A Review of Environmental Risk Factors for African Swine Fever in European Wild Boar
Abstract
:Simple Summary
Abstract
1. Introduction
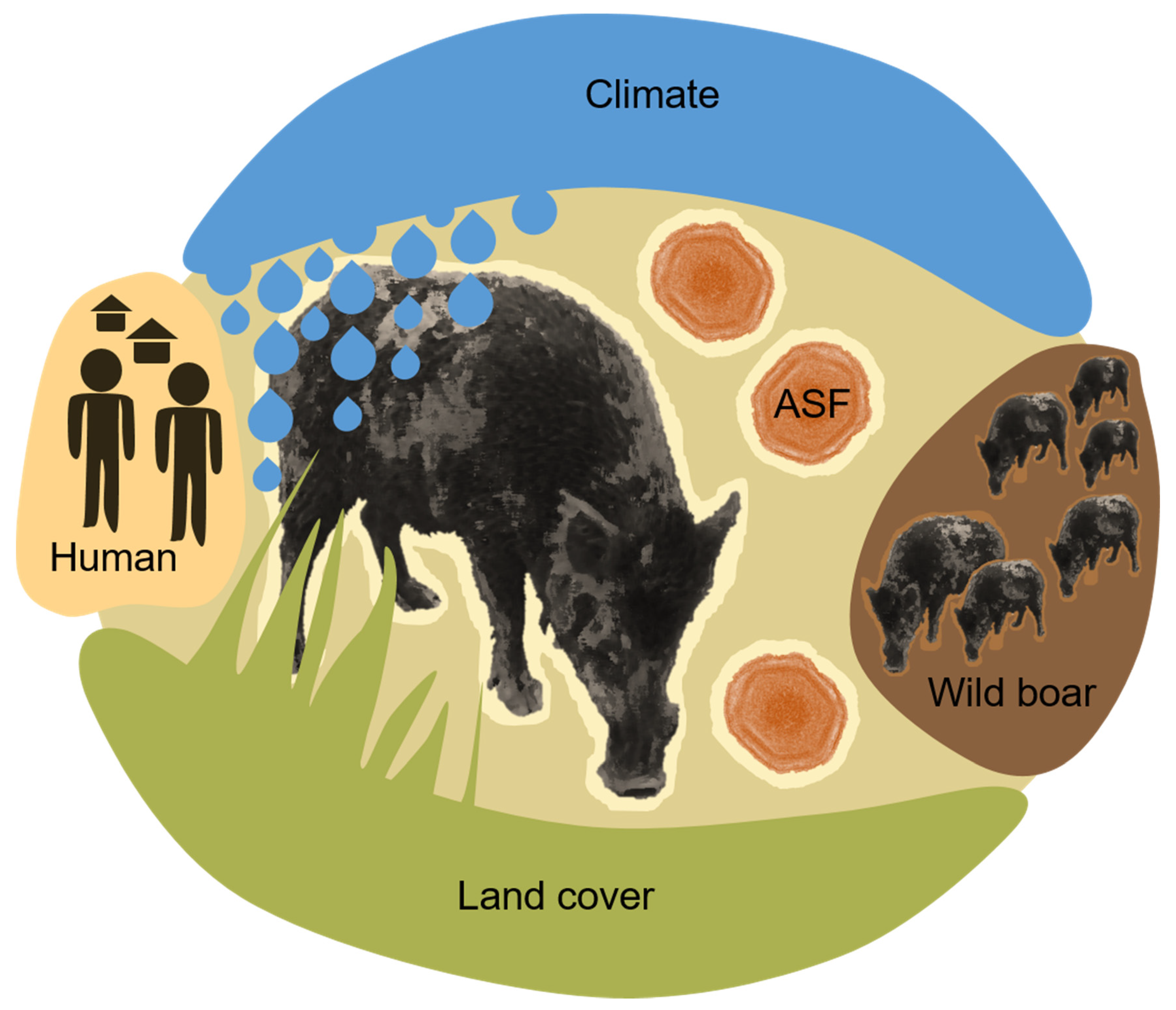
2. Environmental ASF Risk Factors
- Climate factors, such as temperature, precipitation, humidity, wind, cloud coverage, ultra-violet light conditions, climate changes or season;
- Land cover and geomorphology factors, such as vegetation-type, coverage, distribution pattern, altitude, soil type and water availability or type;
- Human activity factors, such as human population density, traffic, pollution, artificial structures, housing, roads, farm density, livestock density as well as human outdoor activity types and levels;
- Wild boar host-related factors, such as wild boar presence in terms of density, distribution or measurable effects as a result of their activity (e.g., crop damage);
- ASF disease factors, such as disease presence, disease type (e.g., a high proportion of ASFV seropositive wild boar present), distribution, distance in space and time from susceptible animals and the viral load, infectious pressure or contamination level.
