Drenching Bovine Colostrum, Quercetin or Fructo-Oligosaccharides Has No Effect on Health or Survival of Low Birth Weight Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animals
2.3. Piglet Selection
2.4. Experimental Treatments
2.5. Data Collection
2.5.1. Skin Lesion Scoring
2.5.2. Blood Sampling
2.5.3. IgG and IGF-1 Analysis
2.5.4. Statistical Analysis
3. Results
3.1. Milk Replacer Compared with Sham Group
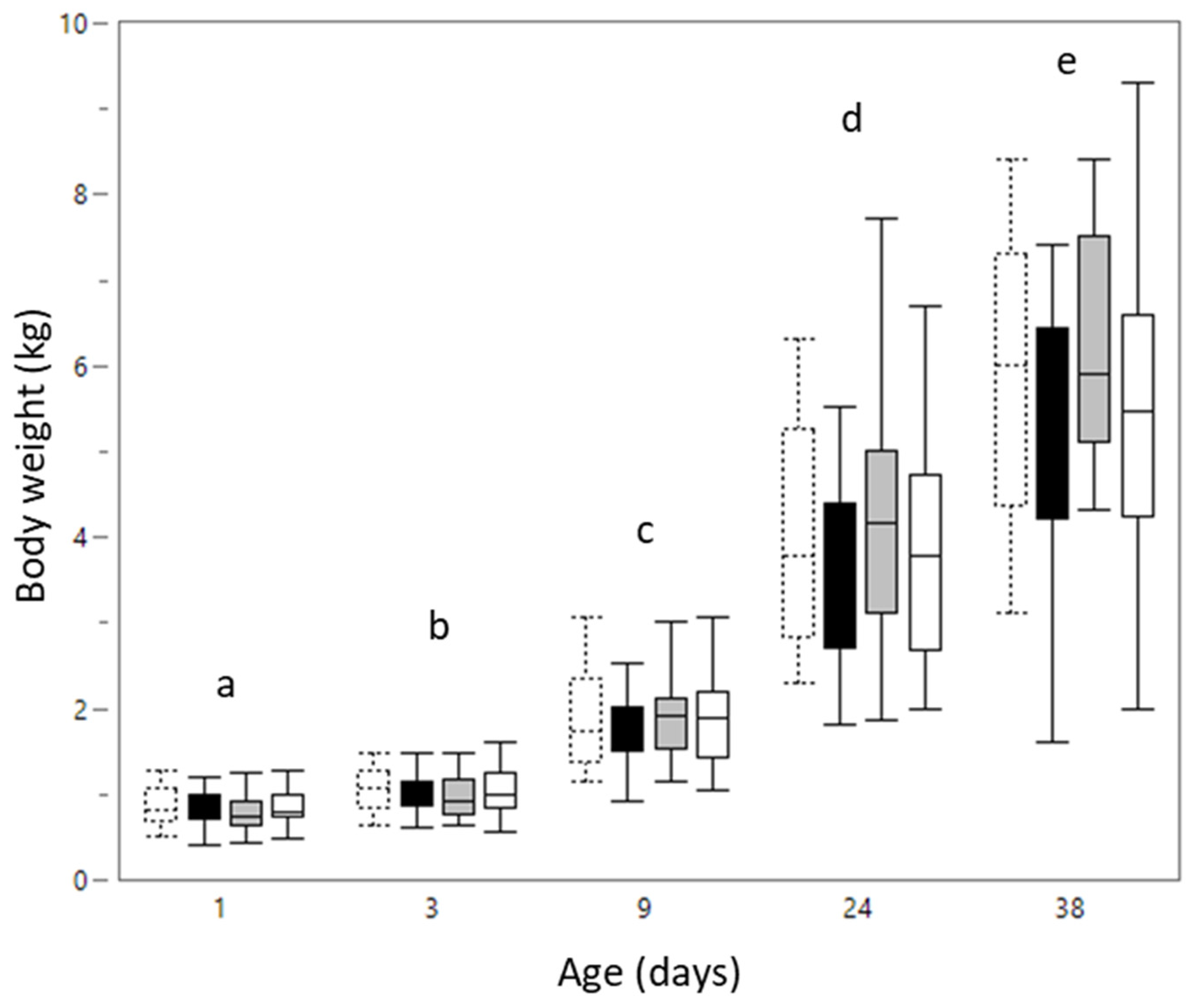
3.1.1. Body Weight
3.1.2. Biochemical Analysis
3.1.3. Hematological Analysis
3.1.4. Skin Lesion Scores
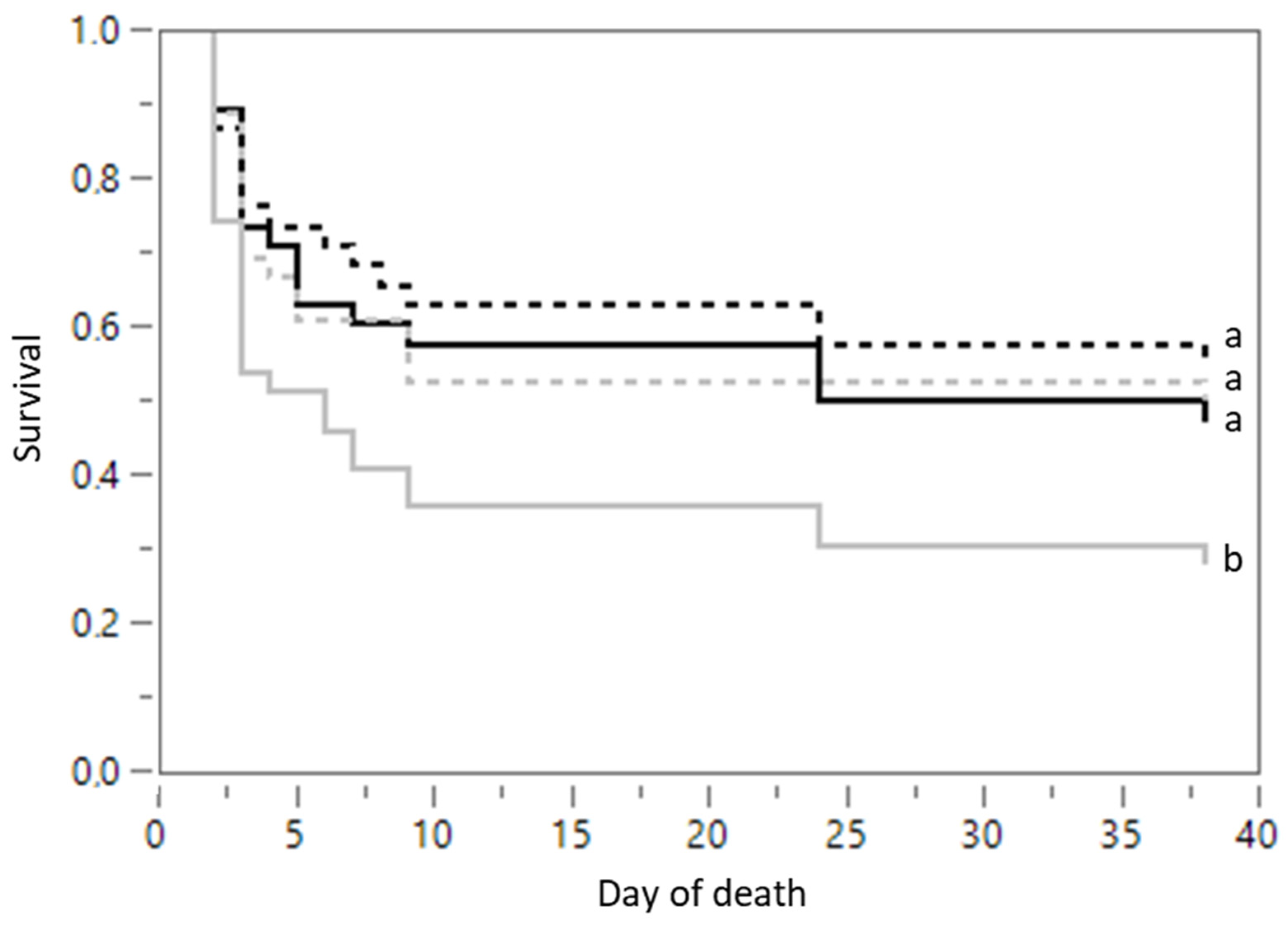
3.1.5. Mortality
3.2. Bioactive Substances Compared with Milk Replacer
3.2.1. Body Weight
3.2.2. Biochemical Analysis
3.2.3. Hematological Analysis
3.2.4. Skin Lesion Scores
3.2.5. Mortality
4. Discussion
4.1. Effect of Milk Replacer
4.2. Effect of Bioactive Substances
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kemp, B.; Da Silva, C.; Soede, N.M. Recent advances in pig reproduction: Focus on impact of genetic selection for female fertility. Reprod. Domest. Anim. 2018, 53, 28–36. [Google Scholar] [CrossRef]
- Bruns, C.E.; Noel, R.J.; McNeil, B.M.; Sonderman, J.P.; Rathje, T.A. 118 Examining Factors That Influence Pig Quality Measured By Weaning Weight. J. Anim. Sci. 2018, 96, 62–63. [Google Scholar] [CrossRef]
- Rutherford, K.; Baxter, E.; D’Eath, R.; Turner, S.P.; Arnott, G.; Roehe, R.; Ask, B.; Sandøe, P.; Moustsen, V.; Thorup, F.; et al. The welfare implications of large litter size in the domestic pig I: Biological factors. Anim. Welf. 2013, 22, 199–218. [Google Scholar] [CrossRef]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Quesnel, H.; Brossard, L.; Valancogne, A.; Quiniou, N. Influence of some sow characteristics on within-litter variation of piglet birth weight. Animal 2008, 2, 1842–1849. [Google Scholar] [CrossRef]
- Beaulieu, A.D.; Aalhus, J.L.; Williams, N.H.; Patience, J.F. Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork. J. Anim. Sci. 2010, 88, 2767–2778. [Google Scholar] [CrossRef] [PubMed]
- Hawe, S.J.; Scollan, N.; Gordon, A.; Magowan, E. What is the current significance of low birthweight pigs on commercial farms in Northern Ireland in terms of impaired growth and mortality? Transl. Anim. Sci. 2020, 4, txaa147. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.A.; Kirkwood, R.N.; Plush, K.J. Are Larger Litters a Concern for Piglet Survival or an Effectively Manageable Trait? Animals 2020, 10, 309. [Google Scholar] [CrossRef]
- Rutherford, K.; Baxter, E.; Ask, B.; Berg, P.; D’Eath, R.; Jarvis, S.; Jensen, K.K.; Lawrence, A.; Moustsen, V.; Robson, S.K.; et al. The ethical and welfare implications of large litter size in domestic pig—Challenges and solutions. Proj. Rep. 2011, 17, 1–148. [Google Scholar]
- Prunier, A.; Heinonen, M.; Quesnel, H. High physiological demands in intensively raised pigs: Impact on health and welfare. Animal 2010, 4, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.M.; Schmitt, O.; Pedersen, L.J. Managing the litter from hyperprolific sows. In The Suckling and Weaned Piglet; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2020; pp. 71–106. [Google Scholar]
