Evaluation of Changes in the Cardiac Function before and after Transcatheter Edge-to-Edge Mitral Valve Repair in Healthy Dogs: Conventional and Novel Echocardiography
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Procedure
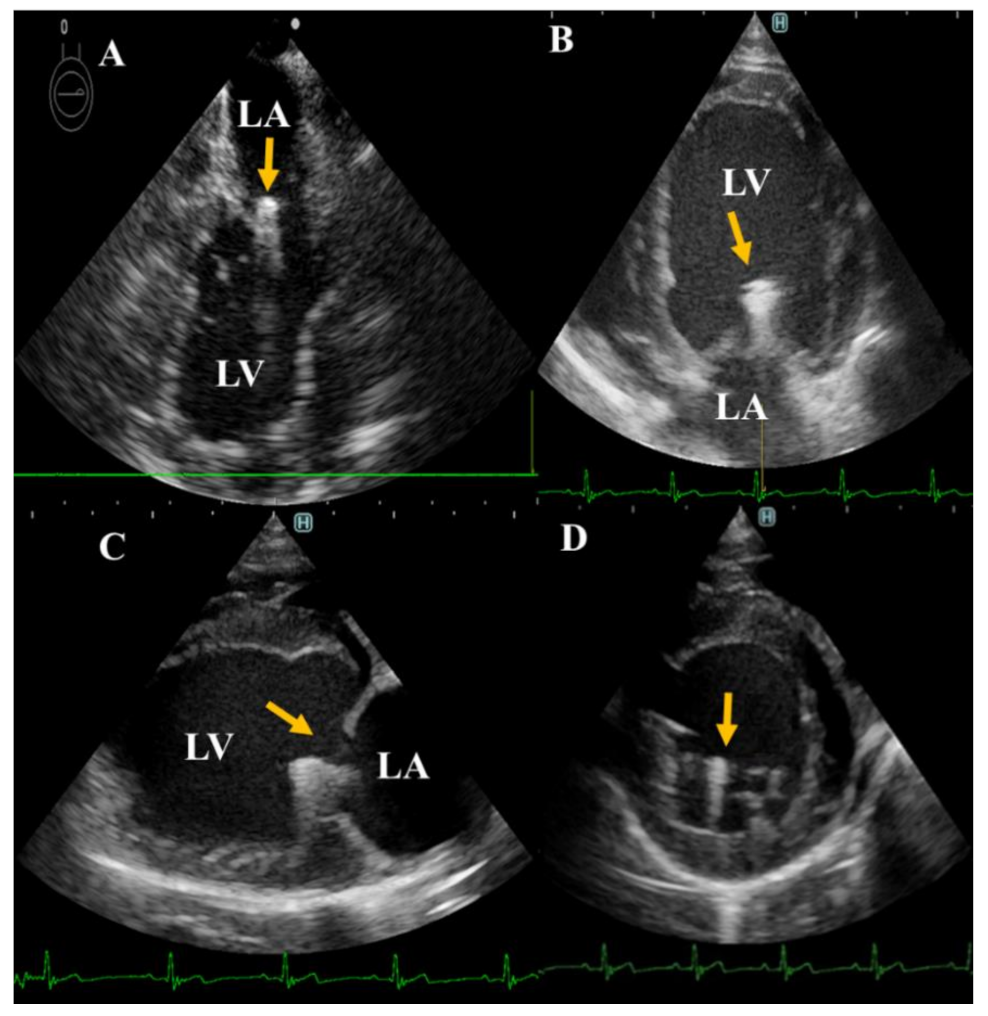
2.2. Echocardiography
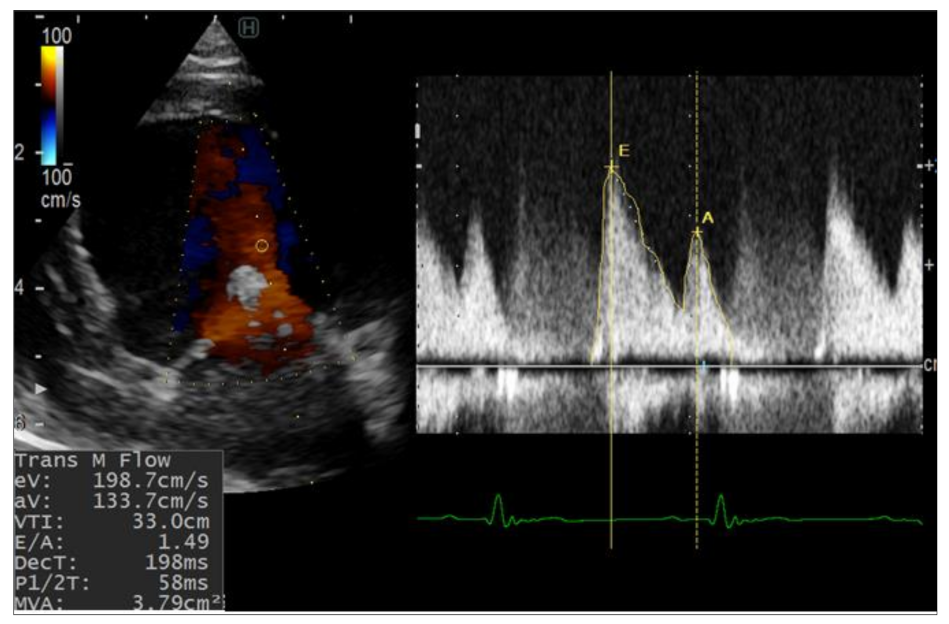
2.2.1. Conventional Echocardiography
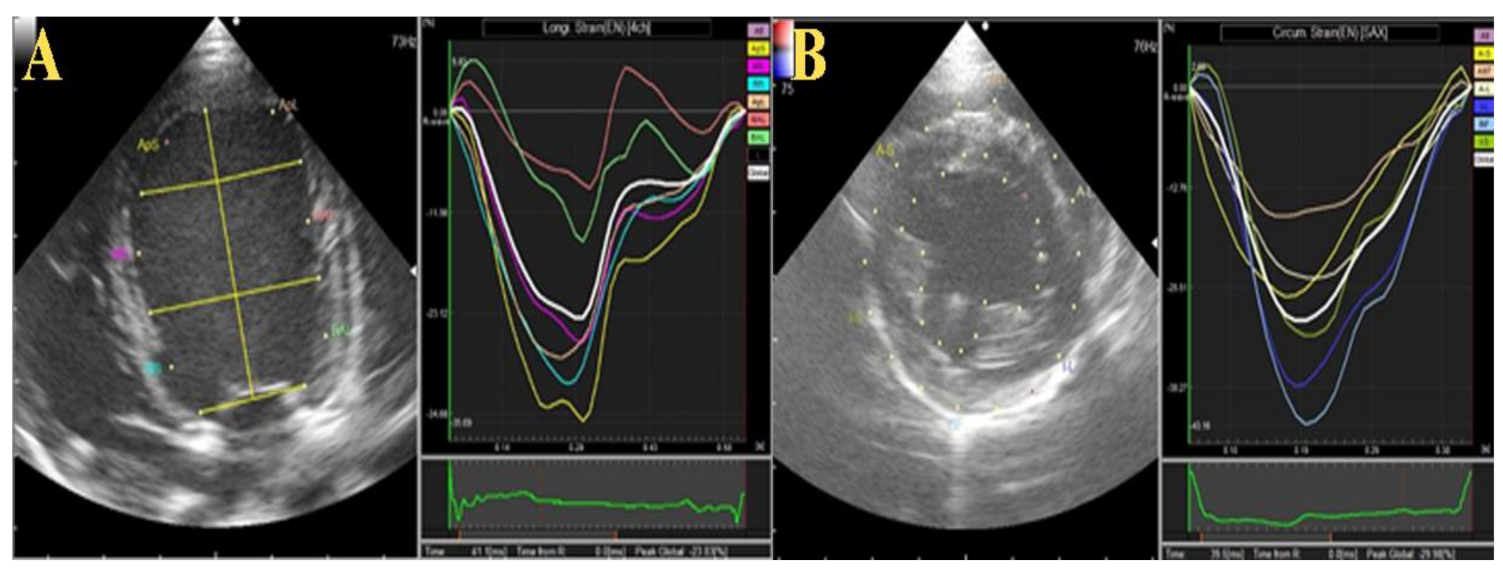
2.2.2. Two-Dimensional Speckle Tracking Echocardiography (2D-STE)
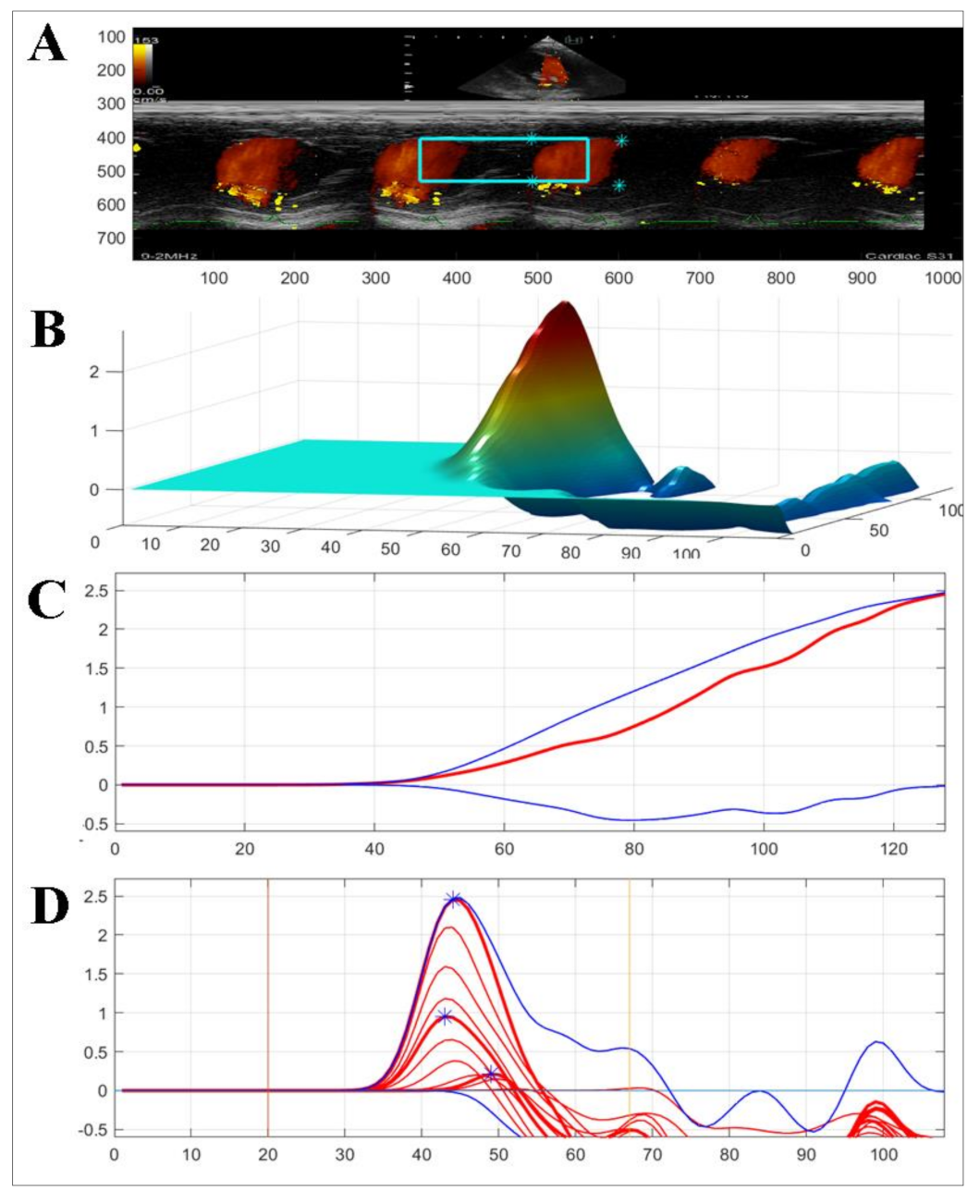
2.2.3. Color M-Mode Echocardiography (CMME)
3. Results
3.1. Echocardiographic Measurement
3.1.1. Conventional Echocardiography
3.1.2. Two-Dimensional Speckle Tracking and Color M-Mode Echocardiography
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Haggstrom, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef]
- Hezzell, M.J.; Boswood, A.; Chang, Y.M.; Moonarmart, W.; Souttar, K.; Elliott, J. The combined prognostic potential of serum high-sensitivity cardiac troponin I and N-terminal pro-B-type natriuretic peptide concentrations in dogs with degenerative mitral valve disease. J. Vet. Intern. Med. 2012, 26, 302–311. [Google Scholar] [CrossRef]
- Martin, M.W.; Stafford Johnson, M.J.; Strehlau, G.; King, J.N. Canine dilated cardiomyopathy: A retrospective study of prognostic findings in 367 clinical cases. J. Small Anim. Pr. 2010, 51, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Mizukoshi, T.; Uechi, M. Long-term outcome in dogs undergoing mitral valve repair with suture annuloplasty and chordae tendinae replacement. J. Small Anim. Pr. 2013, 54, 104–107. [Google Scholar] [CrossRef]
- Orton, E.C.; Hackett, T.B.; Mama, K.; Boon, J.A. Technique and outcome of mitral valve replacement in dogs. J. Am. Vet. Med. Assoc. 2005, 226, 1508–1511. [Google Scholar] [CrossRef]