3. Climate
3.1. Seasonality
3.2. Precipitation and Temperature
4. Land Cover
4.1. Forest
4.2. Water and Meadows
4.3. Wild Boar Habitat Quality
5. Human Activity
5.1. Human Presence and Environmental Impacts
5.2. Hunting
5.3. Farming
6. Wild Boar
7. ASF Disease
8. Discussion
9. Conclusions
9.1. Timing
9.2. Spatial Targeting
9.3. Dynamic Disease Control
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African swine fever epidemiology and control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef] [Green Version]
- Carlson, J.; Fischer, M.; Zani, L.; Eschbaumer, M.; Fuchs, W.; Mettenleiter, T.; Beer, M.; Blome, S. Stability of African swine fever virus in soil and options to mitigate the potential transmission risk. Pathogens 2020, 9, 977. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Zmudzki, J.; Wozniakowski, G. African Swine fever virus—Persistence in different environmental conditions and the possibility of its indirect transmission. J. Vet. Res. 2019, 63, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galindo, I.; Alonso, C. African swine fever virus: A review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef] [PubMed]
- Petrini, S.; Feliziani, F.; Casciari, C.; Giammarioli, M.; Torresi, C.; De Mia, G.M. Survival of African swine fever virus (ASFV) in various traditional Italian dry-cured meat products. Prev. Vet. Med. 2019, 162, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Huhr, J.; Blome, S.; Conraths, F.J.; Probst, C. Stability of African swine fever virus in carcasses of domestic pigs and wild boar experimentally infected with the ASFV “Estonia 2014” isolate. Viruses 2020, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Zani, L.; Masiulis, M.; Busauskas, P.; Dietze, K.; Pridotkas, G.; Globig, A.; Blome, S.; Mettenleiter, T.; Depner, K.; Karveliene, B. African swine fever virus survival in buried wild boar carcasses. Transbound. Emerg. Dis. 2020, 67, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Epidemiological analyses of African swine fever in the European Union (November 2017 until November 2018). EFSA J. 2018, 16, 305494. [Google Scholar] [CrossRef]
- Sanchez-Cordon, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef]
- Dixon, L.K.; Sun, H.; Roberts, H. African swine fever. Antivir. Res. 2019, 165, 34–41. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Terrestrial Animal Health Code. Volume I: General Provisions; Office International des Épizooties: Paris, France, 2019; Volume I. [Google Scholar]
- Penrith, M.L.; Bastos, A.; Chenais, E. With or without a vaccine—A review of complementary and alternative approaches to managing African swine fever in resource-constrained smallholder settings. Vaccines 2021, 9, 116. [Google Scholar] [CrossRef]
- Borca, M.V.; Rai, A.; Ramirez-Medina, E.; Silva, E.; Velazquez-Salinas, L.; Vuono, E.; Pruitt, S.; Espinoza, N.; Gladue, D.P. A cell culture-adapted vaccine virus against the current pandemic African swine fever virus strain. J. Virol. 2021, 95. [Google Scholar] [CrossRef]
- Bellini, S.; Casadei, G.; De Lorenzi, G.; Tamba, M. A Review of Risk Factors of African Swine Fever Incursion in Pig Farming within the European Union Scenario. Pathogens 2021, 10, 84. [Google Scholar] [CrossRef]
- Cukor, J.; Linda, R.; Vaclavek, P.; Satran, P.; Mahlerova, K.; Vacek, Z.; Kunca, T.; Havranek, F. Wild boar deathbed choice in relation to ASF: Are there any differences between positive and negative carcasses? Prev. Vet. Med. 2020, 177, 104943. [Google Scholar] [CrossRef]
- Linden, A.; Licoppe, A.; Volpe, R.; Paternostre, J.; Lesenfants, C.; Cassart, D.; Garigliany, M.; Tignon, M.; van den Berg, T.; Desmecht, D.; et al. Summer 2018: African swine fever virus hits north-western Europe. Transbound. Emerg. Dis. 2019, 66, 54–55. [Google Scholar] [CrossRef] [PubMed]
- OIE. WAHID Database. Disease Information. Available online: http://www.oie.int/ (accessed on 1 June 2021).
