Effects of Concentrate Supplementation on Growth Performance, Rumen Fermentation, and Bacterial Community Composition in Grazing Yaks during the Warm Season
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Animals, Diet, Experimental Design, and Management
2.3. Sample Collection
2.4. Feed Analysis
2.5. Serum and Rumen Fermentation Parameters
2.6. DNA Extraction and Determination
2.7. Data and Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemical Parameters
3.3. Rumen Fermentation Parameters
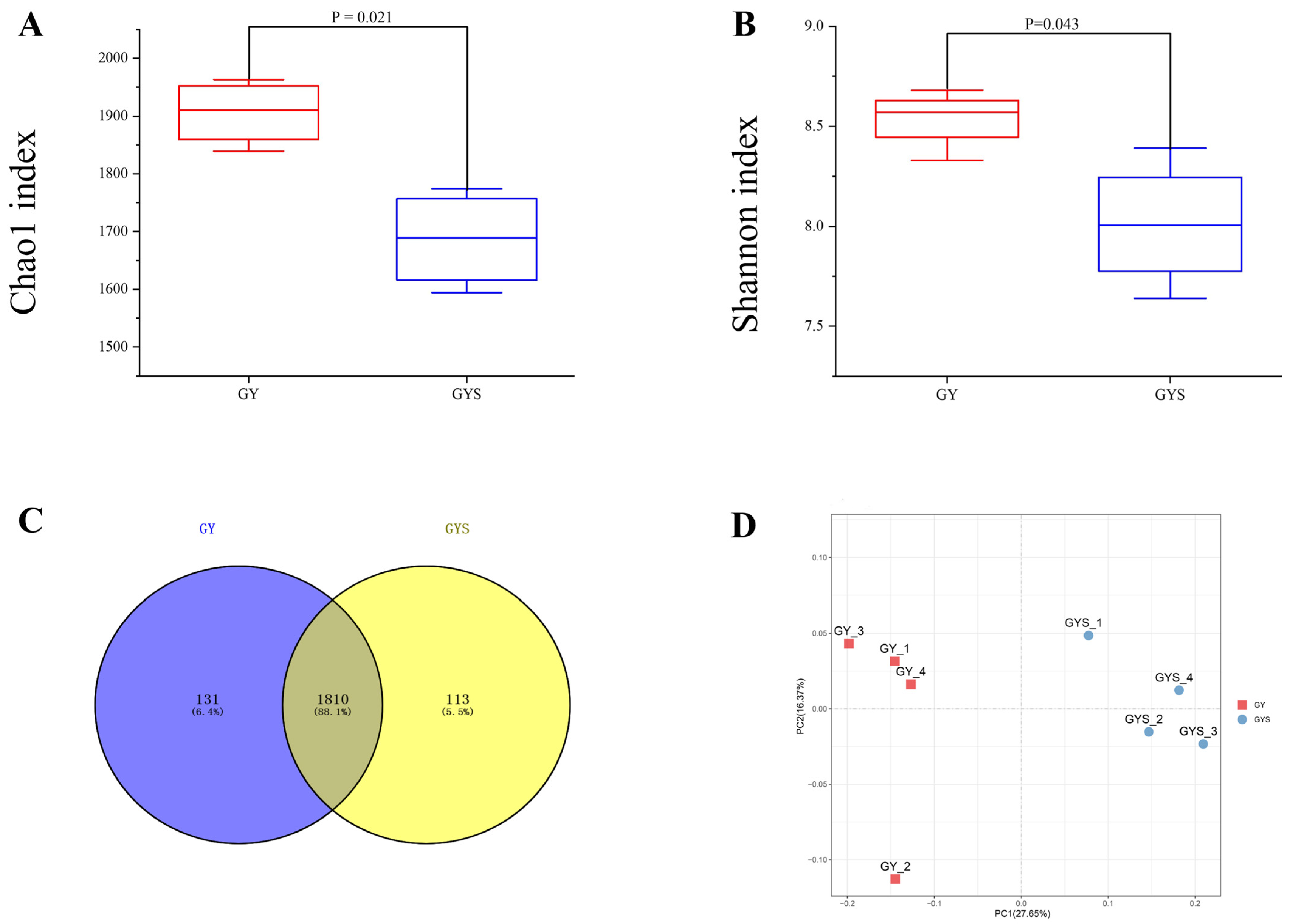
3.4. Alpha Diversity and Composition of Rumen Bacterial Communities
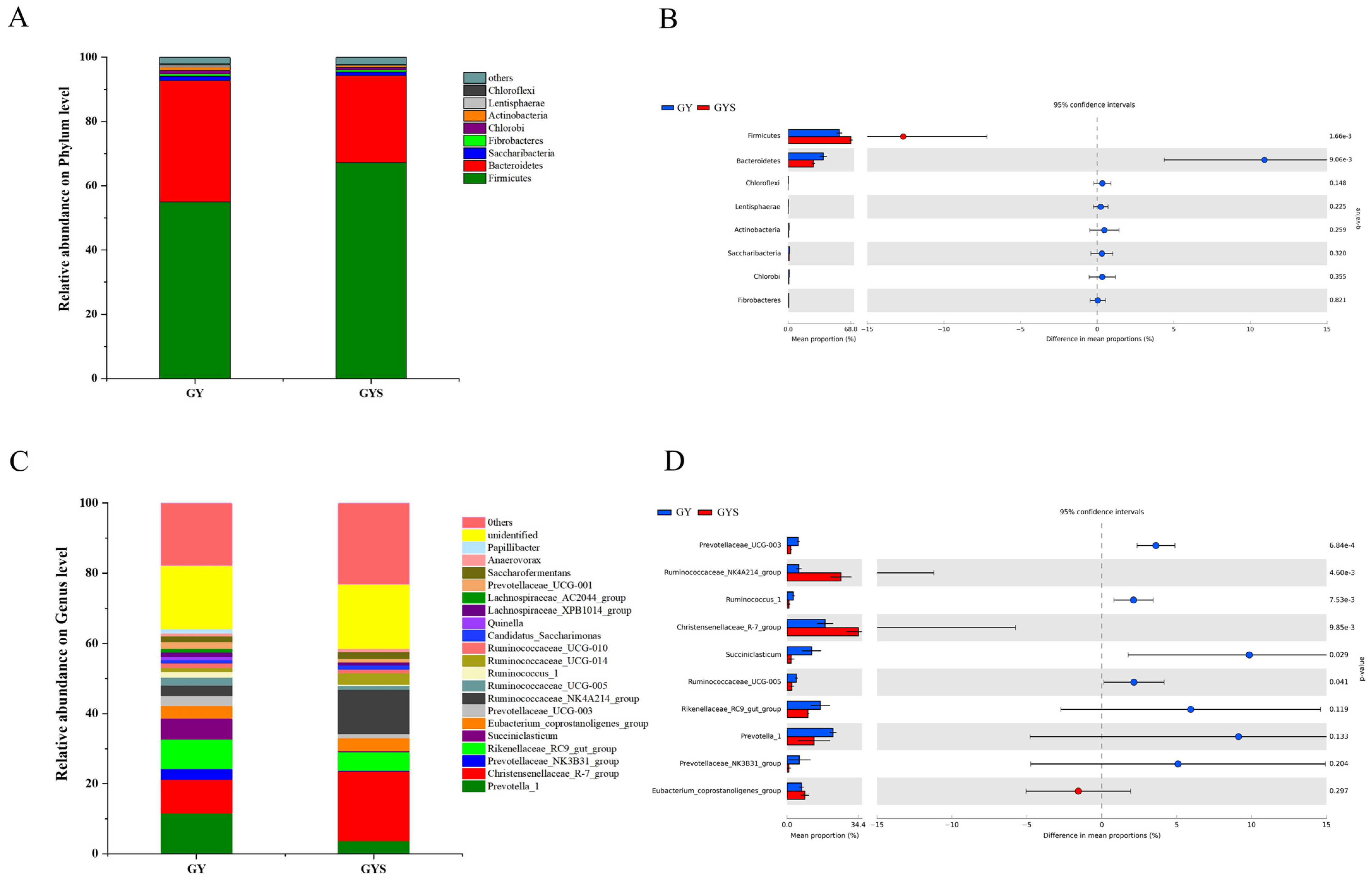
3.5. Differences in Rumen Bacterial Community Composition
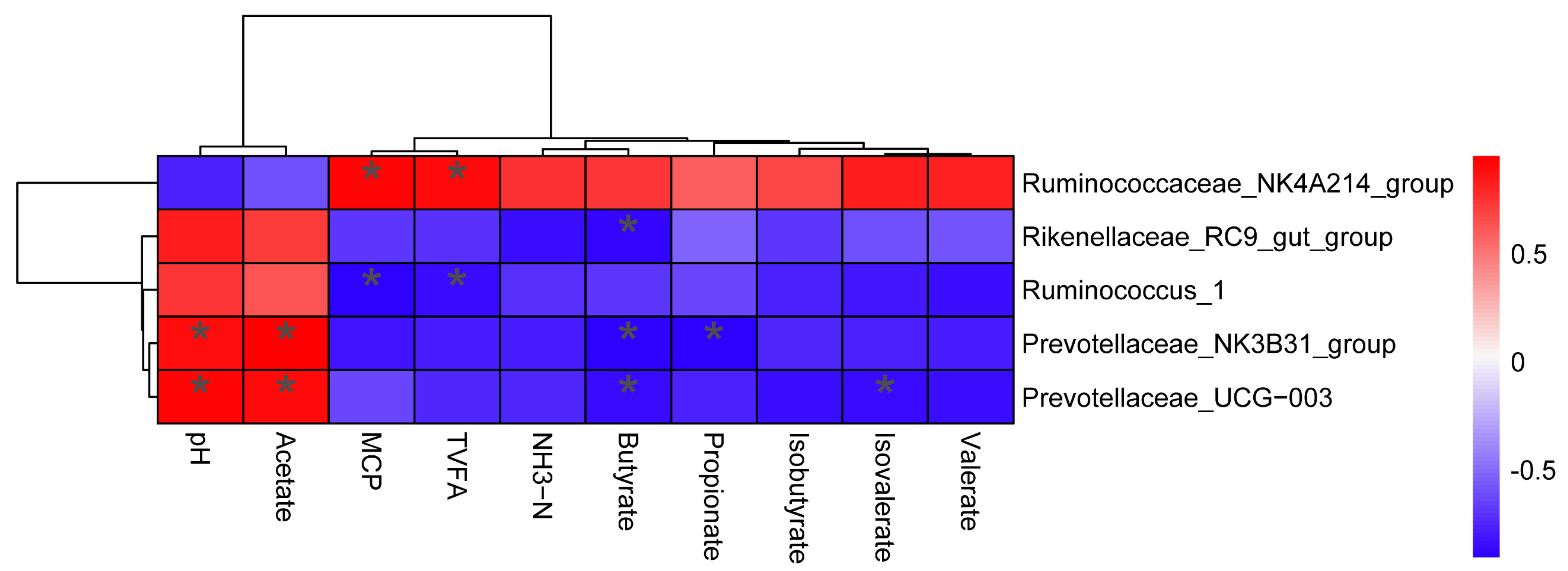
3.6. Correlation between Rumen Fermentation Parameters and Bacteria Community
3.7. Predicted Function of Rumen Bacteria
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, Q.S.; Wanapat, M.; Hou, F.J. Mineral nutritional status of yaks (Bos grunniens) grazing on the Qinghai-Tibetan plateau. Animals 2019, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.S.; Wanapat, M.; Hou, F.J. Rumen bacteria influence milk protein yield of yak grazing on the Qinghai-Tibet plateau. Anim. Biosci. 2021, 34, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.J.; Ma, T.; Wang, K.; Xu, T.; Liu, J.Q.; Qiu, Q. The Yak genome database: An integrative database for studying yak biology and high-altitude adaption. BMC Genom. 2012, 13, 600. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, S.X.; Liu, H.J.; Xu, T.W.; Hu, L.Y.; Zhao, N.; Han, X.P.; Zhang, X.L. Yak rumen microbial diversity at different forage growth stages of an alpine meadow on the Qinghai-Tibet Plateau. PeerJ. 2019, 7, e7645. [Google Scholar] [CrossRef]
- Xu, T.W.; Xu, S.X.; Hu, L.Y.; Zhao, N.; Liu, Z.; Ma, L.; Liu, H.J.; Zhao, X.Q. Effect of Dietary Types on Feed Intakes, Growth Performance and Economic Benefit in Tibetan sheep and Yaks on the Qinghai-Tibet Plateau during Cold Season. PLoS ONE 2017, 12, e169187. [Google Scholar] [CrossRef]
- Zhou, J.; Yue, S.M.; Peng, Q.H.; Wang, L.Z.; Wang, Z.S.; Xue, B. Metabonomic responses of grazing yak to different concentrate supplementations in cold season. Animals 2020, 10, 1595. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Fang, L.; Meng, Q.X.; Li, S.L.; Chai, S.T.; Liu, S.J.; Schonewille, J.T. Assessment of ruminal bacterial and archaeal community structure in yak (Bos grunniens). Front. Microbiol. 2017, 7, 8–17. [Google Scholar] [CrossRef]
- Bergmann, G.T. Microbial community composition along the digestive tract in forage- and grain-fed bison. BMC Vet. Res. 2017, 13, 253. [Google Scholar] [CrossRef]