- Takashima, K.; Soda, A.; Tanaka, R.; Yamane, Y. Short-term performance of mitral valve replacement with porcine bioprosthetic valves in dogs. J. Vet. Med. Sci. 2007, 69, 793–798. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takashima, K.; Soda, A.; Tanaka, R.; Yamane, Y. Long-term clinical evaluation of mitral valve replacement with porcine bioprosthetic valves in dogs. J. Vet. Med. Sci. 2008, 70, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Mamada, K.; Chen, A.; Takamura, K.; Murakami, M.; Uechi, M. Unilateral diaphragmatic paralysis associated with surgical mitral valve repair in dogs. J. Vet. Cardiol. 2020, 29, 33–39. [Google Scholar] [CrossRef]
- Uechi, M. Mitral valve repair in dogs. J. Vet. Cardiol. 2012, 14, 185–192. [Google Scholar] [CrossRef]
- Uechi, M.; Mizukoshi, T.; Mizuno, T.; Mizuno, M.; Harada, K.; Ebisawa, T.; Takeuchi, J.; Sawada, T.; Uchida, S.; Shinoda, A.; et al. Mitral valve repair under cardiopulmonary bypass in small-breed dogs: 48 cases (2006–2009). J. Am. Vet. Med. Assoc. 2012, 240, 1194–1201. [Google Scholar] [CrossRef]
- Zhou, S.; Egorova, N.; Moskowitz, G.; Giustino, G.; Ailawadi, G.; Acker, M.A.; Gillinov, M.; Moskowitz, A.; Gelijns, A. Trends in MitraClip, mitral valve repair, and mitral valve replacement from 2000 to 2016. J. Thorac. Cardiovasc. Surg. 2021, 162, 551–562.e4. [Google Scholar] [CrossRef] [PubMed]
- Uemura, A.; Seijirow, G.; Tanaka, R.; Kocaturk, M.; Yilmaz, Z. Transoesophageal echocardiography-guided hybrid balloon valvuloplasty for severe pulmonic stenosis in small dogs. J. South Afr. Vet. Assoc. 2021, 92, e1–e5. [Google Scholar] [CrossRef]
- Holoshitz, N.; Kenny, D.; Hijazi, Z.M. Hybrid interventional procedures in congenital heart disease. Methodist Debakey Cardiovasc. J. 2014, 10, 93–98. [Google Scholar] [CrossRef]