- Heras-Molina, A.; Pesantez-Pacheco, J.L.; Astiz, S.; Garcia-Contreras, C.; Vazquez-Gomez, M.; Encinas, T.; Óvilo, C.; Isabel, B.; Gonzalez-Bulnes, A. Maternal Supplementation with Polyphenols and Omega-3 Fatty Acids during Pregnancy: Effects on Growth, Metabolism, and Body Composition of the Offspring. Animals 2020, 10, 1946. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.J.; Zhao, X.; Xiao, K.; Deng, M.; Zhang, L.; Qiu, X.; Deng, J.; Yin, Y.; Tan, C. Dietary energy sources during late gestation and lactation of sows: Effects on performance, glucolipid metabolism, oxidative status of sows, and their offspring. J. Anim. Sci. 2019, 97, 4608–4618. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Piazuelo, D.; Gardiner, G.E.; Ranjitkar, S.; Bouwhuis, M.A.; Ham, R.; Phelan, J.P.; Marsh, A.; Lawlor, P.G. Maternal supplementation with Bacillus altitudinis spores improves porcine offspring growth performance and carcass weight. Br. J. Nutr. 2021, First view, 1–18. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, X.; Cui, Y.; Wang, W.; Liu, H.; Li, Z.; Guo, Z.; Ma, S.; Li, D.; Wang, C.; et al. Consumption of Dietary Fiber from Different Sources during Pregnancy Alters Sow Gut Microbiota and Improves Performance and Reduces Inflammation in Sows and Piglets. mSystems 2021, 6, e00591-20. [Google Scholar] [CrossRef]
- Feyera, T.; Højgaard, C.K.; Vinther, J.; Bruun, T.S.; Theil, P.K. Dietary supplement rich in fiber fed to late gestating sows during transition reduces rate of stillborn piglets. J. Anim. Sci. 2017, 95, 5430–5438. [Google Scholar] [CrossRef]
- Baxter, E.M.; Jarvis, S.; D’Eath, R.B.; Ross, D.W.; Robson, S.K.; Farish, M.; Nevison, I.M.; Lawrence, A.B.; Edwards, S.A. Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology 2008, 69, 773–783. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Holyoake, P.K.; Dial, G.D.; Trigg, T.; King, V.L. Reducing pig mortality through supervision during the perinatal period. J. Anim. Sci. 1995, 73, 3543–3551. [Google Scholar] [CrossRef]
- Alexopoulos, J.G.; Lines, D.S.; Hallett, S.; Plush, K.J. A Review of Success Factors for Piglet Fostering in Lactation. Animals 2018, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.; Rutherford, K.; Arnott, G.; D’Eath, R.; Turner, S.P.; Jarvis, S.; Sandøe, P.; Moustsen, V.; Thorup, F.; Edwards, S.; et al. The welfare implications of large litter size in the domestic pig II: Management factors. Anim. Welf. 2013, 22, 219–238. [Google Scholar] [CrossRef]
- Deen, M.G.H.; Bilkei, G. Cross fostering of low-birthweight piglets. Livest. Prod. Sci. 2004, 90, 279–284. [Google Scholar] [CrossRef]
- Calderon Diaz, J.A.; Garcia Manzanilla, E.; Diana, A.; Boyle, L.A. Cross-Fostering Implications for Pig Mortality, Welfare and Performance. Front. Vet. Sci. 2018, 5, 123. [Google Scholar] [CrossRef]
- Douglas, S.L.; Edwards, S.A.; Kyriazakis, I. Management strategies to improve the performance of low birth weight pigs to weaning and their long-term consequences. J. Anim. Sci. 2014, 92, 2280–2288. [Google Scholar] [CrossRef]
- Vergauwen, H.; Degroote, J.; Prims, S.; Wang, W.; Fransen, E.; De Smet, S.; Casteleyn, C.; Van Cruchten, S.; Michiels, J.; Van Ginneken, C. Artificial rearing influences the morphology, permeability and redox state of the gastrointestinal tract of low and normal birth weight piglets. J. Anim. Sci. Biotechnol. 2017, 8, 30. [Google Scholar] [CrossRef]
- Schmitt, O.; Baxter, E.M.; Lawlor, P.G.; Boyle, L.A.; O’Driscoll, K. A Single Dose of Fat-Based Energy Supplement to Light Birth Weight Pigs Shortly After Birth Does Not Increase Their Survival and Growth. Animals 2019, 9, 227. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Effect of oral supplementation with different energy boosters in newborn piglets on pre-weaning mortality, growth and serological levels of IGF-I and IgG. J. Anim. Sci. 2017, 95, 353–360. [Google Scholar] [CrossRef]