- EFSA. Epidemiological analyses of African swine fever in the Baltic States and Poland. EFSA J. 2017, 15, e05068. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.A.; Podgorski, T.; Simons, R.R.L.; Ip, S.; Gale, P.; Kelly, L.A.; Snary, E.L. Predicting spread and effective control measures for African swine fever-Should we blame the boars? Transbound. Emerg. Dis. 2020, 68, 397–416. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, T.; Smietanka, K. Do wild boar movements drive the spread of African swine Fever? Transbound. Emerg. Dis. 2018, 65, 1588–1596. [Google Scholar] [CrossRef]
- EFSA. Epidemiological analyses of African swine fever in the European Union (November 2018 to October 2019). EFSA J. 2020, 18, e05996. [Google Scholar] [CrossRef] [Green Version]
- Mazur-Panasiuk, N.; Walczak, M.; Juszkiewicz, M.; Wozniakowski, G. The spillover of African swine fever in Western Poland revealed its estimated origin on the basis of O174L, K145R, MGF 505-5R and IGR I73R/I329L genomic sequences. Viruses 2020, 12, 1094. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Forth, J.H.; Probst, C.; Staubach, C.; Hlinak, A.; Rudovsky, A.; Holland, D.; Schlieben, P.; Goldner, M.; Schatz, J.; et al. Joining the club: First detection of African swine fever in wild boar in Germany. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef]
- Lange, M.; Thulke, H.H. Elucidating transmission parameters of African swine fever through wild boar carcasses by combining spatio-temporal notification data and agent-based modelling. Stoch. Env. Res. Risk Assess. 2017, 31, 379–391. [Google Scholar] [CrossRef]
- Probst, C.; Globig, A.; Knoll, B.; Conraths, F.J.; Depner, K. Behaviour of free ranging wild boar towards their dead fellows: Potential implications for the transmission of African swine fever. R. Soc. Open Sci. 2017, 4, 170054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morelle, K.; Bubnicki, J.; Churski, M.; Gryz, J.; Podgorski, T.; Kuijper, D.P.J. Disease-induced mortality outweighs hunting in causing wild boar population crash after African swine fever outbreak. Front. Vet. Sci. 2020, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Chenais, E.; Stahl, K.; Guberti, V.; Depner, K. Identification of wild boar-habitat epidemiologic cycle in African swine fever epizootic. Emerg. Infect. Dis. 2018, 24, 810–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenais, E.; Depner, K.; Guberti, V.; Dietze, K.; Viltrop, A.; Stahl, K. Epidemiological considerations on African swine fever in Europe 2014–2018. Porc. Health Manag. 