- Pan, W.; Pan, C.; Jilaihawi, H.; Wei, L.; Tang, Y.; Zhou, D.; Ge, J. A novel user-friendly transcatheter edge-to-edge mitral valve repair device in a porcine model. Catheter. Cardiovasc. Interv. 2019, 93, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Leach, S.B.; Pan, W.; Zheng, F.; Jia, L.; Zhou, X.; Li, J. Preliminary Outcome of a Novel Edge-to-Edge Closure Device to Manage Mitral Regurgitation in Dogs. Front. Vet. Sci. 2020, 7, 597879. [Google Scholar] [CrossRef] [PubMed]
- Hamabe, L.; Mandour, A.S.; Shimada, K.; Uemura, A.; Yilmaz, Z.; Nagaoka, K.; Tanaka, R. Role of Two-Dimensional Speckle-Tracking Echocardiography in Early Detection of Left Ventricular Dysfunction in Dogs. Animals 2021, 11, 2361. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Shiraishi, K.; Mandour, A.S.; Sato, K.; Shimada, K.; Goya, S.; Yoshida, T.; Kitpipatkun, P.; Hamabe, L.; Uemura, A.; et al. The Utility of Intraventricular Pressure Gradient for Early Detection of Chemotherapy-Induced Subclinical Cardiac Dysfunction in Dogs. Animals 2021, 11, 1122. [Google Scholar] [CrossRef]
- Yairo, A.; Mandour, A.S.; Matsuura, K.; Yoshida, T.; Ma, D.; Kitpipatkun, P.; Kato, K.; Cheng, C.J.; El-Husseiny, H.M.; Tanaka, T.; et al. Effect of Loading Changes on the Intraventricular Pressure Measured by Color M-Mode Echocardiography in Rats. Diagnostics 2021, 11, 1403. [Google Scholar] [CrossRef]
- De Madron, E.; Chetboul, V.; Bussadori, C. Clinical Echocardiography of the Dog and Cat-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Rishniw, M.; Caivano, D.; Dickson, D.; Vatne, L.; Harris, J.; Matos, J.N. Two-dimensional echocardiographic left- atrial-to-aortic ratio in healthy adult dogs: A reexamination of reference intervals. J. Vet. Cardiol. 2019, 26, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Schiller, N.B.; Shah, P.M.; Crawford, M.; DeMaria, A.; Devereux, R.; Feigenbaum, H.; Gutgesell, H.; Reichek, N.; Sahn, D.; Schnittger, I.; et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J. Am. Soc. Echocardiogr. 1989, 2, 358–367. [Google Scholar] [CrossRef]
