Social Behaviour in Zoo Bachelor Groups: A Case Study of Related South American Fur Seals
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Enclosure
2.2. Data Collection
2.3. Data Analysis
2.3.1. Frequency of Social Interactions
2.3.2. Assessment of Group-Level Interactions Using Social Matrices
3. Results
3.1. Frequency of Social Interactions
3.1.1. Interactions Given by Individual Seals
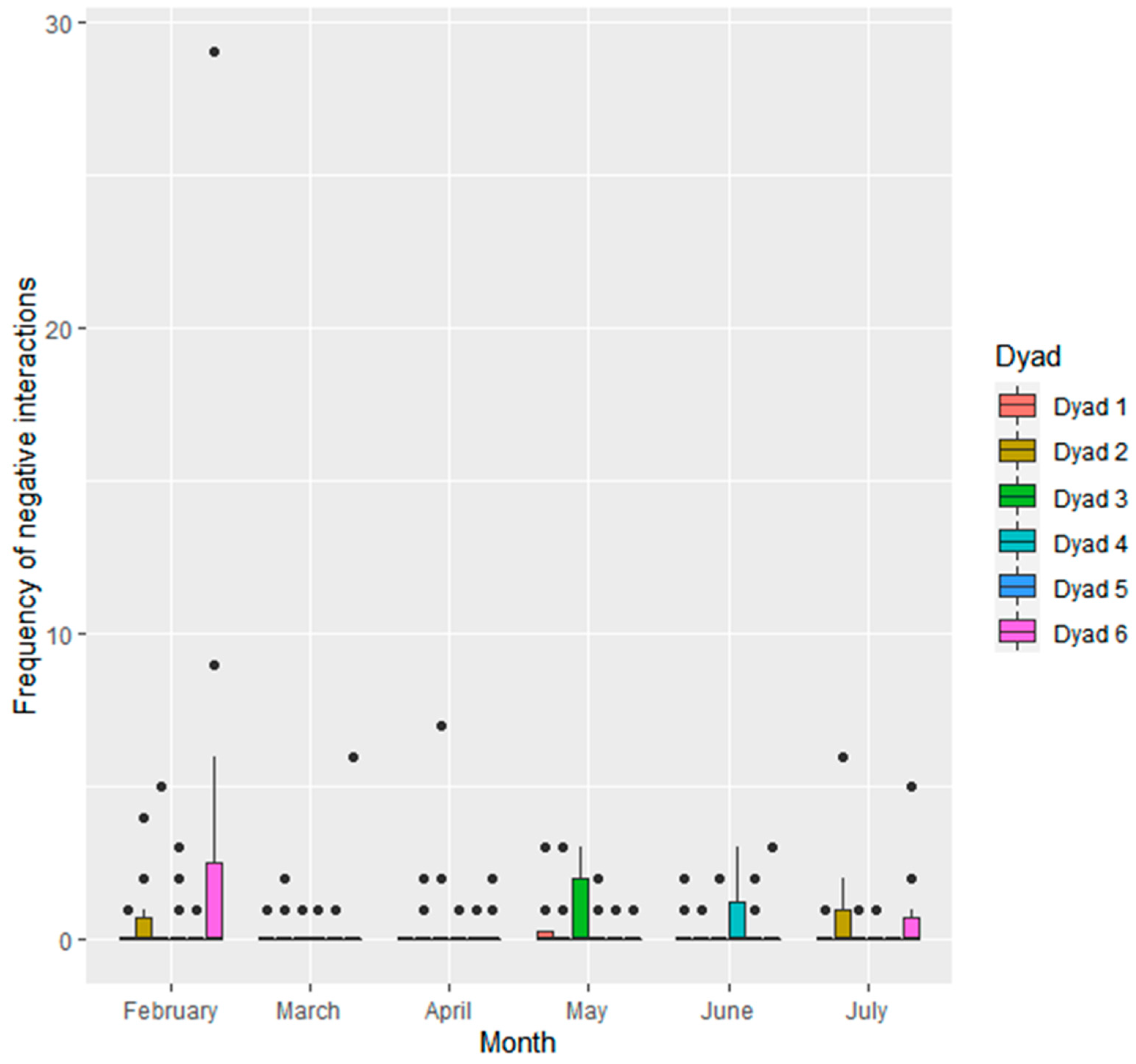
3.1.2. Interactions within Seal Dyads
3.2. Fluidity in Group-Level Interactions over Time and Dyadic Reciprocity
3.3. Nearest Neighbours
4. Discussion
4.1. Positive and Negative Social Interactions
4.2. Temporality in Social Relationships
4.3. Factors Affecting Observed Social Interactions
4.4. Implications for Management of South American Fur Seals and Other Pinniped Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolf, J.B.W.; Mawdsley, D.; Trillmich, F.; James, R. Social structure in a colonial mammal: Unravelling hidden structural layers and their foundations by network analysis. Anim. Behav. 2007, 74, 1293–1302. [Google Scholar] [CrossRef]
- Riedman, M. The pinnipeds: Seals, Sea Lions and the Walruses; University of California Press: Oxford, UK, 1990. [Google Scholar]
- Clutton-Brock, T. Mammal Societies; Wiley Blackwell: West Sussex, UK, 2016. [Google Scholar]
- Cárdenas-Alayza, S.; Oliveira, L.; Crespo, E. Arctocephalus Australis. The IUCN RedList of Threatened Species 2016: E.T2055A45223529; IUCN: Gland, Switzerland, 2016; Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T2055A45223529.en (accessed on 12 August 2021).