- Deusch, S.; Camarinha-Silva, A.; Conrad, J.; Beifuss, U.; Rodehutscord, M.; Seifert, J. A structural and functional elucidation of the rumen microbiome influenced by various diets and microenvironments. Front. Microbiol. 2017, 8, 1605. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Rathahao-Paris, E.; Popova, M.; Boccard, J.; Nielsen, K.F.; Boudra, H. Rumen microbial communities influence metabolic phenotypes in lambs. Front. Microbiol. 2015, 6, 1060. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H.; Bannink, A.; Dieho, K.; Dijkstra, J.; Global, R.C.C.; et al. Sveriges, L. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.I.; Zeng, S.Q.; Zhang, R.; Diao, Q.Y.; Tu, Y. Effects of dietary energy levels on rumen bacterial community composition in Holstein heifers under the same forage to concentrate ratio condition. BMC Microbiol. 2018, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.W.; Vinitchaikul, P.; Deng, M.Y.; Zhang, G.G.; Sun, L.Y.; Wang, H.X.; Gou, X.; Mao, H.M.; Yang, S.L. Exploration of the effects of altitude change on bacteria and fungi in the rumen of yak (Bos grunniens). Arch. Microbiol. 2021, 203, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.S.; Wanapat, M.; Hou, F.J. Chemical Composition of Milk and Rumen Microbiome Diversity of Yak, Impacting by Herbage Grown at Different Phenological Periods on the Qinghai-Tibet Plateau. Animals 2021, 6, 1030. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Yang, C.; Zhang, J.B.; Kalwar, Q.; Liang, Z.; Li, C.; Du, M.; Yan, P.; Long, R.J.; Han, J.L.; et al. Effects of Dietary Energy Levels on Rumen Fermentation, Microbial Diversity, and Feed Efficiency of Yaks (Bos grunniens). Front. Microbiol. 2020, 11, 625. [Google Scholar] [CrossRef]
- Zou, H.W.; Hu, R.; Wang, Z.S.; Shah, A.M.; Zeng, S.Y.; Peng, Q.H.; Xue, B.; Wang, L.S.; Zhang, X.F.; Wang, X.Y.; et al. Effects of Nutritional Deprivation and Re-Alimentation on the Feed Efficiency, Blood Biochemistry, and Rumen Microflora in Yaks (Bos grunniens). Animals 2019, 9, 807. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, H.; Hao, L.Z.; Cao, X.L.; Degen, A.; Zhou, J.W.; Zhang, C.F. Rumen Bacterial Community of Grazing Lactating Yaks (Poephagus grunniens) Supplemented with Concentrate Feed and/or Rumen-Protected Lysine and Methionine. Animals 2021, 11, 2425. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zhang, J.B.; Liang, Z.Y.; Yang, C.; Kalwar, Q.; Shah, T.; Du, M.; Muhammad, I.; Zheng, J.; Yan, P.; et al. Dynamics of rumen bacterial composition of yak (Bos grunniens) in response to dietary supplements during the cold season. PeerJ. 2021, 9, e11520. [Google Scholar] [CrossRef]