- Nakata, T.M.; Matsuura, N.; Kaji, H.; Shimizu, M.; Fukushima, R.; Machida, N.; Tanaka, R. Sequential radial and circumferential strain and oxidative stress assessment in dogs with tachycardia-induced cardiac dysfunction. Int J. Cardiovasc. Imaging 2016, 32, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Bhamra-Ariza, P.; Muller, D.W.M. The MitraClip Experience and Future Percutaneous Mitral Valve Therapies. Heart Lung Circ. 2014, 23, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Chieh-Jen Cheng, A.M.; Yoshida, T.; Watari, T.; Tanaka, R.; Matsuura, K. Change in renin-angiotensin-aldosterone system during cardiac remodeling after mitral valvuloplasty in dogs. J. Vet. Int. Med. 2021, in press. [Google Scholar]
- Ibrahim, M.F.; David, T.E. Mitral stenosis after mitral valve repair for non-rheumatic mitral regurgitation. Ann. Thorac Surg. 2002, 73, 34–36. [Google Scholar] [CrossRef]
- Borgarelli, M.; Tursi, M.; Rosa, G.L.; Savarino, P.; Galloni, M. Anatomic, histologic, and two-dimensional–echocardiographic evaluation of mitral valve anatomy in dogs. Am. J. Vet. Res. 2011, 72, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Mandour, A.S.; Yoshida, T.; Matsuura, K.; Shimada, K.; Kitpipatkun, P.; Uemura, A.; Ifuku, M.; Takahashi, K.; Tanaka, R. Intraventricular pressure gradients change during the development of left ventricular hypertrophy: Effect of salvianolic acid B and beta-blocker. Ultrasound 2021, 29, 229–240. [Google Scholar] [CrossRef]
- Kadappu, K.K.; Thomas, L. Tissue Doppler imaging in echocardiography: Value and limitations. Heart Lung Circ. 2015, 24, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, E.; Maslow, A.D.; Poppas, A. Primary mitral valve regurgitation: Update and review. Glob. Cardiol. Sci. Pract. 2017, 1, e201703. [Google Scholar]