- Crespo, E.A.; Schiavini, A.C.M.; Garcia, N.A.; Franco-Trecu, V.R.; Goodall, N.P.; Rodriguez, D.; Morgante, S.; Rossa de Oliveira, L. Status, population trend and genetic structure of South American fur seals, Arctocephalus australias, in southwestern Atlantic waters. Mar. Mamm. Sci. 2015, 31, 866–890. [Google Scholar] [CrossRef]
- Jones, K.A.; Baylis, A.M.M.; Orben, R.A.; Ratcliffe, N.; Votier, S.C.; Newton, J.; Staniland, I.J. Stable isotope values in south American fur seal pup whiskers as proxies of year-round maternal foraging ecology. Mar. Biol. 2020, 167, 148. [Google Scholar] [CrossRef]
- Pavès, H.J.; Schlatter, R.; Franco-Trecu, V.; Paez, E.; Sielfeld, W.; Araos, V.; Giesecke, R.; Batalles, L.M.; Cappozzo, H.L. Breeding season of the South American fur seal (Arctocephalus australis, Otariidae: Carnivora): New data for establishing independent evolutionary histories? Rev. Biol. Mar. Oceanogr. 2016, 51, 241–253. [Google Scholar] [CrossRef]
- Franco-Trecu, V.; Costa, P.; Schramm, Y.; Tassino, B.; Inchausti, P. Sex on the rocks: Reproductive tactics and breeding success of South American fur seal males. Behav. Ecol. 2014, 25, 1513–1523. [Google Scholar] [CrossRef]
- Gili, C.; Meijer, G.; Lacave, G. EAZA and EAAM Best Practice Guidelines for Otariidae and Phocidae; EAZA: Genova, Italy, 2018. [Google Scholar]
- Sha, J.C.M.; Alagappasamy, S.; Chandran, S.; Cho, K.M.; Guha, B. Establishment of a Captive All-male Group of Proboscis Monkey (Nasalis larvatus) at the Singapore Zoo. Zoo Biol. 2013, 32, 281–290. [Google Scholar] [CrossRef]
- Stoinski, T.S.; Lukas, K.E.; Kuhar, C.W.; Maple, T.L. Factors influencing the formation and maintenance of all-male gorilla groups in captivity. Zoo Biol. 2004, 23, 189–203. [Google Scholar] [CrossRef]
- Gartland, K.N.; Carrigan, J.; White, F.J. Preliminary relationship between overnight separation and wounding in bachelor groups of western lowland gorillas (Gorilla gorilla gorilla). Appl. Anim. Behav. Sci. 2021, 241, 105388. [Google Scholar] [CrossRef]
- Gilbert, T.; Woodfine, T. The Biology, Husbandry and Conservation of Scimitar-Horned Oryx (Oryx Dammah); Marwell Preservation Trust: Hampshire, UK, 2004. [Google Scholar]
- Stoinski, T.S.; Hoff, M.P.; Lukas, K.E.; Maple, T.L. A preliminary behavioral comparison of two captive all-male gorilla groups. Zoo Biol. 2001, 20, 27–40. [Google Scholar] [CrossRef]
- Held, S.D.E.; Špinka, M. Animal play and animal welfare. Anim. Behav. 2011, 81, 891–899. [Google Scholar] [CrossRef]
- Williams, E.; Bremner-Harrison, S.; Ward, S.J. Can we meet the needs of social species in zoos? An overview of the impact of group housing on welfare in socially housed zoo mammals. In Zoo Animals: Husbandry, Welfare and Public Interactions; Berger, M., Corbett, S., Eds.; Nova Science Publishers: New York, NY, USA, 2018. [Google Scholar]
- Rose, P.; Croft, D.P. The potential of Social Network Analysis as a tool for the management of zoo animals. Anim. Welf. 2015, 24, 123–138. [Google Scholar] [CrossRef]
- Williams, E.; Bremner-Harrison, S.; Hall, C.; Carter, A. Understanding Temporal Social Dynamics in Zoo Animal Management: An Elephant Case Study. Animals 2020, 10, 882. [Google Scholar] [CrossRef]
- Brando, S.; Broom, D.M.; Acasuso-Rivero, C.; Clark, F. Optimal marine mammal welfare under human care: Current efforts and future directions. Behav. Proc. 2018, 156, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Zims. Species360 Zoological Information Management System (ZIMS). 2021. Available online: zims.Species360.org (accessed on 18 August 2021).