- Jia, J.L.; Liang, C.N.; Wu, X.Y.; Xiong, L.; Bao, P.J.; Chen, Q.; Yan, P. Effect of high proportion concentrate dietary on Ashdan Yak jejunal barrier and microbial function in cold season. Res. Vet. Sci. 2021, 140, 259–267. [Google Scholar] [CrossRef]
- Bünemann, K.; Johannes, M.; Schmitz, R.; Hartwiger, J.; von Soosten, D.; Hüther, L.; Meyer, U.; Westendarp, H.; Hummel, J.; Zeyner, A.; et al. Effects of different concentrate feed proportions on ruminal ph parameters, duodenal nutrient flows and efficiency of microbial crude protein synthesis in dairy cows during early lactation. Animals 2020, 10, 267. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists, 19th ed.; Association of Official Analytical Chemists Inc.: Arlington, VA, USA, 1999; pp. 1048–1049. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Degen, A.; Hao, L.Z.; Huang, Y.Y.; Niu, J.Z.; Wang, X.; Chai, S.T.; Liu, S.J. An increase in dietary lipid content from different forms of double-low rapeseed reduces enteric methane emission in Datong yaks on the Qinghai-Tibetan Plateau. Anim. Sci. J. 2020, 11, e13489. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Becker, K. Purine quantification in digesta from ruminants by spectrophotometric and HPLC methods. Br. J. Nutr. 1999, 81, 107–112. [Google Scholar] [CrossRef]
- Kuczynski, J.; Stombaugh, J.; Walters, W.A.; González, A.; Caporaso, J.G.; Knight, R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr. Protoc. Bioinform. 2011, 10, 36–63. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.J.; Huasai, S.; Chen, A. Dietary concentrate supplementation alters serum metabolic profiles related to energy and amino acid metabolism in grazing simmental heifers. Front. Vet. Sci. 2021, 8, e743410. [Google Scholar] [CrossRef]
- Mcfarlane, Z.D.; Barbero, R.P.; Nave, R.L.G.; Maheiros, E.B.; Reis, R.A.; Mulliniks, J.T. Effect of forage species and supplement type on rumen kinetics and serum metabolites in growing beef heifers grazing winter forage1. J. Anim. Sci. 2017, 95, 5301–5308. [Google Scholar] [CrossRef]
- Xue, B.C.; Zhang, J.X.; Wang, Z.S.; Wang, L.Z.; Peng, Q.H.; Da, L.C.; Bao, S.K.; Kong, X.Y.; Xue, B. Metabolism response of grazing yak to dietary concentrate supplementation in warm season. Animal 2021, 15, 100175. [Google Scholar] [CrossRef]
- Xie, R.; Zheng, Q.; Luo, G. Finishing effect of maiwa yak by supply feed in warm season. Grass Feed. Livest. 2004, 4, 56–58. [Google Scholar]