- de Greeff, A.; Resink, J.W.; van Hees, H.M.; Ruuls, L.; Klaassen, G.J.; Rouwers, S.M.; Stockhofe-Zurwieden, N. Supplementation of piglets with nutrient-dense complex milk replacer improves intestinal development and microbial fermentation. J. Anim. Sci. 2016, 94, 1012–1019. [Google Scholar] [CrossRef]
- De Vos, M.; Che, L.; Huygelen, V.; Willemen, S.; Michiels, J.; Van Cruchten, S.; Van Ginneken, C. Nutritional interventions to prevent and rear low-birthweight piglets. J. Anim. Physiol. Anim. Nutr. 2014, 98, 609–619. [Google Scholar] [CrossRef]
- Madsen, J.G.; Mueller, S.; Kreuzer, M.; Bigler, M.B.; Silacci, P.; Bee, G. Milk replacers supplemented with either L-arginine or L-carnitine potentially improve muscle maturation of early reared low birth weight piglets from hyperprolific sows. Animal 2018, 12, 43–53. [Google Scholar] [CrossRef]
- Declerck, I.; Dewulf, J.; Decaluwé, R.; Maes, D. Effects of energy supplementation to neonatal (very) low birth weight piglets on mortality, weaning weight, daily weight gain and colostrum intake. Livest. Sci. 2016, 183, 48–53. [Google Scholar] [CrossRef]
- Manzke, N.E.; Gomes, B.K.; Xavier, E.G.; de Lima, G. Efficacy of energy supplementation on growth performance and immune response of suckling pigs. J. Anim. Sci. 2018, 96, 4723–4730. [Google Scholar] [CrossRef]
- Santos, L.S.; Caldara, F.R.; Machado, S.T.; Nääs, I.A.; Foppa, L.; Garcia, R.G.; Moura, R.; Machado, S.P. Sows’ parity and coconut oil postnatal supplement on piglets performance. Rev. MVZ Córdoba 2015, 20, 4513–4521. [Google Scholar] [CrossRef][Green Version]
- Sinkora, M.; Butler, J.E. The ontogeny of the porcine immune system. Dev. Comp. Immunol. 2009, 33, 273–283. [Google Scholar] [CrossRef]
- Declerck, I.; Dewulf, J.; Sarrazin, S.; Maes, D. Long-term effects of colostrum intake in piglet mortality and performance. J. Anim. Sci. 2016, 94, 1633–1643. [Google Scholar] [CrossRef]
- Moreira, L.P.; Menegat, M.B.; Barros, G.P.; Bernardi, M.L.; Wentz, I.; Bortolozzo, F.P. Effects of colostrum, and protein and energy supplementation on survival and performance of low-birth-weight piglets. Livest. Sci. 2017, 202, 188–193. [Google Scholar] [CrossRef]
- Lo Verso, L.; Matte, J.J.; Lapointe, J.; Talbot, G.; Bissonnette, N.; Blais, M.; Guay, F.; Lessard, M. Impact of birth weight and neonatal nutritional interventions with micronutrients and bovine colostrum on the development of piglet immune response during the peri-weaning period. Vet. Immunol. Immunopathol. 2020, 226, 110072. [Google Scholar] [CrossRef]
- Rathe, M.; Müller, K.; Sangild, P.T.; Husby, S. Clinical applications of bovine colostrum therapy: A systematic review. Nutr. Rev. 2014, 72, 237–254. [Google Scholar] [CrossRef]
- Poulsen, A.R.; de Jonge, N.; Sugiharto, S.; Nielsen, J.L.; Lauridsen, C.; Canibe, N. The microbial community of the gut differs between piglets fed sow milk, milk replacer or bovine colostrum. Br. J. Nutr. 2017, 117, 964–978. [Google Scholar] [CrossRef]
- Huang, S.; Li, N.; Liu, C.; Li, T.; Wang, W.; Jiang, L.; Li, Z.; Han, D.; Tao, S.; Wang, J. Characteristics of the gut microbiota colonization, inflammatory profile, and plasma metabolome in intrauterine growth restricted piglets during the first 12 hours after birth. J. Microbiol. 2019, 57, 748–758. [Google Scholar] [CrossRef]