- Mohamed, A.; Marciniak, M.; Williamson, W.; Huckstep, O.J.; Lapidaire, W.; McCance, A.; Neubauer, S.; Leeson, P.; Lewandowski, A.J. Association of Systolic Blood Pressure Elevation With Disproportionate Left Ventricular Remodeling in Very Preterm-Born Young Adults: The Preterm Heart and Elevated Blood Pressure. JAMA Cardiol. 2021, 6, 821–829. [Google Scholar] [CrossRef]
- Smith, F.W.; Tilley, L.P.; Oyama, M.; Sleeper, M.M. Manual of Canine and Feline Cardiology-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Zhang, K.; Sheu, R.; Zimmerman, N.M.; Alfirevic, A.; Sale, S.; Gillinov, A.M.; Duncan, A.E. A Comparison of Global Longitudinal, Circumferential, and Radial Strain to Predict Outcomes After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Mandoli, G.E.; Sciaccaluga, C.; Mondillo, S. More than 10 years of speckle tracking echocardiography: Still a novel technique or a definite tool for clinical practice? Echocardiography 2019, 36, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Matsumoto, H.; Teshima, T.; Koyama, H. Clinical assessment of systolic myocardial deformations in dogs with chronic mitral valve insufficiency using two-dimensional speckle-tracking echocardiography. J. Vet. Cardiol. 2013, 15, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Iwano, H.; Kamimura, D.; Fox, E.; Hall, M.; Vlachos, P.; Little, W.C. Altered spatial distribution of the diastolic left ventricular pressure difference in heart failure. J. Am. Soc. Echocardiogr. 2015, 28, 597–605.e1. [Google Scholar] [CrossRef] [PubMed]
| Parameters | 1st Dog | 2nd Dog | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre | 1 w | 2 w | 4 w | Pre | 1 w | 2 w | 4 w | |
| HR (bpm) | 142 | 162 | 168 | 108 | 116 | 136 | 157 | 112 |
| IVSd (mm) | 7.7 | 8.9 | 6.6 | 7.4 | 6.6 | 10.2 | 7.2 | 7.2 |