2019, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.M.; Golnar, A.J.; Abdo, Z.; Podgorski, T. Ecological drivers of African swine fever virus persistence in wild boar populations: Insight for control. Ecol. Evol. 2020, 10, 2846–2859. [Google Scholar] [CrossRef] [Green Version]
- Rothman, K.J. Causes. Am. J. Epidemiol. 1976, 104, 587–592. [Google Scholar] [CrossRef]
- Thrusfield, M. Veterinary Epidemiology; John Wiley & Sons Ltd.: Edinburgh, Scotland, UK, 2018; Volume 4. [Google Scholar]
- Probst, C.; Gethmann, J.; Amendt, J.; Lutz, L.; Teifke, J.P.; Conraths, F.J. Estimating the postmortem interval of wild boar carcasses. Vet. Sci. 2020, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, S.I.; Bouhsira, E.; De Regge, N.; Fite, J.; Etore, F.; Garigliany, M.M.; Jori, F.; Lempereur, L.; Le Potier, M.F.; Quillery, E.; et al. Putative Role of Arthropod Vectors in African Swine Fever Virus Transmission in Relation to Their Bio-Ecological Properties. Viruses-Basel 2020, 12, 778. [Google Scholar] [CrossRef]
- Smietanka, K.; Wozniakowski, G.; Kozak, E.; Niemczuk, K.; Fraczyk, M.; Bocian, L.; Kowalczyk, A.; Pejsak, Z. African swine fever epidemic, Poland, 2014–2015. Emerg. Infect. Dis. 2016, 22, 1201–1207. [Google Scholar] [CrossRef] [Green Version]
- Podgorski, T.; Borowik, T.; Lyjak, M.; Wozniakowski, G. Spatial epidemiology of African swine fever: Host, landscape and anthropogenic drivers of disease occurrence in wild boar. Prev. Vet. Med. 2020, 177, 104691. [Google Scholar] [CrossRef] [PubMed]
- Pautienius, A.; Grigas, J.; Pileviciene, S.; Zagrabskaite, R.; Buitkuviene, J.; Pridotkas, G.; Stankevicius, R.; Streimikyte, Z.; Salomskas, A.; Zienius, D.; et al. Prevalence and spatiotemporal distribution of African swine fever in Lithuania, 2014–2017. Virol. J. 2018, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Maciulskis, P.; Masiulis, M.; Pridotkas, G.; Buitkuviene, J.; Jurgelevicius, V.; Jaceviciene, I.; Zagrabskaite, R.; Zani, L.; Pileviciene, S. The African swine fever epidemic in wild boar (Sus scrofa) in Lithuania (2014–2018). Vet. Sci. 2020, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Epidemiological analyses on African swine fever in the Baltic countries and Poland. EFSA J. 2017, 15, e04732. [Google Scholar] [CrossRef]