- Pluske, J.R.; Turpin, D.L.; Kim, J.C. Gastrointestinal tract (gut) health in the young pig. Anim. Nutr. 2018, 4, 187–196. [Google Scholar] [CrossRef]
- D’Inca, R.; Gras-Le Guen, C.; Che, L.; Sangild, P.T.; Le Huërou-Luron, I. Intrauterine growth restriction delays feeding-induced gut adaptation in term newborn pigs. Neonatology 2011, 99, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Iwasaki, Y.; Nakayama, K.; Ushida, K. Stimulation of butyrate production in the large intestine of weaning piglets by dietary fructooligosaccharides and its influence on the histological variables of the large intestinal mucosa. J. Nutr. Sci. Vitam. 2003, 49, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Le Bourgot, C.; Ferret-Bernard, S.; Le Normand, L.; Savary, G.; Menendez-Aparicio, E.; Blat, S.; Appert-Bossard, E.; Respondek, F.; Le Huerou-Luron, I. Maternal short-chain fructooligosaccharide supplementation influences intestinal immune system maturation in piglets. PLoS ONE 2014, 9, e107508. [Google Scholar] [CrossRef] [PubMed]
- Le Bourgot, C.; Ferret-Bernard, S.; Blat, S.; Apper, E.; Le Huërou-Luron, I. Short-chain fructooligosaccharide supplementation during gestation and lactation or after weaning differentially impacts pig growth and IgA response to influenza vaccination. J. Funct. Foods 2016, 24, 307–315. [Google Scholar] [CrossRef]
- Ayuso, M.; Michiels, J.; Wuyts, S.; Yan, H.; Degroote, J.; Lebeer, S.; Le Bourgot, C.; Apper, E.; Majdeddin, M.; Van Noten, N.; et al. Short-chain fructo-oligosaccharides supplementation to suckling piglets: Assessment of pre- and post-weaning performance and gut health. PLoS ONE 2020, 15, e0233910. [Google Scholar] [CrossRef]
- Apper, E.; Meymerit, C.; Bodin, J.C.; Respondek, F.; Wagner, A. Effect of Dietary Supplementation with Short-Chain Fructooligosaccharides in Lactating Sows and Newly Weaned Piglets on Reproductive Performance of Sows, Immune Response, and Growth Performance of Piglets from Birth to Slaughter. J. Anim. Res. Nutr. 2016, 1, 1. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Liu, G.; Duan, J.; Yang, G.; Wu, L.; Li, T.; Yin, Y. Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free. Radic. Res. 2013, 47, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.; De Vos, M.; Missotten, J.; Ovyn, A.; De Smet, S.; Van Ginneken, C. Maturation of digestive function is retarded and plasma antioxidant capacity lowered in fully weaned low birth weight piglets. Br. J. Nutr. 2013, 109, 65–75. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Li, D.; Yin, Y.; Wang, X.; Li, P.; Dangott, L.J.; Hu, W.; Wu, G. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J. Nutr. 2008, 138, 60–66. [Google Scholar] [CrossRef]
- Yu, L.C.; Wang, J.T.; Wei, S.C.; Ni, Y.H. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World J. Gastrointest. Pathophysiol. 2012, 3, 27–43. [Google Scholar] [CrossRef]
- Nazli, A.; Yang, P.C.; Jury, J.; Howe, K.; Watson, J.L.; Söderholm, J.D.; Sherman, P.M.; Perdue, M.H.; McKay, D.M. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am. J. Pathol. 2004, 164, 947–957. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Vergauwen, H.; Tambuyzer, B.; Jennes, K.; Degroote, J.; Wang, W.; De Smet, S.; Michiels, J.; Van Ginneken, C. Trolox and ascorbic acid reduce direct and indirect oxidative stress in the IPEC-J2 cells, an in vitro model for the porcine gastrointestinal tract. PLoS ONE 2015, 10, e0120485. [Google Scholar] [CrossRef]