| LVIDd (mm) | 34.5 | 29.8 | 33.9 | 34.7 | 26.9 | 35.4 | 33.4 | 35.0 |
| LVPWd (mm) | 6.2 | 7.2 | 7.0 | 6.4 | 13.9 | 7.9 | 8.1 | 7.0 |
| IVSs (mm) | 10.8 | 12.2 | 12.9 | 11.5 | 11.3 | 11.5 | 11.9 | 12.2 |
| LVIDs (mm) | 19.8 | 15.7 | 16.1 | 17.6 | 20.5 | 23.5 | 14.5 | 16.8 |
| LVPWs (mm) | 10.0 | 11.3 | 12.5 | 11.2 | 11.9 | 11.8 | 14.9 | 13.4 |
| FS % | 42.6 | 47.3 | 52.6 | 49.2 | 23.8 | 34.1 | 56.6 | 52.0 |
| E (cm/s) | 64.8 | 151.5 | 215.4 | 198.7 | 59.9 | 98.6 | 127.2 | 113.9 |
| A (cm/s) | 22.1 | 102.1 | 101.2 | 133.7 | 36.8 | 100.3 | 85.9 | 87.9 |
| S’ (cm/s) | 7.9 | 9.4 | 8.0 | 7.9 | 11.5 | 7.5 | 9.5 | 7.6 |
| é (cm/s) | 10.6 | 8.9 | 7.4 | 6.3 | 10.0 | 8.6 | 8.8 | 9.5 |
| á (cm/s) | 7.5 | 8.1 | 6.6 | 5.8 | 9.5 | 8.4 | 7.3 | 7.9 |
| E/é (cm/s) | 6.1 | 17.0 | 29.1 | 31.5 | 6.0 | 11.5 | 14.5 | 12.1 |
| MR velocity (cm/s) | - | 484.8 | 461.2 | 564.8 | - | 479.8 | 561.9 | 576.5 |
| MR dP/dt max | - | 1340 | 846 | 3217 | - | 5358 | 4021 | 4021 |
| LA/Ao | 1.0 | 1.2 | 1.1 | 1.3 | 1.1 | 1.1 | 1.1 | 1.1 |
| MOAA (mm) | 24.2 | 22.1 | 22.5 | 23.3 | 23.8 | 20.6 | 20.8 | 22.3 |
| MOCA (mm) | 19.9 | 19.0 | 23.0 | 23.5 | 20.7 | 20.6 | 20.4 | 22.5 |
| SAP | 105 | 115 | 112 | 135 | 110 | 112 | 132 | 134 |
| DAP | 85 | 90 | 85 | 91 | 86 | 81 | 93 | 89 |
| MAP | 98 | 107 | 103 | 120 | 102 | 102 | 119 | 119 |
| Variables | 1st Dog | 2nd Dog | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre | 1 w | 2 w | 4 w | Pre | 1 w | 2 w | 4 w | |
| Speckle tracking echocardiography | ||||||||
| GRS % | 16.8 | 18.5 | 13.2 | 19.7 | 10.3 | 12.0 | 12.6 | 13.2 |
| GCS % | −16.0 | −13.4 | −14.8 | −14.4 | −14.8 | −14.4 | −17.3 | −19.7 |
| GCSR % | −2.2 | −1.5 | −1.8 | −2.2 | −2.2 | −2.1 | −2.2 | −2.5 |
| LS % | −17.5 | −14.4 | −15.5 | −15.7 | −23.8 | −21.3 | −23.7 | −26.9 |
| LSR % | 1.4 | 1.4 | 2.4 | 2.8 | 3.0 | 2.2 | 2.2 | 1.9 |
| Color M-mode echocardiography | ||||||||
