Electrocardiographic Changes in a Horse with Induced Myocardial Infarction
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Acute Myocardial Infarction
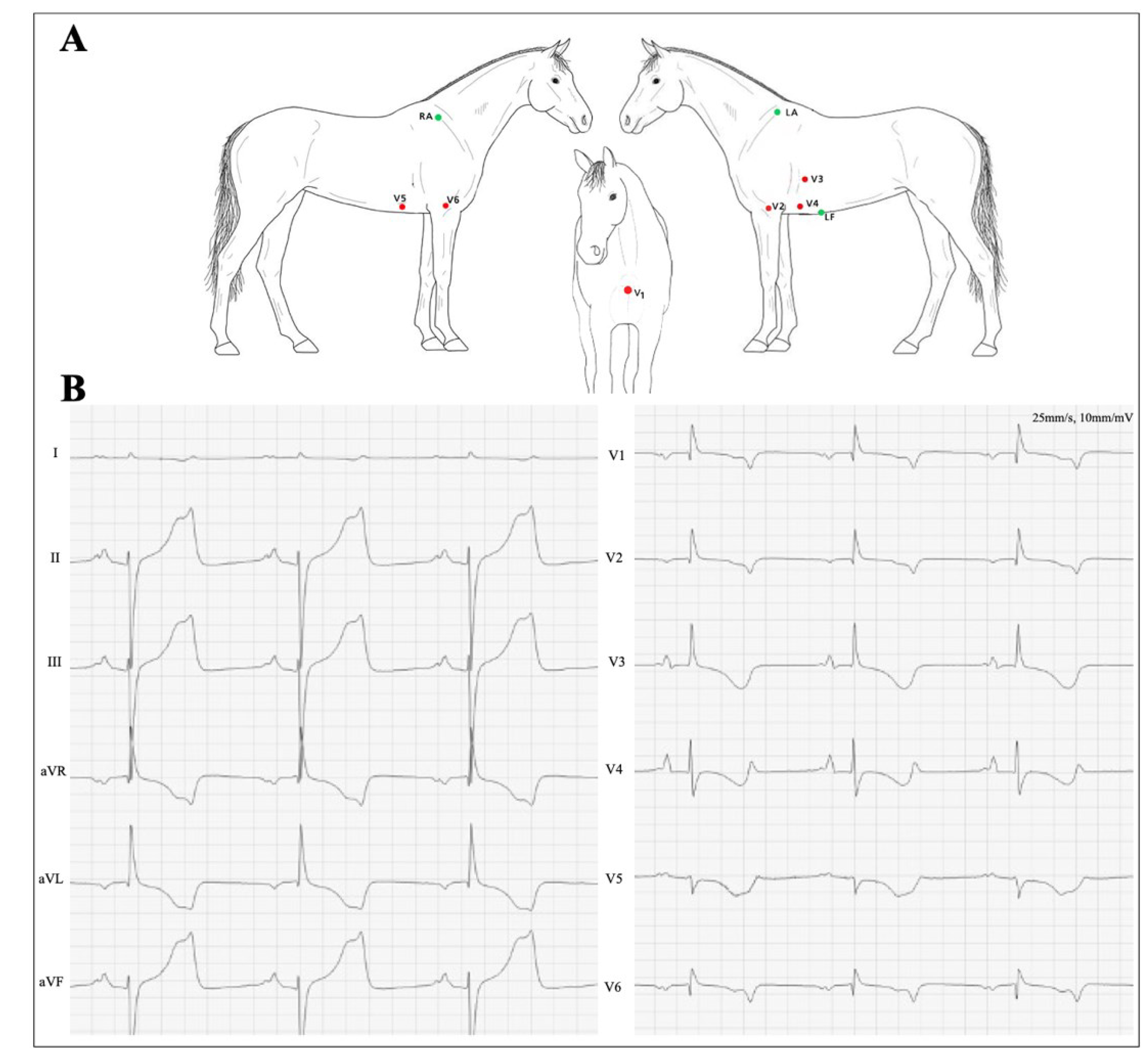
2.2. ECG Recording and Measurements
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [Green Version]
- Sokolow, M.; Lyon, T.P. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am. Heart J. 1949, 37, 161–186. [Google Scholar] [CrossRef]
- Sattler, S.M.; Skibsbye, L.; Linz, D.; Lubberding, A.F.; Tfelt-Hansen, J.; Jespersen, T. Ventricular arrhythmias in first acute myocardial infarction: Epidemiology, mechanisms, and interventions in large animal models. Front. Cardiovasc. Med. 2019, 6, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thygesen, K.; Alpert, J.S.; White, H.D. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 2173–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reef, V.B.; Bonagura, J.; Buhl, R.; McGurrin, M.K.J.; Schwarzwald, C.C.; van Loon, G.; Young, L. Recommendations for management of equine athletes with cardiovascular abnormalities. J. Vet. Intern. Med. 2014, 28, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Hesselkilde, E.M.; Isaksen, J.L.; Petersen, B.V.; Carstensen, H.; Jespersen, T.; Pehrson, S.; Kanters, J.K.; Buhl, R. A novel approach for obtaining 12-lead electrocardiograms in horses. J. Vet. Intern. Med. 2020, 35, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Haugaard, M.M.; Hesselkilde, E.Z.; Pehrson, S.; Carstensen, H.; Flethøj, M.; Præstegaard, K.F.; Sørensen, U.S.; Diness, J.G.; Grunnet, M.; Buhl, R.; et al. Pharmacologic inhibition of small-conductance calcium-activated potassium (SK) channels by NS8593 reveals atrial antiarrhythmic potential in horses. Heart Rhythm 2015, 12, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Marr, C.M.; Bowen, I.M. Cardiology of the Horse, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Williams, D.O.; Boatwright, R.B.; Rugh, K.S.; Ross, C.R.; Sarazan, R.D.; Garner, H.E.; Griggs, D.M. Equine coronary hemodynamics during brief coronary occlusions at three levels of collateral function. Am. J. Physiol. 1996, 270, H1893–H1904. [Google Scholar] [CrossRef] [PubMed]
