 16–20. [Google Scholar] [CrossRef]
- Vasilic, B.; Wehrli, F.W. A Novel Local Thresholding Algorithm for Trabecular Bone Volume Fraction Mapping in the Limited Spatial Resolution Regime of in Vivo MRI. IEEE Trans. Med. Imaging 2005, 24, 1574–1585. [Google Scholar] [CrossRef]
- Champely, S. pwr: Basic Functions for Power Analysis. R Package Version 1.3-0. Available online: https://CRAN.R-project.org/package=pwr (accessed on 23 March 2021).
- Mair, P.; Wilcox, R. Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 2019, 52, 464–488. [Google Scholar] [CrossRef]
- Trentham, D.E.; Dynesius-Trentham, R.A.; Orav, E.J.; Combitchi, D.; Lorenzo, C.; Sewell, K.L.; Hafler, D.A.; Weiner, H.L. Effects of Oral Administration of Type II Collagen on Rheumatoid Arthritis. Science 1993, 261, 1727–1730. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, D.; Misner, B.; Bagchi, M.; Kothari, S.C.; Downs, B.W.; Fafard, R.D.; Preuss, H.G. Effects of Orally Administered Undenatured Type II Collagen against Arthritic Inflammatory Diseases: A Mechanistic Exploration. Int. J. Clin. Pharm. Res. 2002, 22, 101–110. [Google Scholar]
- D’Altilio, M.; Peal, A.; Alvey, M.; Simms, C.; Curtsinger, A.; Gupta, R.C.; Canerdy, T.D.; Goad, J.T.; Bagchi, M.; Bagchi, D. Therapeutic Efficacy and Safety of Undenatured Type II Collagen Singly or in Combination with Glucosamine and Chondroitin in Arthritic Dogs. Toxicol. Mech. Methods 2007, 17, 189–196. [Google Scholar] [CrossRef]
- Peal, A.; D’Altilio, M.; Simms, C.; Alvey, M.; Gupta, R.C.; Goad, J.T.; Canerdy, T.D.; Bagchi, M.; Bagchi, D. Therapeutic Efficacy and Safety of Undenatured Type-II Collagen (UC-II) Alone or in Combination with (−)-hydroxycitric Acid and Chromemate in Arthritic Dogs. J. Vet. Pharmacol. Ther. 2007, 30, 275–278. [Google Scholar] [CrossRef]
- Weiner, H.L. Oral Tolerance for the Treatment of Autoimmune Diseases. Annu. Rev. Med. 1997, 48, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Honvo, G.; Lengelé, L.; Charles, A.; Reginster, J.-Y.; Bruyere, O. Role of Collagen Derivatives in Osteoarthritis and Cartilage Repair: A Systematic Scoping Review with Evidence Mapping. Rheumatol. Ther. 2020, 7, 703–740. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Mahmoudian, A.; Kalvaityte, U.; Uzieliene, I.; Larder, C.E.; Iskandar, M.M.; Kubow, S.; Hamdan, P.C.; de Almeida, C.S.; Favazzo, L.J.; et al. A White Paper on Collagen Hydrolyzates and Ultrahydrolyzates: Potential Supplements to Support Joint Health in Osteoarthritis? Curr. Rheumatol. Rep. 2021, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, M.; Lurati, A.; Vignati, G.; Marrazza, M.G.; Telese, F.; Re, K.; Bellistri, A. Biomarkers, Type II Collagen, Glucosamine and Chondroitin Sulfate in Osteoarthritis Follow-up: The “Magenta Osteoarthritis Study”. J. Orthop. Traumatol. 2008, 9, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabezas, M.; Benito, J.; Ortega, A.; Pedraza, E. Long-Term Supplementation with an Undenatured Type-II Collagen (UC-II®) Formulation in Dogs with Degenerative Joint Disease: Exploratory Study. Open Vet. J. 2022, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Gencoglu, H.; Orhan, C.; Sahin, E.; Sahin, K. Undenatured Type II Collagen (UC-II) in Joint Health and Disease: A Review on the Current Knowledge of Companion Animals. Animals 2020, 10, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugo, J.P.; Saiyed, Z.M.; Lane, N.E. Efficacy and Tolerability of an Undenatured Type II Collagen Supplement in Modulating Knee Osteoarthritis Symptoms: A Multicenter Randomized, Double-Blind, Placebo-Controlled Study. Nutr. J. 2016, 15, 14–15. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.C.; Canerdy, T.D.; Lindley, J.; Konemann, M.; Minniear, J.; Carroll, B.A.; Hendrick, C.; Goad, J.T.; Rohde, K.; Doss, R.; et al. Comparative Therapeutic Efficacy and Safety of Type-II Collagen (Uc-II), Glucosamine and Chondroitin in Arthritic Dogs: Pain Evaluation by Ground Force Plate. J. Anim. Physiol. Anim. Nutr. 2012, 96, 770–777. [Google Scholar] [CrossRef]
- Zhu, X.; Sang, L.; Wu, D.; Rong, J.; Jiang, L. Effectiveness and Safety of Glucosamine and Chondroitin for the Treatment of Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. J. Orthop. Surg. Res. 2018, 13, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The Role of Hyaluronic Acid in Cartilage Boundary Lubrication. Cells 2020, 9, 1606. [Google Scholar] [CrossRef]