- Liang, R.; Lu, Y.; Qu, X.; Su, Q.; Li, C.; Xia, S.; Liu, Y.; Zhang, Q.; Cao, X.; Chen, Q.; et al. Prediction for global African swine fever outbreaks based on a combination of random forest algorithms and meteorological data. Transbound. Emerg. Dis. 2020, 67, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Iglesias, I.; Munoz, M.J.; de la Torre, A. A Cartographic tool for managing african swine fever in Eurasia: Mapping wild boar distribution based on the quality of available habitats. Transbound. Emerg. Dis. 2017, 64, 1720–1733. [Google Scholar] [CrossRef]
- Alexander, N.S.; Massei, G.; Wint, W. The European distribution of Sus Scrofa. Model outputs from the project described within the poster—where are all the boars? An attempt to gain a continental perspective. Open Health Data 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Ballari, S.A.; Barrios-Garcia, M.N. A review of wild boar Sus scrofa diet and factors affecting food selection in native and introduced ranges. Mammal. Rev. 2014, 44, 124–134. [Google Scholar] [CrossRef]
- Loi, F.; Laddomada, A.; Coccollone, A.; Marrocu, E.; Piseddu, T.; Masala, G.; Bandino, E.; Cappai, S.; Rolesu, S. Socio-economic factors as indicators for various animal diseases in Sardinia. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Podgorski, T.; Bas, G.; Jedrzejewska, B.; Sonnichsen, L.; Sniezko, S.; Jedrzejewski, W.; Okarma, H. Spatiotemporal behavioral plasticity of wild boar (Sus scrofa) under contrasting conditions of human pressure: Primeval Forest and metropolitan area. J. Mammal. 2013, 94, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Schulz, K.; Conraths, F.J.; Staubach, C.; Viltrop, A.; Olsevskis, E.; Nurmoja, I.; Lamberga, K.; Sauter-Louis, C. To sample or not to sample? Detection of African swine fever in wild boar killed in road traffic accidents. Transbound. Emerg. Dis. 2020, 67, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Nurmoja, I.; Schulz, K.; Staubach, C.; Sauter-Louis, C.; Depner, K.; Conraths, F.J.; Viltrop, A. Development of African swine fever epidemic among wild boar in Estonia—Two different areas in the epidemiological focus. Sci. Rep. 2017, 7, 12562. [Google Scholar] [CrossRef] [Green Version]