- Vergauwen, H.; Prims, S.; Degroote, J.; Wang, W.; Casteleyn, C.; van Cruchten, S.; de Smet, S.; Michiels, J.; van Ginneken, C. In Vitro Investigation of Six Antioxidants for Pig Diets. Antioxidants 2016, 5, 41. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, Q.; Xu, G.; Chen, H.; Lei, H.; Su, J. Effects of Quercetin on Proliferation and H2O2-Induced Apoptosis of Intestinal Porcine Enterocyte Cells. Molecules 2018, 23, 2012. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhang, Y.; Si, X.; Jin, Y.; Jiang, D.; Dai, Z.; Wu, Z. Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes. Nutrients 2021, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, H.; Van Ginneken, C.; Michiels, J. Intestinal Oxidative status, Barrier and Regeneration in Piglets: From In Vitro to In Vivo. Ph.D. Thesis, University of Antwerp, Antwerp, Belgium, 2018. [Google Scholar]
- Degroote, J.; Vergauwen, H.; Van Noten, N.; Wang, W.; De Smet, S.; Van Ginneken, C.; Michiels, J. The Effect of Dietary Quercetin on the Glutathione Redox System and Small Intestinal Functionality of Weaned Piglets. Antioxidants 2019, 8, 312. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wei, H.K.; Xiang, Q.H.; Wang, J.; Zhou, Y.F.; Peng, J. Protective effect of quercetin on pig intestinal integrity after transport stress is associated with regulation oxidative status and inflammation. J. Vet. Med. Sci. 2016, 78, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Luehring, M.; Blank, R.; Wolffram, S. Vitamin E-sparing and vitamin E-independent antioxidative effects of the flavonol quercetin in growing pigs. Anim. Feed. Sci. Technol. 2011, 169, 199–207. [Google Scholar] [CrossRef]
- Bieger, J.; Cermak, R.; Blank, R.; de Boer, V.C.; Hollman, P.C.; Kamphues, J.; Wolffram, S. Tissue distribution of quercetin in pigs after long-term dietary supplementation. J. Nutr. 2008, 138, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Burdeos, G.C.; Blank, R.; Wolffram, S. Influence of quercetin on the global DNA methylation pattern in pigs. Food Funct. 2020, 11, 7421–7426. [Google Scholar] [CrossRef] [PubMed]
- Van Le Thanh, B.; Lemay, M.; Bastien, A.; Lapointe, J.; Lessard, M.; Chorfi, Y.; Guay, F. The potential effects of antioxidant feed additives in mitigating the adverse effects of corn naturally contaminated with Fusarium mycotoxins on antioxidant systems in the intestinal mucosa, plasma, and liver in weaned pigs. Mycotoxin Res. 2016, 32, 99–116. [Google Scholar] [CrossRef]
- King, M.R.; Morel, P.C.H.; Revell, D.K.; Pluske, J.R.; Birtles, M.J. Dietary Bovine Colostrum Increases Villus Height and Decreases Small Intestine Weight in Early-weaned Pigs. Asian-Australas J. Anim. Sci. 2008, 21, 567–573. [Google Scholar] [CrossRef]
- Huguet, A.; Le Dividich, J.; Le Huërou-Luron, I. Improvement of growth performance and sanitary status of weaned piglets fed a bovine colostrum-supplemented diet. J. Anim. Sci. 2012, 90, 1513–1520. [Google Scholar] [CrossRef]
- Viehmann, V.; Unterweger, C.; Ganter, M.; Metzler-Zebeli, B.U.; Ritzmann, M.; Hennig-Pauka, I. Effects of bovine colostrum on performance, survival, and immunoglobulin status of suckling piglets during the first days of life. Czech J. Anim. Sci. 2015, 60, 351–358. [Google Scholar] [CrossRef]
