Yearly Variations in GCM Concentrations in Female Mountain Hares (Lepus timidus) and the Effect of Pregnancy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Noninvasive Method for Measuring States of Pregnancy
2.2. Fieldwork
2.3. Genetic Analyses
2.4. GCM Analyses
2.5. Pregnancy State Analyses
2.6. Statistical Analyses
3. Results
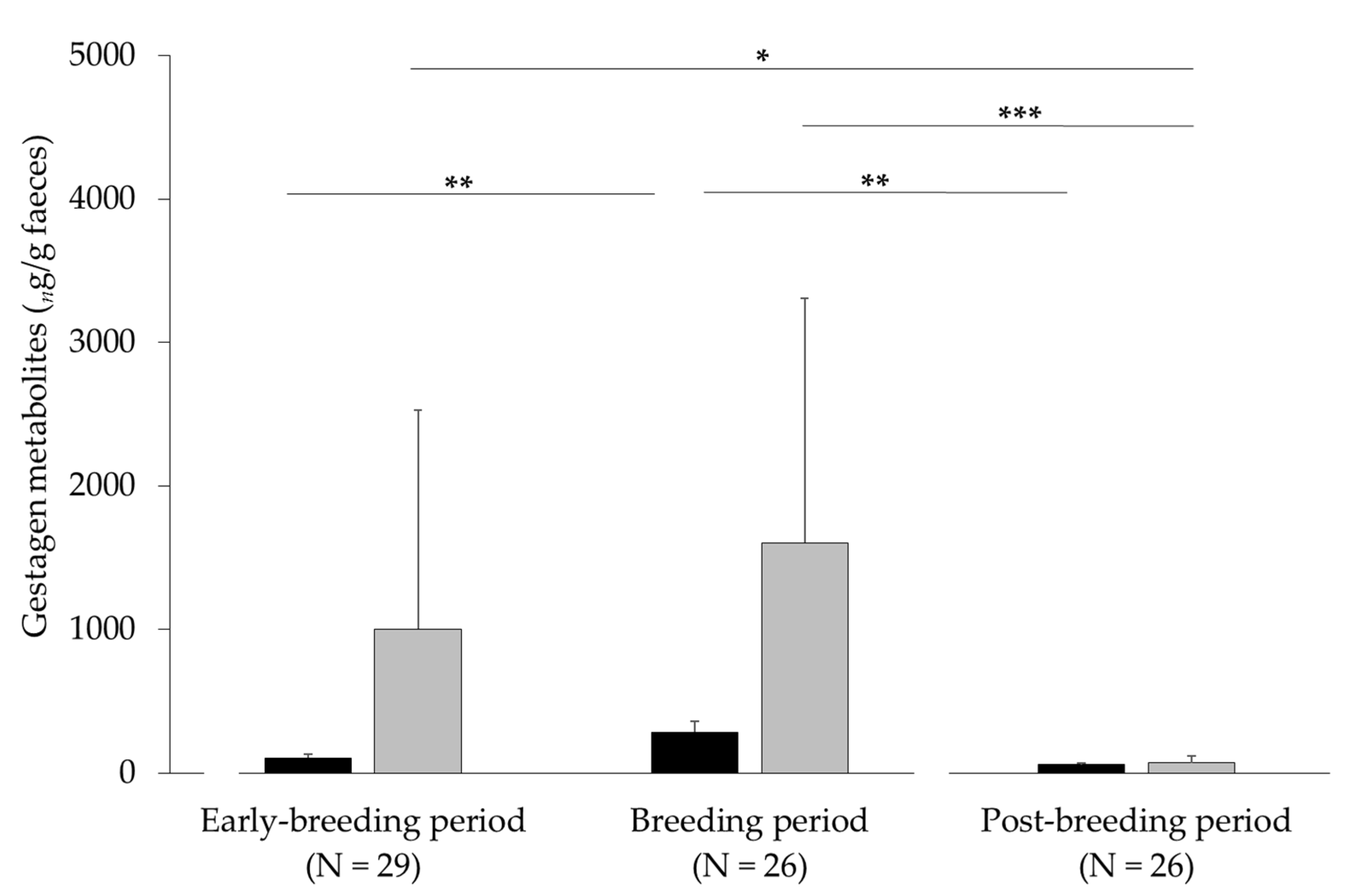
3.1. Noninvasive Method for Measuring States of Pregnancy
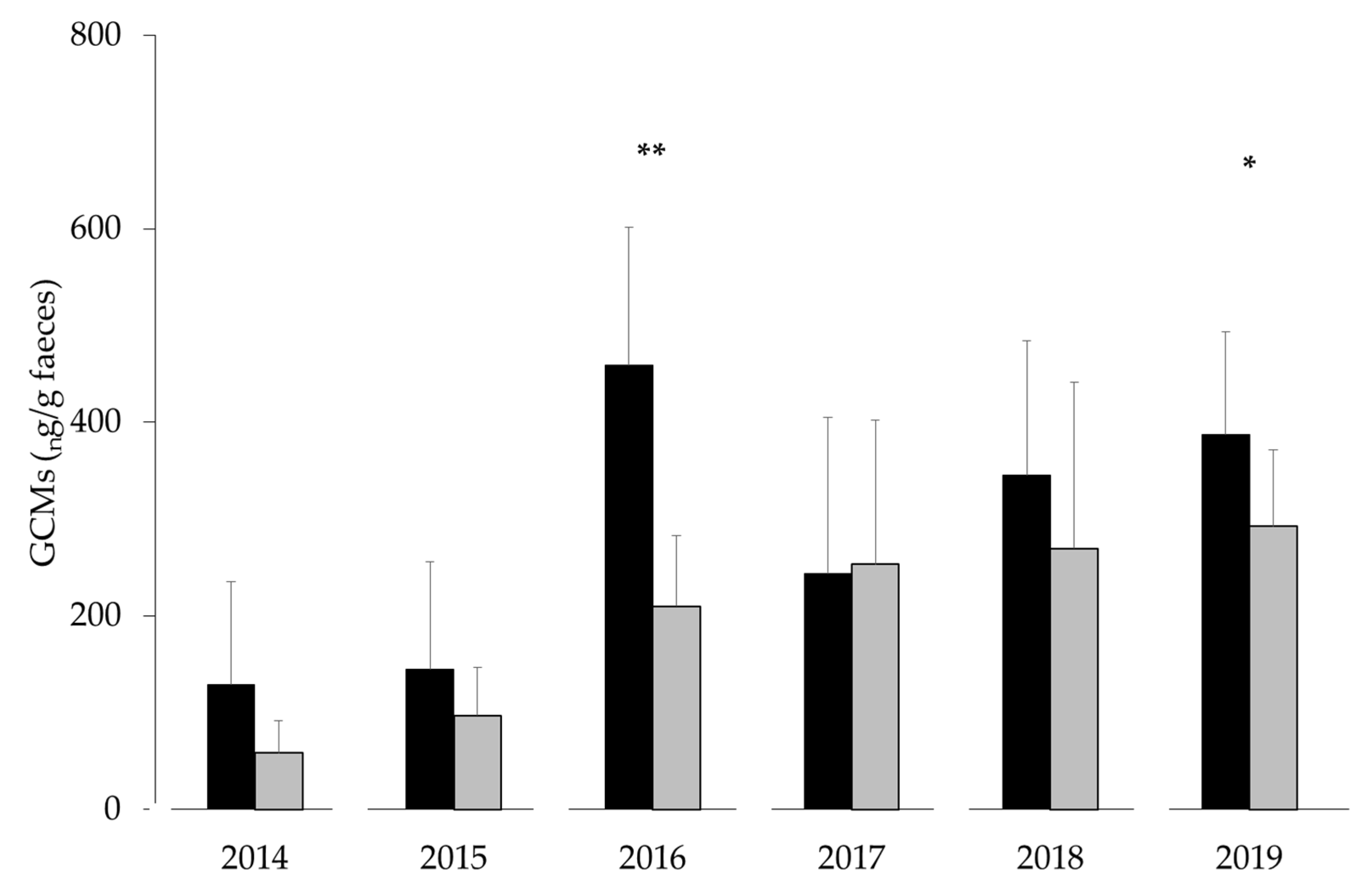
3.2. Fieldwork
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palme, R. Measuring faecal steroids: Guidelines for practical application. Ann. N. Y. Acad. Sci. 2005, 1046, 75–80. [Google Scholar] [CrossRef]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Boonstra, R. Reality as the leading cause of stress: Rethinking the impact of chronic stress in nature. Funct. Ecol. 2012, 27, 11–23. [Google Scholar] [CrossRef]
- Edwards, P.D.; Boonstra, R. Glucocorticoids and CBG during pregnancy in mammals: Diversity, pattern, and function. Gen. Comp. Endocrinol. 2018, 259, 122–130. [Google Scholar] [CrossRef]
- Rehnus, M.; Bollmann, K. Non-invasive genetic population density estimation of mountain hares (Lepus timidus) in the Alps: Systematic or opportunistic sampling? Eur. J. Wildl. Res. 2016, 62, 737–747. [Google Scholar] [CrossRef]
- Rehnus, M.; Hackländer, K.; Palme, R. A non-invasive method for measuring glucocorticoid metabolites (GCM) in Mountain hares (Lepus timidus). Eur. J. Wildl. Res. 2009, 55, 615–620. [Google Scholar] [CrossRef]
- Rehnus, M.; Palme, R. How genetic data improve the interpretation of results of faecal glucocorticoid metabolite measurements in a free-living population. PLoS ONE 2017, 12, e0183718. [Google Scholar] [CrossRef] [Green Version]
- Rehnus, M.; Palme, R.; Filli, F.; Hackländer, K. Seasonal glucocorticoid secretion in mountain hares (Lepus timidus). Mammalia 2010, 74, 347–350. [Google Scholar] [CrossRef]
- Rehnus, M.; Wehrle, M.; Palme, R. Mountain hares Lepus timidus and tourism: Stress events and reactions. J. Appl. Ecol. 2014, 51, 6–12. [Google Scholar] [CrossRef]
- Rehnus, M. Der Schneehase in den Alpen–Ein Überlebenskünstler mit Ungewisser Zukunft; Haupt Verlag: Bern Stuttgart Wien, Austria, 2013; pp. 1–93. [Google Scholar]
- Thulin, C.-G.; Flux, J.E.C. Lepus timidus Linnaeus, 1758—Schneehase. In Handbuch der Säugetiere Europas Band, 3/II: Hasenartige Lagomorpha; Krapp, F., Ed.; Aula Publisher: Wiebelsheim, Germany, 2003; pp. 155–185. [Google Scholar]