- Ebert, C.; Knauer, F.; Spielberger, B.; Thiele, B.; Hohmann, U. Estimating wild boar Sus scrofa population size using faecal DNA and capture-recapture modelling. Wildl. Biol. 2012, 18, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.S.; Vergne, T.; Pak, S.I.; Kim, E. Modelling the Spatial Distribution of ASF-Positive wild boar carcasses in South Korea using 2019–2020 national surveillance data. Animals 2021, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sanchez, R.; Astigarraga, A.; Oleaga-Perez, A.; Encinas-Grandes, A. Relationship between the Persistence of African Swine Fever and the Distribution of Ornithodoros-Erraticus in the Province of Salamanca, Spain. Vet. Rec. 1994, 135, 207–209. [Google Scholar] [CrossRef]
- Boklund, A.; Dhollander, S.; Chesnoiu Vasile, T.; Abrahantes, J.C.; Botner, A.; Gogin, A.; Gonzalez Villeta, L.C.; Gortazar, C.; More, S.J.; Papanikolaou, A.; et al. Risk factors for African swine fever incursion in Romanian domestic farms during 2019. Sci. Rep. 2020, 10, 10215. [Google Scholar] [CrossRef] [PubMed]
- Costard, S.; Mur, L.; Lubroth, J.; Sanchez-Vizcaino, J.M.; Pfeiffer, D.U. Epidemiology of African swine fever virus. Virus Res. 2013, 173, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.; Staubach, C.; Blome, S.; Viltrop, A.; Nurmoja, I.; Conraths, F.J.; Sauter-Louis, C. Analysis of Estonian surveillance in wild boar suggests a decline in the incidence of African swine fever. Sci.Rep. 2019, 9, 8490. [Google Scholar] [CrossRef]
- Dellicour, S.; Desmecht, D.; Paternostre, J.; Malengreaux, C.; Licoppe, A.; Gilbert, M.; Linden, A. Unravelling the dispersal dynamics and ecological drivers of the African swine fever outbreak in Belgium. J. Appl. Ecol. 2020, 57, 1619–1629. [Google Scholar] [CrossRef]
- Dellicour, S.; Rose, R.; Pybus, O.G. Explaining the geographic spread of emerging epidemics: A framework for comparing viral phylogenies and environmental landscape data. BMC Bioinform. 2016, 17, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morelle, K.; Jezek, M.; Licoppe, A.; Podgorski, T. Deathbed choice by ASF-infected wild boar can help find carcasses. Transbound. Emerg. Dis. 2019, 66, 1821–1826. [Google Scholar] [CrossRef]