- Yang, C.; Zhang, J.B.; Ahmad, A.A.; Bao, P.J.; Guo, X.; Long, R.J.; Ding, X.Z.; Yan, P. Dietary energy levels affect growth performance through growth hormone and insulin-like growth factor 1 in yak (Bos grunniens). Animals 2019, 9, 39. [Google Scholar] [CrossRef]
- Detmann, E.; Queiroz, A. In vitro degradation of neutral detergent fiber of high-quality tropical forage according to supplementation with different nitrogenous compounds. Rev. Bras. Zootec. 2009, 38, 964–971. [Google Scholar]
- Chai, J.M.; Diao, Q.Y.; Wang, H.C.; Tu, Y.; Tao, X.J.; Zhang, N.F. Effects of weaning age on growth, nutrient digestibility and metabolism, and serum parameters in Hu lambs. Anim. Nutr. 2015, 1, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Rodríguez, I.J.; Alvarado-Espino, A.; Véliz, F.G.; Mellado, J.; García, J.E. Short communication: Reproductive response to concentrate supplementation of mixed-breed goats on rangeland. Trop. Anim. Health Prod. 2020, 52, 2737–2741. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.C.; Williams, C.C.; Jenny, B.F.; Fernandez, J.M.; Bateman, H.G.; Nipper, W.A. Effects of feeding milk replacer once versus twice daily on glucose metabolism in Holstein and Jersey calves. J. Dairy Sci. 2002, 85, 2335–2343. [Google Scholar] [CrossRef]
- Jin, Y.D.; Zhou, Y.X. Effects of concentrate level and chromium methionine supplementation on the performance, nutrient digestibility, rumen fermentation, blood metabolites, and meat quality of Tan lambs. Anim. Biosci. 2021, 35, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Song, S.D.; Wang, B.X.; Zhang, Z.F.; Peng, Z.L.; Guo, C.H.; Zhong, J.C.; Wang, Y. Effects of forage: Concentrate ratio on growth performance, ruminal fermentation and blood metabolites in housing-feeding yaks. Asian Australas. J. Anim. Sci. 2015, 28, 1736–1741. [Google Scholar] [CrossRef][Green Version]
- Melo, L.Q.; Costa, S.F.; Lopes, F.; Guerreiro, M.C.; Armentano, L.E.; Pereira, M.N. Rumen morphometrics and the effect of digesta pH and volume on volatile fatty acid absorption1. J. Anim. Sci. 2013, 91, 1775–1783. [Google Scholar] [CrossRef]
- Geishauser, T.; Linhart, N.; Neidl, A.; Reimann, A. Factors associated with ruminal pH at herd level. J. Dairy Sci. 2012, 95, 4556–4567. [Google Scholar] [CrossRef]
- Eun, J.S.; Beauchemin, K.A. Effects of a proteolytic feed enzyme on intake, digestion, ruminal fermentation, and milk production. J. Dairy Sci. 2005, 88, 2140–2153. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, Y.; Zhang, Y.G.; Wang, L.H. The effects of different Concentrate-to-Forage ratio diets on rumen bacterial microbiota and the structures of holstein cows during the feeding cycle. Animals 2020, 10, 957. [Google Scholar] [CrossRef]