- Bisi, F.; Nodari, M.; Oliveira, N.M.D.S.; Masseroni, E.; Preatoni, D.; Wauters, L.; Tosi, G.; Martinoli, A. Space use patterns of mountain hare (Lepus timidus) on the Alps. Eur. J. Wildl. Res. 2010, 57, 305–312. [Google Scholar] [CrossRef]
- Schai-Braun, S.; Gander, J.; Jenny, H.; Hackländer, K. Is reproductive strategy of Alpine mountain hares adapted to different elevations? Mamm. Biol. 2017, 85, 55–59. [Google Scholar] [CrossRef]
- Leach, K.; Kelly, R.; Cameron, A.; Montgomery, W.I.; Reid, N. Expertly validated models and phylogenetically-controlled analysis suggests responses to climate change are related to species traits in the order lagomorpha. PLoS ONE 2015, 10, e0122267. [Google Scholar] [CrossRef]
- Rehnus, M.; Bollmann, K.; Schmatz, D.R.; Hackländer, K.; Braunisch, V. Alpine glacial relict species losing out to climate change: The case of the fragmented mountain hare population (Lepus timidus) in the Alps. Glob. Chang. Biol. 2018, 24, 3236–3253. [Google Scholar] [CrossRef]
- IUCN Protected Areas Category Ia. Available online: https://www.iucn.org/about/work/programmes/gpap_home/gpap_quality/gpap_pacategories/gpap_cat1a/ (accessed on 19 October 2016).
- Lotz, A. (Ed.) Alpine Habitat Diversity—HABITALP—Project Report 2002–2006; Nationalpark Berchtesgaden: Berchtesgaden, Germany, 2006; pp. 1–196. [Google Scholar]
- Haller, H.; Eisenhut, A.; Haller, R. Atlas des Schweizerischen Nationalparks. In Die ersten 100 Jahre; Haupt: Bern, Switzerland, 2013; pp. 1–247. [Google Scholar]
- Sloane, M.A.; Sunnucks, P.; Alpers, D.; Beheregaray, L.B.; Taylor, A.C. Highly reliable genetic identification of individual northern hairy-nosed wombats from single remotely collected hairs: A feasible censusing method. Mol. Ecol. 2000, 9, 1233–1240. [Google Scholar] [CrossRef]
- Kryger, U.; Robinson, T.J.; Bloomer, P. Isolation and characterization of six polymorphic microsatellite loci in South African hares (Lepus saxatilis F. Cuvier, 1823 and Lepus capensis Linnaeus, 1758). Mol. Ecol. Notes 2002, 2, 422–424. [Google Scholar] [CrossRef]
- Mougel, F.; Mounolou, J.C.; Monnerot, M. Nine polymorphic microsatellite loci in the rabbit, Oryctolagus cuniculus. Anim. Genet. 1997, 28, 58–59. [Google Scholar]
- Rico, C.; Rico, I.; Webb, N.; Smith, S.; Bell, D.; Hewitt, G. Four polymorphic microsatellite loci for the European wild rabbit, Oryctolagus cuniculus. Anim. Genet. 2009, 25, 367. [Google Scholar] [CrossRef]