- Guinat, C.; Vergne, T.; Jurado-Diaz, C.; Sanchez-Vizcaino, J.M.; Dixon, L.; Pfeiffer, D.U. Effectiveness and practicality of control strategies for African swine fever: What do we really know? Vet. Rec. 2017, 180, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
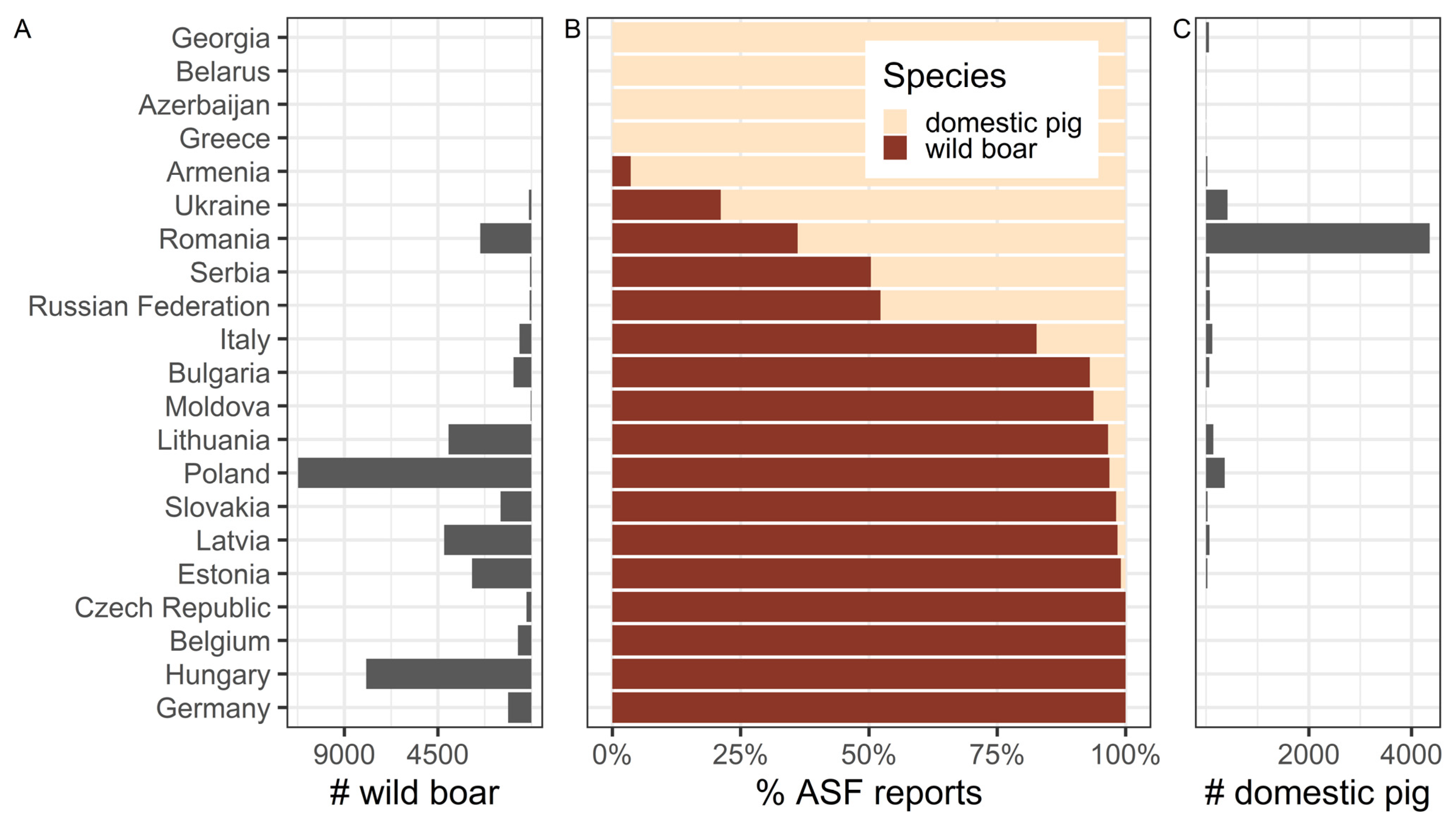
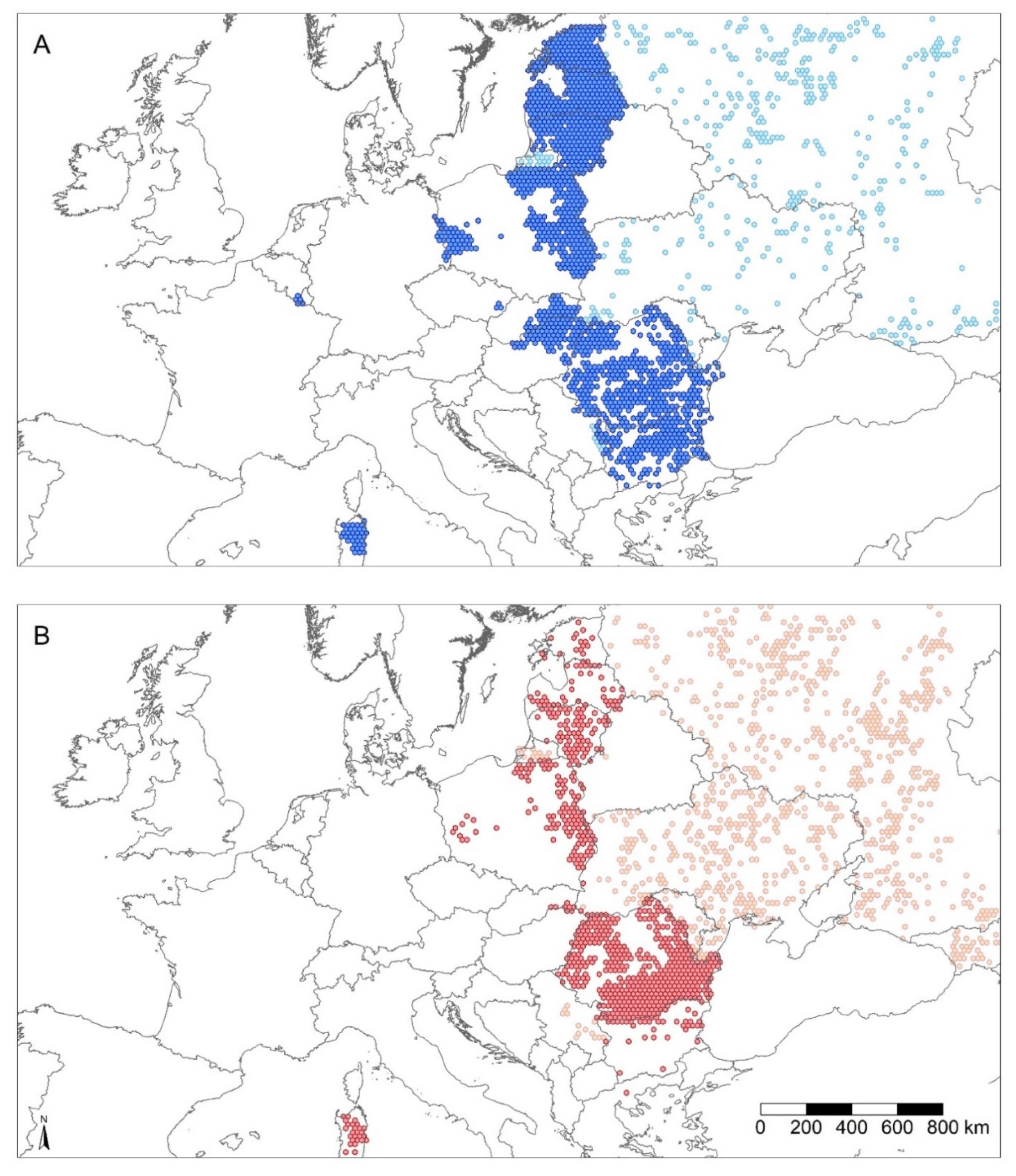
| Risk Factor | Summary of the Possible Effect | Reference |
|---|---|---|
| Seasonality | Seasonal disease patterns of disease occurrence observed | Podgorski et al., 2018 [21]; Smietanka et al., 2016 [35]; Podgorski et al., 2020 [36]; Pautienius et al., 2018 [37]; Maciulskis et al., 2020 [38]; EFSA, 2018 [9]; EFSA (Abrahantes et al.), 2017 [39]; EFSA, 2020 [22]; EFSA (Depner et al.), 2017 [19] |
| Precipitation | Precipitation during extreme dry, wet or cold periods influences disease occurrence | Liang et al., 2020 [40] |
| Temperature | Temperatures, particularly during extremely cold periods, may influence disease occurrence and spatial association with water sources | Liang et al., 2020 [40]; EFSA, 2018 [9]; EFSA, 2020 [22]; Cukor et al., 2020 [16] |
| Forest | More forest, proximity to forest, younger tree ages of broad-leafed forest associated with disease | EFSA (Abrahantes et al.), 2017 [39]; Loi et al., 2019 [44]; Podgorski et al., 2020 [36]; Cukor et al., 2020 [16] |
| Water | Presence and proximity to surface water associated with disease | EFSA (Abrahantes et al.), 2017 [39]; Cukor et al., 2020 [16] |
| Meadows | Growth height of meadow vegetation between 1 and 1.2 m associated with disease detection | Cukor et al., 2020 [16] |
| Wild boar habitat quality | High wild boar habitat suitability likely associated with disease | EFSA, 2018 [9]; EFSA (Depner et al.), 2017 [19]; EFSA, 2020 [22] |
| Human population density | Greater human population density may be associated with disease | EFSA (Abrahantes et al.), 2017 [39] |
| Human settlements | Human settlements unlikely associated with disease | EFSA (Abrahantes et al.), 2017 [39]; EFSA (Depner et al.), 2017 [19]; EFSA, 2018 [9]; EFSA, 2020 [22]; Podgorski et al., 2020 [36]; Cukor et al., 2020 [16] |