- Le Bourgot, C.; Ferret-Bernard, S.; Apper, E.; Taminiau, B.; Cahu, A.; Le Normand, L.; Respondek, F.; Le Huërou-Luron, I.; Blat, S. Perinatal short-chain fructooligosaccharides program intestinal microbiota and improve enteroinsular axis function and inflammatory status in high-fat diet-fed adult pigs. FASEB J. 2019, 33, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Cermak, R.; Landgraf, S.; Wolffram, S. The bioavailability of quercetin in pigs depends on the glycoside moiety and on dietary factors. J. Nutr. 2003, 133, 2802–2807. [Google Scholar] [CrossRef] [PubMed]
- Wein, S.; Wolffram, S. Concomitant intake of quercetin with a grain-based diet acutely lowers postprandial plasma glucose and lipid concentrations in pigs. BioMed Res. Int. 2014, 2014, 748742. [Google Scholar] [CrossRef]
- Rundgren, M.; Löfquist, I. Effects on performance and behaviour of mixing 20-kg pigs fed individually. Anim. Sci. 1989, 49, 311–315. [Google Scholar] [CrossRef]
- Pluske, J.R.; Williams, I.H. Reducing stress in piglets as a means of increasing production after weaning: Administration of amperozide or co-mingling of piglets during lactation? Anim. Sci. 1996, 62, 121–130. [Google Scholar] [CrossRef]
- Parratt, C.A.; Chapman, K.J.; Turner, C.; Jones, P.H.; Mendl, M.T.; Miller, B.G. The fighting behaviour of piglets mixed before and after weaning in the presence or absence of a sow. Appl. Anim. Behav. Sci. 2006, 101, 54–67. [Google Scholar] [CrossRef]
- Huting, A.M.S.; Middelkoop, A.; Guan, X.; Molist, F. Using Nutritional Strategies to Shape the Gastro-Intestinal Tracts of Suckling and Weaned Piglets. Animals 2021, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Muns, R.; Silva, C.; Manteca, X.; Gasa, J. Effect of cross-fostering and oral supplementation with colostrums on performance of newborn piglets. J. Anim. Sci. 2014, 92, 1193–1199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cabrera, R.A.; Lin, X.; Campbell, J.M.; Moeser, A.J.; Odle, J. Influence of birth order, birth weight, colostrum and serum immunoglobulin G on neonatal piglet survival. J. Anim. Sci. Biotechnol. 2012, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Machado-Neto, R.; Graves, C.N.; Curtis, S.E. Immunoglobulins in piglets from sows heat-stressed prepartum. J. Anim. Sci. 1987, 65, 445–455. [Google Scholar] [CrossRef]
- Müller, R.; Thorup, F.; Hansen, C.F. Supplementing new born piglets with 50 mL sow colostrum failed to influence piglet survival. In Proceedings of the 4th European Symposium of Porcine Health Management, Bruges, Belgium, 25–27 April 2012; p. 118. [Google Scholar]
- Schokker, D.; Fledderus, J.; Jansen, R.; Vastenhouw, S.A.; de Bree, F.M.; Smits, M.A.; Jansman, A. Supplementation of fructooligosaccharides to suckling piglets affects intestinal microbiota colonization and immune development. J. Anim. Sci. 2018, 96, 2139–2153. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Sureshkumar, S.; Kim, I.H. Influences of dietary flavonoid (quercetin) supplementation on growth performance and immune response of growing pigs challenged with Escherichia coli lipopolysaccharide. J. Anim. Sci. Technol. 2020, 62, 605–613. [Google Scholar] [CrossRef] [PubMed]
| Analytical Constituents | Nutritional Additives | ||
|---|---|---|---|
| Crude protein (%) | 19.9 | Vitamin A (IU/kg) | 25,000 |
| Crude fat (%) | 15.9 | Vitamin D3 (IU/kg) | 5000 |
| Crude ash (%) | 7.6 | Vitamin E (mg/kg) | 80 |
| Crude fibre (%) | 0 | Vitamin K (mg/kg) | 4 |
| Moisture (%) | 3.1 | Vitamin C (mg/kg) | 158 |