- Surridge, A.K.; Bell, D.J.; Rico, C.; Hewitt, G.M. Polymorphic microsatellite loci in the European rabbit (Oryctolagus cuniculus) are also amplified in other lagomorph species. Anim. Genet. 1997, 28, 302–305. [Google Scholar] [CrossRef]
- R Development Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Schenker, L.; Bollmann, K.; Rehnus, M.; Brodbeck, S.; Gugerli, F. Hare’s affairs: Lessons learnt from a noninvasive genetic monitoring for tracking mountain hare individuals. Ecol. Evol. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Wallner, B.; Huber, S.; Achmann, R. Non-invasive PCR sexing of rabbits (Oryctolagus cuniculus) and hares (Lepus europaeus). Mamm. Biol. 2001, 66, 190–192. [Google Scholar]
- Galpern, P.; Manseau, M.; Fall, A. Patch-based graphs of landscape connectivity: A guide to construction, analysis and application for conservation. Biol. Conserv. 2011, 144, 44–55. [Google Scholar] [CrossRef]
- Möstl, E.; Maggs, J.; Schrötter, G.; Besenfelder, U.; Palme, R. Measurement of cortisol metabolites in faeces of ruminants. Veter-Res. Commun. 2002, 26, 127–139. [Google Scholar] [CrossRef]
- Palme, R.; Touma, C.; Arias, N.; Dominchin, M.F.; Lepschy, M. Steroid extraction: Get the best out of faecal samples. Wien Tierarztl Mon. 2013, 100, 238–246. [Google Scholar]
- Schwarzenberger, F.; Rietschel, W.; Vahala, J.; Holečková, D.; Thomas, P.; Maltzan, J.; Baumgartner, K.; Schaftenaar, W. Fecal progesterone, estrogen, and androgen metabolites for noninvasive monitoring of reproductive function in the female Indian rhinoceros, Rhinoceros unicornis. Gen. Comp. Endocrinol. 2000, 119, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Schwarzenberger, F.; Tomášová, K.; Holečková, D.; Matern, B.; Möstl, E. Measurement of fecal steroids in the black rhinoceros (Diceros bicornis) using group-specific enzyme immunoassays for 20-oxo-pregnanes. Zoo Biol. 1996, 15, 159–171. [Google Scholar] [CrossRef]
- Oli, M.K.; Krebs, C.J.; Kenney, A.J.; Boonstra, R.; Boutin, S.; Hines, J.E. Demography of snowshoe hare population cycles. Ecology 2020, 101, e02969. [Google Scholar] [CrossRef] [PubMed]
- Hewson, R. Variation in reproduction and shooting bags of mountain hares on two moors in north-east Scotland. J. Appl. Ecol. 1970, 7, 243. [Google Scholar] [CrossRef]