- Mckay, Z.C.; Lynch, M.B.; Mulligan, F.J.; Rajauria, G.; Miller, C.; Pierce, K.M. The effect of concentrate supplementation type on milk production, dry matter intake, rumen fermentation, and nitrogen excretion in late-lactation, spring-calving grazing dairy cows. J. Dairy Sci. 2019, 102, 5042–5053. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.F.; Tian, J.; Tian, P.; Cong, R.H.; Luo, Y.W.; Geng, Y.L.; Tao, S.Y.; Ni, Y.; Zhao, R.Q. Feeding a high concentration diet induces unhealthy alterations in the composition and metabolism of ruminal microbiota and host response in a goat model. Front. Microbiol. 2017, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Ariko, T.; Kass, M.; Henno, M.; Fievez, V.; Kärt, O.; Kaart, T.; Ots, M. The effect of replacing barley with glycerol in the diet of dairy cows on rumen parameters and milk fatty acid profile. Anim. Feed Sci. Technol. 2015, 209, 69–78. [Google Scholar] [CrossRef]
- Qiu, Q.H.; Zhu, Y.X.; Qiu, X.J.; Gao, C.Y.; Wang, J.J.; Wang, H.B.; He, Y.; Rahman, M.A.U.; Cao, B.H.; Su, H.W. Dynamic variations in fecal bacterial community and fermentation profile of holstein steers in response to three stepwise density diets. Animals 2019, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.C.; Jeong, C.D.; Mamuad, L.L.; Kim, S.H.; Kang, S.H.; Kim, E.T.; Cho, Y.I.; Lee, S.S.; Lee, S.S. Diet transition from High-Forage to High-Concentrate alters rumen bacterial community composition, epithelial transcriptomes and ruminal fermentation parameters in dairy cows. Animals 2021, 11, 838. [Google Scholar] [CrossRef]
- Sutton, J.D.; Dhanoa, M.S.; Morant, S.V.; France, J.; Napper, D.J.; Schuller, E. Rates of production of acetate, propionate, and butyrate in the rumen of lactating dairy cows given normal and Low-Roughage diets. J. Dairy Sci. 2003, 86, 3620–3633. [Google Scholar] [CrossRef]
- Li, R.H.; Teng, Z.W.; Lang, C.L.; Zhou, H.Z.; Zhong, W.G.; Ban, Z.B.; Yan, X.G.; Yang, H.M.; Farouk, M.H.; Lou, Y.J. Effect of different forage-to-concentrate ratios on ruminal bacterial structure and real-time methane production in sheep. PLoS ONE 2019, 14, e214777. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H.T.; Wang, Y.J.; Li, S.L.; Cao, Z.J.; Ji, S.K.; He, Y.; Zhang, H.T. Effect of dietary forage to concentrate ratios on dynamic profile changes and interactions of ruminal microbiota and metabolites in holstein heifers. Front. Microbiol. 2017, 8, 2206. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Jiang, L.; Mao, S.Y. Effect of high-concentrate diets on microbial composition, function, and the VFAs formation process in the rumen of dairy cows. Anim. Feed Sci. Technol. 2020, 269, 114619. [Google Scholar] [CrossRef]