| Total IVPG (mmHg) | 0.75 | 1.26 | 0.66 | 0.73 | 0.91 | 0.93 | 0.98 | 0.95 |
| Basal IVPG (mmHg) | 0.44 | 0.6 | 0.23 | 0.23 | 0.43 | 0.63 | 0.66 | 0.59 |
| Mid-apical IVPG (mmHg) | 0.31 | 0.66 | 0.43 | 0.5 | 0.48 | 0.30 | 0.31 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, K.; Ma, D.; Mandour, A.S.; Ozai, Y.; Yoshida, T.; Matsuura, K.; Takeuchi, A.; Cheng, C.-J.; El-Husseiny, H.M.; Hendawy, H.; et al. Evaluation of Changes in the Cardiac Function before and after Transcatheter Edge-to-Edge Mitral Valve Repair in Healthy Dogs: Conventional and Novel Echocardiography. Animals 2022, 12, 56. https://doi.org/10.3390/ani12010056
Sasaki K, Ma D, Mandour AS, Ozai Y, Yoshida T, Matsuura K, Takeuchi A, Cheng C-J, El-Husseiny HM, Hendawy H, et al. Evaluation of Changes in the Cardiac Function before and after Transcatheter Edge-to-Edge Mitral Valve Repair in Healthy Dogs: Conventional and Novel Echocardiography. Animals. 2022; 12(1):56. https://doi.org/10.3390/ani12010056
Chicago/Turabian StyleSasaki, Kenta, Danfu Ma, Ahmed S. Mandour, Yusuke Ozai, Tomohiko Yoshida, Katsuhiro Matsuura, Aki Takeuchi, Chieh-Jen Cheng, Hussein M. El-Husseiny, Hanan Hendawy, and et al. 2022. "Evaluation of Changes in the Cardiac Function before and after Transcatheter Edge-to-Edge Mitral Valve Repair in Healthy Dogs: Conventional and Novel Echocardiography" Animals 12, no. 1: 56. https://doi.org/10.3390/ani12010056
APA StyleSasaki, K., Ma, D., Mandour, A. S., Ozai, Y., Yoshida, T., Matsuura, K., Takeuchi, A., Cheng, C.-J., El-Husseiny, H. M., Hendawy, H., Shimada, K., Hamabe, L., Uemura, A., & Tanaka, R. (2022). Evaluation of Changes in the Cardiac Function before and after Transcatheter Edge-to-Edge Mitral Valve Repair in Healthy Dogs: Conventional and Novel Echocardiography. Animals, 12(1), 56. https://doi.org/10.3390/ani12010056