- Flux, J.E.C. Life history of the Mountain hare (Lepus timidus scoticus) in north-east Scotland. J. Zoöl. 1970, 161, 75–123. [Google Scholar] [CrossRef]
- Rehnus, M.; Bollmann, K. Quantification of sex-related diet use by free-ranging mountain hares (Lepus timidus). Hystrix 2020, 31, 80–82. [Google Scholar]
- Angerbjörn, A. Reproduction of Mountain hares (Lepus timidus) in relation to density and physical condition. J. Zoöl. 1986, 208, 559–568. [Google Scholar] [CrossRef]
- Clark, W.R. Reproduction, Survival and Density of Snowshoe Hares in Northeastern Utah; Utah State University: Logan, UT, USA, 1973. [Google Scholar]
- Conaway, C.H.; Wight, H.M. Onset of reproductive season and first pregnancy of the season in cottontails. J. Wildl. Manag. 1962, 26, 278. [Google Scholar] [CrossRef]
- Stefan, C.I.; Krebs, C.J. Reproductive changes in a cyclic population of snowshoe hares. Can. J. Zool. 2001, 79, 2101–2108. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1–1132. [Google Scholar]
- Matiu, M.; Crespi, A.; Bertoldi, G.; Carmagnola, C.M.; Marty, C.; Morin, S.; Schöner, W.; Berro, D.C.; Chiogna, G.; De Gregorio, L.; et al. Observed snow depth trends in the European Alps: 1971 to 2019. Cryosphere 2021, 15, 1343–1382. [Google Scholar] [CrossRef]
- Rehnus, M.; Bollmann, K. Mountain hares (Lepus timidus) follow the green-up wave in the pursuit of high-quality food. Wildl. Biol. 2020, 3, 1–5. [Google Scholar] [CrossRef]
- Cavigelli, S.; Dubovick, T.; Levash, W.; Jolly, A.; Pitts, A. Female dominance status and fecal corticoids in a cooperative breeder with low reproductive skew: Ring-tailed lemurs (Lemur catta). Horm. Behav. 2003, 43, 166–179. [Google Scholar] [CrossRef]
- Saltzman, W.; Schultz-Darken, N.J.; Wegner, F.H.; Wittwer, D.J.; Abbott, D.H. Suppression of cortisol levels in subordinate female marmosets: Reproductive and social contributions. Horm. Behav. 1998, 33, 58–74. [Google Scholar] [CrossRef] [Green Version]
- Boudreau, M.R.; Seguin, J.L.; Boonstra, R.; Palme, R.; Boutin, S.; Krebs, C.J.; Murray, D.L. Experimental increase in predation risk causes a cascading stress response in free-ranging snowshoe hares. Oecologia 2019, 191, 311–323. [Google Scholar] [CrossRef]
- Lavergne, S.; Smith, K.; Kenney, A.; Krebs, C.; Palme, R.; Boonstra, R. Physiology and behaviour of juvenile snowshoe hares at the start of the 10-year cycle. Anim. Behav. 2019, 157, 141–152. [Google Scholar] [CrossRef]