- Shen, H.; Lu, Z.Y.; Xu, Z.H.; Chen, Z.; Shen, Z.M. Associations among dietary non-fiber carbohydrate, ruminal microbiota and epithelium G-protein-coupled receptor, and histone deacetylase regulations in goats. Microbiome 2017, 5, 123. [Google Scholar] [CrossRef]
- Guo, W.; Li, Y.; Wang, L.Z.; Wang, J.W.; Xu, Q.; Yan, T.H.; Xue, B. Evaluation of composition and individual variability of rumen microbiota in yaks by 16S rRNA high-throughput sequencing technology. Anaerobe 2015, 34, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Costa-Roura, S.; Balcells, J.; de la Fuente, G.; Mora-Gil, J.; Llanes, N.; Villalba, D. Nutrient utilization efficiency, ruminal fermentation and microbial community in Holstein bulls fed concentrate-based diets with different forage source. Anim. Feed Sci. Technol. 2020, 269, 114662. [Google Scholar] [CrossRef]
- Escobar, J.S.; Klotz, B.; Valdes, B.E.; Agudelo, G.M. The gut microbiota of Colombians differs from that of Americans, Europeans and Asians. BMC Microbiol. 2014, 14, 311. [Google Scholar] [CrossRef]
- Perea, K.; Perz, K.; Olivo, S.K.; Williams, A.; Lachman, M.; Ishaq, S.L.; Thomson, J.; Yeoman, C.J. Feed efficiency phenotypes in lambs involve changes in ruminal, colonic, and small-intestine-located microbiota1. J. Anim. Sci. 2017, 95, 2585–2592. [Google Scholar] [CrossRef] [PubMed]
- Gagen, E.J.; Padmanabha, J.; Denman, S.E.; Mcsweeney, C.S. Hydrogenotrophic culture enrichment reveals rumen Lachnospiraceae and Ruminococcaceae acetogens and hydrogen-responsive Bacteroidetes from pasture-fed cattle. FEMS Microbiol. Lett. 2015, 362, v104. [Google Scholar] [CrossRef] [PubMed]
- Opdahl, L.; Gonda, M.; St-Pierre, B. Identification of uncultured bacterial species from firmicutes, bacteroidetes and CANDIDATUS saccharibacteria as candidate cellulose utilizers from the rumen of beef cows. Microorganisms 2018, 6, 17. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.J.; Huasai, S.J.; Chen, A. Effects of dietary forage to concentrate ratio on nutrient digestibility, ruminal fermentation and rumen bacterial composition in Angus cows. Sci. Rep. 2021, 11, 17023. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Liu, S.J.; Chai, S.T.; Meng, Q.X.; Zhou, Z.M. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front. Microbiol. 2019, 10, 1116–1134. [Google Scholar] [CrossRef]
- Morotomi, M.; Nagai, F.; Sakon, H.; Tanaka, R. Paraprevotella clara gen. Nov., Sp. Nov. And Paraprevotella xylaniphila sp. Nov., Members of the family ‘Prevotellaceae’ isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2009, 59, 1895–1900. [Google Scholar] [CrossRef]