| Roads | More roads may increase detection of disease, but roads may also have a dispersing effect on disease occurrence | EFSA (Abrahantes et al.), 2017 [39]; Podgorski et al., 2020 [36]; EFSA (Depner et al.), 2017 [19]; EFSA, 2018 [9]; EFSA, 2020 [22]; Cukor et al., 2020 [16]; Schulz et al., 2020 [46] |
| Renewable energy production | More energy production from renewable resources may reduce disease | Loi et al., 2019 [44] |
| Waste production | More waste production likely associated with disease | Loi et al., 2019 [44] |
| Hunting | Hunting was not found to associate with disease | EFSA, 2018 [9]; EFSA, 2020 [22] |
| Farming | More domestic pigs and pig farms, particularly smaller pig farms, associated with disease occurrence | EFSA (Abrahantes et al.), 2017 [39]; EFSA (Depner et al.), 2017 [19]; EFSA, 2018 [9]; EFSA, 2020 [22] |
| Wild boar presence | High wild boar density associated with disease | EFSA (Abrahantes et al.), 2017 [39]; Pautienius et al., 2018 [37]; Nurmoja et al., 2017 [47]; EFSA (Depner et al.), 2017 [19]; EFSA, 2018 [9]; EFSA, 2020 [22]; Smietanka et al., 2016 [35]; Podgorski et al., 2020 [36] |
| ASF nearness in wild boar | Proximity to ASF in wild boar associated with disease | EFSA, 2018 [9]; EFSA, 2020 [22]; Podgorski et al., 2018 [21]; Podgorski et al., 2020 [36]; Lim et al., 2021 [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergmann, H.; Schulz, K.; Conraths, F.J.; Sauter-Louis, C. A Review of Environmental Risk Factors for African Swine Fever in European Wild Boar. Animals 2021, 11, 2692. https://doi.org/10.3390/ani11092692
Bergmann H, Schulz K, Conraths FJ, Sauter-Louis C. A Review of Environmental Risk Factors for African Swine Fever in European Wild Boar. Animals. 2021; 11(9):2692. https://doi.org/10.3390/ani11092692
Chicago/Turabian StyleBergmann, Hannes, Katja Schulz, Franz J. Conraths, and Carola Sauter-Louis. 2021. "A Review of Environmental Risk Factors for African Swine Fever in European Wild Boar" Animals 11, no. 9: 2692. https://doi.org/10.3390/ani11092692
APA StyleBergmann, H., Schulz, K., Conraths, F. J., & Sauter-Louis, C. (2021). A Review of Environmental Risk Factors for African Swine Fever in European Wild Boar. Animals, 11(9), 2692. https://doi.org/10.3390/ani11092692