- Fang, H.; Beier, F. Mouse Models of Osteoarthritis: Modelling Risk Factors and Assessing Outcomes. Nat. Rev. Rheumatol. 2014, 10, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.-P.; Boileau, C.; Altman, R.D.; Martel-Pelletier, J. Experimental Models of Osteoarthritis: Usefulness in the Development of Disease-Modifying Osteoarthritis Drugs/Agents. Therapy 2010, 7, 621–634. [Google Scholar] [CrossRef]
- Arzi, B.; Wisner, E.R.; Huey, D.J.; Kass, P.H.; Hu, J.; Athanasiou, K.A. A Proposed Model of Naturally Occurring Osteoarthritis in the Domestic Rabbit. Lab Anim. 2012, 41, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.E.; Lu, Y.; Tortorella, M.D.; Malfait, A.-M. Genetically Engineered Mouse Models Reveal the Importance of Proteases as Osteoarthritis Drug Targets. Curr. Rheumatol. Rep. 2013, 15, 350. [Google Scholar] [CrossRef]
- Adams, M.E.; Lussier, A.J.; Peyron, J.G. A Risk-Benefit Assessment of Injections of Hyaluronan and Its Derivatives in the Treatment of Osteoarthritis of the Knee. Drug Saf. 2000, 23, 115–130. [Google Scholar] [CrossRef]
- Tashiro, T.; Seino, S.; Sato, T.; Matsuoka, R.; Masuda, Y.; Fukui, N. Oral Administration of Polymer Hyaluronic Acid Alleviates Symptoms of Knee Osteoarthritis: A Double-Blind, Placebo-Controlled Study over a 12-Month Period. Sci. World J. 2012, 2012, 167928. [Google Scholar] [CrossRef] [Green Version]
- Kyostio-Moore, S.; Nambiar, B.; Hutto, E.; Ewing, P.J.; Piraino, S.; Berthelette, P.; Sookdeo, C.; Matthews, G.; Armentano, D. STR/Ort Mice, a Model for Spontaneous Osteoarthritis, Exhibit Elevated Levels of Both Local and Systemic Inflammatory Markers. Comp. Med. 2011, 61, 346–355. [Google Scholar]
- Mosher, T.J.; Zhang, Z.; Reddy, R.; Boudhar, S.; Milestone, B.N.; Morrison, W.B.; Kwoh, C.K.; Eckstein, F.; Witschey, W.R.T.; Borthakur, A. Knee Articular Cartilage Damage in Osteoarthritis: Analysis of MR Image Biomarker Reproducibility in ACRIN-PA 4001 Multicenter Trial. Radiology 2011, 258, 832–842. [Google Scholar] [CrossRef]
- Hunter, D.J.; Guermazi, A.; Lo, G.H.; Grainger, A.J.; Conaghan, P.G.; Boudreau, R.M.; Roemer, F.W. Evolution of Semi-Quantitative Whole Joint Assessment of Knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthr. Cartil. 2011, 19, 990–1002. [Google Scholar] [CrossRef] [Green Version]
- Du Souich, P. Absorption, Distribution and Mechanism of Action of SYSADOAS. Pharmacol. Ther. 2014, 142, 362–374. [Google Scholar] [CrossRef]




| 0 Days | 28 Days | 56 Days | 84 Days | |
|---|---|---|---|---|
| Healthy | 243.30 | - | - | - |
| CTR | - | 53.21 | 48.46 | 71.54 |
| CGH | - | 61.35 | 67.85 | 59.52 |
| CGH-NC | - | 119.62 | 68.57 | 90.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sifre, V.; Soler, C.; Segarra, S.; Redondo, J.I.; Doménech, L.; Ten-Esteve, A.; Vilalta, L.; Pardo-Marín, L.; Serra, C.I. Improved Joint Health Following Oral Administration of Glycosaminoglycans with Native Type II Collagen in a Rabbit Model of Osteoarthritis. Animals 2022, 12, 1401. https://doi.org/10.3390/ani12111401
Sifre V, Soler C, Segarra S, Redondo JI, Doménech L, Ten-Esteve A, Vilalta L, Pardo-Marín L, Serra CI. Improved Joint Health Following Oral Administration of Glycosaminoglycans with Native Type II Collagen in a Rabbit Model of Osteoarthritis. Animals. 2022; 12(11):1401. https://doi.org/10.3390/ani12111401
Chicago/Turabian StyleSifre, Vicente, Carme Soler, Sergi Segarra, José Ignacio Redondo, Luis Doménech, Amadeo Ten-Esteve, Laura Vilalta, Luis Pardo-Marín, and Claudio Iván Serra. 2022. "Improved Joint Health Following Oral Administration of Glycosaminoglycans with Native Type II Collagen in a Rabbit Model of Osteoarthritis" Animals 12, no. 11: 1401. https://doi.org/10.3390/ani12111401
APA StyleSifre, V., Soler, C., Segarra, S., Redondo, J. I., Doménech, L., Ten-Esteve, A., Vilalta, L., Pardo-Marín, L., & Serra, C. I. (2022). Improved Joint Health Following Oral Administration of Glycosaminoglycans with Native Type II Collagen in a Rabbit Model of Osteoarthritis. Animals, 12(11), 1401. https://doi.org/10.3390/ani12111401