- Seshadri, R.; Leahy, S.C.; Attwood, G.T.; Teh, K.H.; Lambie, S.C.; Cookson, A.L.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Hadjithomas, M.; Varghese, N.J.; et al. Cultivation and sequencing of rumen microbiome members from the Hungate1000 Collection. Nat. Biotechnol. 2018, 36, 359–367. [Google Scholar] [CrossRef]
- Shabat, S.K.; Sasson, G.; Doron-Faigenboim, A.; Durman, T.; Yaacoby, S.; Berg, M.M.; White, B.A.; Shterzer, N.; Mizrahi, I. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016, 10, 2958–2972. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Gao, Y.F.; Lu, Y.Y.; Qu, M.G.; Xiong, X.W.; Xu, L.J.; Zhao, X.H.; Pan, K.; Ouyang, K.H. Niacin alters the ruminal microbial composition of cattle under high-concentrate condition. Anim. Nutr. 2017, 3, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef] [PubMed]
| Items | Forage | Concentrate Supplement |
|---|---|---|
| Nutrient composition, % of DM | ||
| DM (1) | 32.51 | 87.64 |
| Crude protein | 11.35 | 18.76 |
| Crude fat | 2.53 | 2.63 |
| Neutral detergent fiber | 54.16 | 16.03 |
| Acid detergent fiber | 26.74 | 7.83 |
| Rumen degradable protein | - | 9.32 |
| Soluble carbohydrates | - | 48.56 |
| Calcium | 2.15 | 0.60 |
| Phosphorus | 0.08 | 0.73 |
| ME, MJ/kg (2) | 8.66 | 12.49 |
| Items 1 | Group 2 | SEM 3 | p-Value | |
|---|---|---|---|---|
| GY | GYS | |||
| Initial body weight (kg) | 124.05 | 124.60 | 3.293 | 0.624 |
| Final body weight (kg) | 137.00 | 159.60 | 4.126 | 0.041 |
| ADG (g) | 215.83 | 583.33 | 35.487 | 0.007 |
| GLU(mmol/L) | 4.15 | 4.54 | 0.135 | 0.004 |
| TG (mmol/L) | 0.17 | 0.20 | 0.012 | 0.086 |
| TP (mmol/L) | 73.17 | 78.92 | 1.255 | 0.029 |
| UN (mmol/L) | 5.79 | 5.45 | 0.389 | 0.056 |
| AST(mmol/L) | 80.38 | 94.31 | 3.241 | 0.032 |
| ALP (mmol/L) | 189.75 | 206.36 | 6.125 | 0.860 |
| Items 1 | Group 2 | SEM 3 | p-Value | |
|---|---|---|---|---|
| GY | GYS | |||
| pH | 7.40 | 6.86 | 0.794 | <0.001 |
| NH3-N, mg/dL | 8.10 | 10.42 | 0.383 | 0.001 |
| MCP, mg/L | 2.07 | 2.75 | 0.116 | 0.002 |
| TVFA, mmol/L | 67.98 | 80.76 | 1.252 | <0.001 |
| VFA, % | ||||
| Acetate | 76.44 | 68.80 | 0.658 | <0.001 |
| Propionate | 15.06 | 19.29 | 0.487 | 0.004 |
| Isobutyrate | 1.22 | 1.60 | 0.094 | 0.007 |
| Butyrate | 5.65 | 9.31 | 0.381 | 0.002 |
| Isovalerate | 1.01 | 1.74 | 0.012 | 0.001 |
| Valerate | 0.61 | 1.23 | 0.117 | 0.002 |
| A:P | 5.07 | 3.08 | 0.117 | <0.001 |
| Item | Group 1 | SEM 2 | p-Value | |
|---|---|---|---|---|
| GY | GYS | |||