- Lavergne, S.G.; Krebs, C.J.; Kenney, A.J.; Boutin, S.; Murray, D.; Palme, R.; Boonstra, R. The impact of variable predation risk on stress in snowshoe hares over the cycle in North America’s boreal forest: Adjusting to change. Oecologia 2021, 1–18. [Google Scholar] [CrossRef]
| Parameters | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Number of females with 20a-OHP levels ≥ 3000 ng/g | 0 | 1 | 4 | 2 | 0 | 1 |
| Number of females with 20α-OHP levels 2000–2999 ng/g | 0 | 0 | 1 | 0 | 0 | 0 |
| Number of females with 20α-OHP levels 1000–1999 ng/g | 1 | 0 | 0 | 0 | 1 | 0 |
| Number of females with 20α-OHP levels < 1000 ng/g | 11 | 3 | 2 | 7 | 4 | 7 |
| Snow height (mm) in January | 132 | 48 | 35 | 50 | 130 | 84 |
| Snow height (mm) in February | 136 | 66 | 70 | 62 | 114 | 89 |
| Snow height (mm) in March | 93 | 60 | 61 | 21 | 124 | 88 |
| Number of days with snowfall in January | 13 | 13 | 5 | 15 | 11 | 5 |
| Number of days with snowfall in February | 11 | 14 | 8 | 5 | 6 | 9 |
| Number of days with snowfall in March | 9 | 10 | 5 | 12 | 9 | 11 |
| Average daily temperature (°C) in January | –7.2 | –7.9 | –8.5 | –11.9 | –6.7 | –11.3 |
| Average daily temperature (°C) in February | –6.0 | –8.9 | –5.1 | –5.2 | –11.3 | –6.5 |
| Average daily temperature (°C) in March | –2.8 | –2.8 | –5.1 | –0.8 | –4.6 | –3.6 |
| Number of days with air frost in January | 31 | 31 | 31 | 31 | 31 | 31 |
| Number of days with air frost in February | 28 | 28 | 29 | 28 | 28 | 28 |
| Number of days with air frost in March | 31 | 31 | 31 | 29 | 31 | 31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehnus, M.; Palme, R. Yearly Variations in GCM Concentrations in Female Mountain Hares (Lepus timidus) and the Effect of Pregnancy. Animals 2021, 11, 2710. https://doi.org/10.3390/ani11092710
Rehnus M, Palme R. Yearly Variations in GCM Concentrations in Female Mountain Hares (Lepus timidus) and the Effect of Pregnancy. Animals. 2021; 11(9):2710. https://doi.org/10.3390/ani11092710
Chicago/Turabian StyleRehnus, Maik, and Rupert Palme. 2021. "Yearly Variations in GCM Concentrations in Female Mountain Hares (Lepus timidus) and the Effect of Pregnancy" Animals 11, no. 9: 2710. https://doi.org/10.3390/ani11092710
APA StyleRehnus, M., & Palme, R. (2021). Yearly Variations in GCM Concentrations in Female Mountain Hares (Lepus timidus) and the Effect of Pregnancy. Animals, 11(9), 2710. https://doi.org/10.3390/ani11092710