| Lactose (%) | 38.5 | Vitamin B1 (mg/kg) | 6 |
| Lysine (%) | 1.75 | Vitamin B2 (mg/kg) | 6 |
| Methionine (%) | 0.62 | Vitamin B6 (mg/kg) | 4 |
| Cystine + Methionine (%) | 1 | Vitamin B12 (µg/kg) | 40 |
| Calcium (%) | 0.55 | Iodine (mg/kg) | 1 |
| Sodium (%) | 0.62 | Manganese (mg/kg) | 45 |
| Phosphorus (%) | 0.5 | Zinc (mg/kg) | 84 |
| Magnesium (%) | 0.12 | Selenium (mg/kg) | 0.30 |
| Iron (mg/kg) | 76 | Propyl gallate (mg/kg) | 3 |
| Copper (mg/kg) | 155 | Butylated hydroxyanisole (mg/kg) | 3 |
| Energetic value | |||
| Metabolizable energy (MJ/kg|kcal/kg) | 17.9|4280 | ||
| Net energy (MJ/kg|kcal/kg) | 14.3|3420 | ||
| Dependent Variable | Treatment | ||
|---|---|---|---|
| Milk Replacer | Sham | ||
| Median ± SD (n) | Median ± SD (n) | p-Value | |
| Glucose (mmol/L) | 6.30 ± 0.86 (27) | 6.56 ± 1.32 (20) | 0.400 |
| NEFA (mmol/L) | 0.32 ± 0.56 (29) | 0.33 ± 0.82 (19) | 0.562 |
| Urea (mmol/L) | 2.46 ± 1.37 (27) | 2.32 ± 1.40 (20) | 0.495 |
| IgG (mg/mL) | 5.41 ± 2.81 (12) | 2.55 ± 1.79 (12) | 0.057 |
| IGF-1 (ng/mL) | 27.46 ± 15.31 (12) | 20.17 ± 17.62 (12) | 0.647 |
| RBC (1012/L) | 5.29 ± 0.99 (20) | 5.46 ± 0.88 (16) | 0.151 |
| HCT (%) | 33.20 ± 5.51 (20) | 33.20 ± 4.15 (16) | 0.665 |
| HGB (g/dL) | 9.40 ± 1.67 (21) | 9.65 ± 1.17 (16) | 0.858 |
| WBC (103/µL) | 16.30 ± 5.74 (21) | 17.81 ± 4.39 (16) | 0.606 |
| Lymphocytes (103/µL) | 7.17 ± 2.11 (21) | 6.93 ± 1.90 (16) | 0.960 |
| Monocytes (103/µL) | 1.49 ± 0.78 (21) | 1.12 ± 0.64 (16) | 0.600 |
| Neutrophils (103/µL) | 7.23 ± 3.93 (21) | 8.40 ± 2.52 (16) | 0.984 |
| Eosinophils (103/µL) | 0.16 ± 0.12 (21) | 0.19 ± 0.17 (16) | 0.361 |
| Basophils (103/µL) | 0.01 ± 0.01 (21) | 0.02 ± 0.01 (16) | 0.752 |
| Thrombocytes (103/µL) | 404 ± 329.13 (21) | 596 ± 334.41 (21) | 0.053 |
| Dependent Variable | Treatment | ||||
|---|---|---|---|---|---|
| Milk Replacer | Colostrum | Quercetin | scFOS | p-Value | |
| Median ± SD (n) | Median ± SD (n) | Median ± SD (n) | Median ± SD (n) | ||
| Glucose (mmol/L) | 6.30 ± 0.86 (27) | 6.00 ± 1.20 (24) | 6.17 ± 1.37 (23) | 6.23 ± 1.07 (15) | 0.466 |
| NEFA (mmol/L) | 0.32 ± 0.56 (29) | 0.38 ± 0.56 (24) | 0.45 ± 0.42 (22) | 0.46 ± 0.72 (16) | 0.799 |
| Urea (mmol/L) | 2.46 ± 1.37 (27) | 2.73 ± 1.23 (23) | 2.32 ± 1.09 (21) | 1.84 ± 1.60 (15) | 0.121 |
| IgG (mg/mL) | 5.41 ± 2.81 (12) | 3.11 ± 1.17 (10) | 4.07 ± 3.09 (12) | 2.62 ± 2.37 (14) | 0.146 |
| IGF-1 (ng/mL) | 27.46 ± 15.31 (12) | 16.87 ± 15.55 (9) | 18.59 ± 13.39 (11) | 13.51 ± 17.73 (14) | 0.292 |
| RBC (1012/L) | 5.29 ± 0.99 (20) | 5.37 ± 0.96 (15) | 5.01 ± 1.11 (18) | 5.28 ± 1.07 (10) | 0.580 |
| HCT (%) | 33.20 ± 5.51 (20) | 35.50 ± 3.82 (15) | 33.90 ± 4.59 (18) | 33.90 ± 4.23 (10) | 0.096 |
| HGB (g/dL) | 9.40 ± 1.67 (21) | 9.80 ± 1.32 (15) | 9.05 ± 1.80 (18) | 9.85 ± 1.25 (10) | 0.151 |
| WBC (103/µL) | 16.30 ± 5.74 (21) | 19.45 ± 6.39 (15) | 16.50 ± 5.27 (18) | 15.50 ± 6.07 (10) | 0.324 |
| Lymphocytes (103/µL) | 7.17 ± 2.11 (21) | 8.71 ± 2.10 (15) | 6.84 ± 2.23 (18) | 6.91 ± 3.31 (10) | 0.362 |
| Monocytes (103/µL) | 1.49 ± 0.78 (21) | 1.06 ± 0.43 (15) | 1.39 ± 0.75 (18) | 1.18 ± 0.64 (10) | 0.295 |
| Neutrophils (103/µL) | 7.23 ± 3.93 (21) | 8.82 ± 3.30 (14) | 8.25 ± 3.47 (18) | 8.42 ± 3.02 (10) | 0.833 |
| Eosinophils (103/µL) | 0.16 ± 0.12 (21) | 0.14 ± 0.10 (15) | 0.10 ± 0.10 (18) | 0.23 ± 0.16 (9) | 0.158 |
| Basophils (103/µL) | 0.01 ± 0.01 (21) | 0.01 ± 0.01 (15) | 0.02 ± 0.01 (18) | 0.01 ± 0.01 (9) | 0.823 |