| Amino acid metabolism | 13.09 | 13.28 | 0.043 | 0.022 |
| Biosynthesis of other secondary metabolites | 2.28 | 2.06 | 0.067 | 0.119 |
| Carbohydrate metabolism | 13.50 | 13.86 | 0.088 | 0.040 |
| Cell growth and death | 1.62 | 1.58 | 0.013 | 0.056 |
| Cell motility | 2.44 | 3.15 | 0.139 | <0.001 |
| Cellular community prokaryotes | 0.18 | 0.19 | 0.003 | 0.047 |
| Digestive system | 0.07 | 0.04 | 0.005 | 0.008 |
| Endocrine system | 0.25 | 0.24 | 0.005 | 0.299 |
| Energy metabolism | 5.75 | 5.78 | 0.016 | 0.860 |
| Environmental adaptation | 0.22 | 0.24 | 0.005 | 0.006 |
| Folding, sorting and degradation | 3.25 | 3.27 | 0.010 | 0.945 |
| Glycan biosynthesis and metabolism | 4.45 | 3.73 | 0.147 | 0.003 |
| Immune system | 0.09 | 0.09 | 0.001 | 0.092 |
| Infectious disease | 0.18 | 0.18 | 0.001 | 0.093 |
| Lipid metabolism | 4.72 | 5.39 | 0.137 | 0.005 |
| Membrane transport | 1.52 | 1.58 | 0.022 | 0.236 |
| Metabolism of cofactors and vitamins | 13.49 | 12.63 | 0.178 | 0.006 |
| Metabolism of other amino acids | 7.11 | 6.68 | 0.123 | 0.112 |
| Metabolism of terpenoids and polyketides | 9.73 | 10.29 | 0.115 | 0.003 |
| Nucleotide metabolism | 2.12 | 2.10 | 0.009 | 0.288 |
| Replication and repair | 6.45 | 6.46 | 0.024 | 0.813 |
| Translation | 5.03 | 5.11 | 0.022 | 0.055 |
| Xenobiotics biodegradation and metabolism | 2.23 | 1.81 | 0.234 | 0.412 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, D.; Pang, K.; Liu, S.; Wang, X.; Yang, Y.; Chai, S.; Wang, S. Effects of Concentrate Supplementation on Growth Performance, Rumen Fermentation, and Bacterial Community Composition in Grazing Yaks during the Warm Season. Animals 2022, 12, 1398. https://doi.org/10.3390/ani12111398
Dai D, Pang K, Liu S, Wang X, Yang Y, Chai S, Wang S. Effects of Concentrate Supplementation on Growth Performance, Rumen Fermentation, and Bacterial Community Composition in Grazing Yaks during the Warm Season. Animals. 2022; 12(11):1398. https://doi.org/10.3390/ani12111398
Chicago/Turabian StyleDai, Dongwen, Kaiyue Pang, Shujie Liu, Xun Wang, Yingkui Yang, Shatuo Chai, and Shuxiang Wang. 2022. "Effects of Concentrate Supplementation on Growth Performance, Rumen Fermentation, and Bacterial Community Composition in Grazing Yaks during the Warm Season" Animals 12, no. 11: 1398. https://doi.org/10.3390/ani12111398
APA StyleDai, D., Pang, K., Liu, S., Wang, X., Yang, Y., Chai, S., & Wang, S. (2022). Effects of Concentrate Supplementation on Growth Performance, Rumen Fermentation, and Bacterial Community Composition in Grazing Yaks during the Warm Season. Animals, 12(11), 1398. https://doi.org/10.3390/ani12111398