- Van Steenkiste, G.; Delhaas, T.; Hermans, B.; Vera, L.; Decloedt, A.; van Loon, G. An exploratory study on vectorcardiographic identification of the site of origin of focally induced premature depolarizations in horses, part I: The atria. Animals 2022, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Van Steenkiste, G.; Delhaas, T.; Hermans, B.; Vera, L.; Decloedt, A.; van Loon, G. An exploratory study on vectorcardiographic identification of the site of origin of focally induced premature depolarizations in horses, part II: The ventricles. Animals 2022, 12, 550. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Fan, H.; Liu, Z.; He, J.-Q. Large mammalian animal models of heart disease. J. Cardiovasc. Dev. Dis. 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weis, R.; Carstensen, H.; Sattler, S.M.; Buhl, R.; Hesselkilde, E.M. Electrocardiographic Changes in a Horse with Induced Myocardial Infarction. Animals 2022, 12, 1272. https://doi.org/10.3390/ani12101272
Weis R, Carstensen H, Sattler SM, Buhl R, Hesselkilde EM. Electrocardiographic Changes in a Horse with Induced Myocardial Infarction. Animals. 2022; 12(10):1272. https://doi.org/10.3390/ani12101272
Chicago/Turabian StyleWeis, Rikke, Helena Carstensen, Stefan M. Sattler, Rikke Buhl, and Eva M. Hesselkilde. 2022. "Electrocardiographic Changes in a Horse with Induced Myocardial Infarction" Animals 12, no. 10: 1272. https://doi.org/10.3390/ani12101272
APA StyleWeis, R., Carstensen, H., Sattler, S. M., Buhl, R., & Hesselkilde, E. M. (2022). Electrocardiographic Changes in a Horse with Induced Myocardial Infarction. Animals, 12(10), 1272. https://doi.org/10.3390/ani12101272