| Thrombocytes (103/µL) | 404 ± 329.13 (21) | 546 ± 316.93 (15) | 484 ± 335.40 (18) | 402 ± 346.78 (10) | 0.590 |
| Dependent Variable | Age | Sex | ||||
|---|---|---|---|---|---|---|
| Day 9 | Day 38 | p-Value | Female | Male | p-Value | |
| Median ± SD (n) | Median ± SD (n) | Median ± SD (n) | Median ± SD (n) | |||
| Glucose (mmol/L) | 6.38 ± 1.14 (54) | 5.90 ± 1.07 (35) | 0.009 | 6.17 ± 1.10 (42) | 6.18 ± 1.16 (47) | 0.466 |
| NEFA (mmol/L) | 0.57 ± 0.58 (55) | 0.08 ± 0.24 (36) | <0.001 | 0.43 ± 0.59 (44) | 0.39 ± 0.54 (47) | 0.974 |
| Urea (mmol/L) | 2.65 ± 1.19 (49) | 1.76 ± 1.29 (37) | <0.001 | 2.47 ± 1.29 (40) | 2.47 ± 1.34 (46) | 0.409 |
| IgG (mg/mL) | 4.27 ± 2.66 (24) | 2.63 ± 2.37 (24) | 0.029 | 3.91 ± 2.90 (18) | 3.22 ± 2.42 (30) | 0.843 |
| IGF-1 (ng/mL) | 9.19 ± 12.11 (24) | 25.35 ± 20.13 (22) | <0.001 | 20.81 ± 23.12 (18) | 15.66 ± 16.17 (28) | 0.363 |
| RBC (1012/L) | 4.27 ± 0.62 (30) | 6.09 ± 0.64 (33) | <0.001 | 5.35 ± 1.03 (32) | 5.19 ± 1.03 (31) | 0.631 |
| HCT (%) | 31.90 ± 4.17 (31) | 37.50 ± 3.83 (33) | <0.001 | 33.55 ± 4.31 (32) | 33.90 ± 5.28 (32) | 0.359 |
| HGB (g/dL) | 8.50 ± 1.26 (31) | 10.40 ± 1.07 (33) | <0.001 | 9.55 ± 1.46 (32) | 9.35 ± 1.68 (32) | 0.255 |
| WBC (103/µL) | 13.51 ± 4.13 (31) | 20.38 ± 4.95 (33) | <0.001 | 16.21 ± 5.66 (32) | 17.76 ± 5.98 (32) | 0.771 |
| Lymphocytes (103/µL) | 5.52 ± 2.00 (31) | 8.58 ± 1.70 (33) | <0.001 | 7.58 ± 2.39 (32) | 7.33 ± 2.31 (32) | 0.363 |
| Monocytes (103/µL) | 0.91 ± 0.33 (31) | 1.56 ± 0.72 (33) | <0.001 | 1.33 ± 0.72 (32) | 1.20 ± 0.65 (32) | 0.283 |
| Neutrophils (103/µL) | 6.85 ± 2.94 (31) | 9.42 ± 3.41 (32) | 0.001 | 8.11 ± 3.48 (32) | 8.04 ± 3.51 (31) | 0.942 |
| Eosinophils (103/µL) | 0.08 ± 0.07 (31) | 0.21 ± 0.12 (32) | <0.001 | 0.16 ± 0.12 (31) | 0.10 ± 0.12 (32) | 0.553 |
| Basophils (103/µL) | 0.01 ± 0.01 (31) | 0.01 ± 0.01 (32) | 0.762 | 0.02 ± 0.01 (31) | 0.01 ± 0.01 (32) | 0.064 |
| Thrombocytes (103/µL) | 815 ± 367.24 (31) | 364 ± 204.30 (33) | 0.001 | 529 ± 330.26 (32) | 463 ± 321.85 (32) | 0.235 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Tichelen, K.; Prims, S.; Ayuso, M.; Van Kerschaver, C.; Vandaele, M.; Degroote, J.; Van Cruchten, S.; Michiels, J.; Van Ginneken, C. Drenching Bovine Colostrum, Quercetin or Fructo-Oligosaccharides Has No Effect on Health or Survival of Low Birth Weight Piglets. Animals 2022, 12, 55. https://doi.org/10.3390/ani12010055
Van Tichelen K, Prims S, Ayuso M, Van Kerschaver C, Vandaele M, Degroote J, Van Cruchten S, Michiels J, Van Ginneken C. Drenching Bovine Colostrum, Quercetin or Fructo-Oligosaccharides Has No Effect on Health or Survival of Low Birth Weight Piglets. Animals. 2022; 12(1):55. https://doi.org/10.3390/ani12010055
Chicago/Turabian StyleVan Tichelen, Kevin, Sara Prims, Miriam Ayuso, Céline Van Kerschaver, Mario Vandaele, Jeroen Degroote, Steven Van Cruchten, Joris Michiels, and Chris Van Ginneken. 2022. "Drenching Bovine Colostrum, Quercetin or Fructo-Oligosaccharides Has No Effect on Health or Survival of Low Birth Weight Piglets" Animals 12, no. 1: 55. https://doi.org/10.3390/ani12010055
APA StyleVan Tichelen, K., Prims, S., Ayuso, M., Van Kerschaver, C., Vandaele, M., Degroote, J., Van Cruchten, S., Michiels, J., & Van Ginneken, C. (2022). Drenching Bovine Colostrum, Quercetin or Fructo-Oligosaccharides Has No Effect on Health or Survival of Low Birth Weight Piglets. Animals, 12(1), 55. https://doi.org/10.3390/ani12010055