- Farine, D.R.; Whitehead, H. Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 2015, 84, 1144–1163. [Google Scholar] [CrossRef] [PubMed]
- Bertram, B. WAZA ZooLex Galley: Bristol Zoo Gardens Seal and Penguin Coast. 2003. Available online: https://zoolex.org/gallery/show/569/ (accessed on 6 September 2021).
- Eakin, E. Understanding the Effect of Zoo Environments: Assessing the Effect of Environmental Variables on South American Fur Seal (Arctocephalus australis) Behaviour. Master’s Thesis, University of Bristol, Bristol, UK, 2019. [Google Scholar]
- Martin, P.; Bateson, P. Measuring Behavior: An Introductory Guide; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- De Vere, A.J.; Lilley, M.K.; Highfill, L. Do Pinnipeds have Personality? Broad Dimensions and Contextual Consistency of Behavior in Harbor Seals (Phoca vitulina) and California Sea Lions (Zalophus californianus). Int. J. Comp. Psyc. 2017, 30. [Google Scholar]
- Borgatti, S.P. NetDraw Software for Network Visualization; Analytic Technologies: Lexington, KY, USA, 2002. [Google Scholar]
- Oldham, S.; Fulcher, B.; Parkes, L.; Arnatkevičiūtė, A.; Suo, C.; Fornito, A. Consistency and differences between centrality measures across distinct classes of networks. PLoS ONE 2019, 14, e0220061. [Google Scholar] [CrossRef]
- Lusseau, D.; Newman, M.E.J. Identifying the role that animals play in their social networks. Proc. R. Soc. B Biol. Sci. 2004, 271 (Suppl. 6), S477–S481. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 12 July 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.5.4. 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 12 July 2021).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Oksanen, J.F.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.M.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 12 July 2021).
- Leeds, A.; Boyer, D.; Ross, S.R.; Lukas, K.E. The effects of group type and young silverbacks on wounding rates in western lowland gorilla (gorilla gorilla gorilla) groups in North American Zoos. Zoo Biol. 2015, 34, 296–304. [Google Scholar] [CrossRef]
- Heitor, F.; Vicente, L. Dominance relationships and patterns of aggression in a bachelor group of Sorraia horses (Equus caballus). J. Ethol. 2010, 28, 35–44. [Google Scholar] [CrossRef]
- Qi, X.G.; Huang, K.; Fang, G.; Grueter, C.C.; Dunn, D.W.; Li, Y.L.; Ji, W.; Wang, X.Y.; Wang, R.T.; Garber, P.A.; et al. Male cooperation for breeding opportunities contributes to the evolution of multilevel societies. Proc. R. Soc. B Biol. Sci. 2017, 284, 1863. [Google Scholar] [CrossRef]
- Faust, L.J.; Thompson, S.D. Birth sex ratios in captive mammals: Patterns, biases, and the implications for management and conservation. Zoo Biol. 2000, 19, 11–25. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W.S.M. Assessment of welfare in zoo animals: Towards optimum quality of life. Animals 2018, 8, 110. [Google Scholar] [CrossRef]
- Stirling, I. Observations on the behaviour of the New Zealand fur seal. J. Mamm. 1970, 51, 766–778. [Google Scholar] [CrossRef]
- Stoinski, T.S.; Lukas, K.E.; Kuhar, C.W.; Maple, T.L. Social dynamics of captive western lowland gorillas living in all-male groups. Behaviour 2004, 141, 169–195. [Google Scholar]
- Gartland, K.; McDonald, M.; Braccini Slade, S.; White, F.; Sanz, C. Behavioral changes following alterations in the composition of a captive bachelor group of western lowland gorillas (Gorilla gorilla gorilla). Zoo Biol. 2018, 37, 391–398. [Google Scholar] [CrossRef]
- Marino, A. Indirect measures of reproductive effort in a resource-defense polygynous ungulate: Territorial defense by male guanacos. J. Ethol. 2012, 30, 83–91. [Google Scholar] [CrossRef]
- Pullen, P.K. Male-Male Social Interactions in Breeder and Bachelor Groups of Gorillas (Gorilla gorilla): An Indication of Behavioural Flexibility. Ph.D. Thesis, University of Exeter, Exeter, UK, 2009. [Google Scholar]
- Garaï, M.E. Special Relationships between Female Asian Elephants (Elephas maximus) in Zoological Gardens. Ethology 1992, 90, 187–205. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Hall, C.; Bremner-Harrison, S. Social Interactions in Zoo-Housed Elephants: Factors Affecting Social Relationships. Animals 2019, 9, 747. [Google Scholar] [CrossRef]
- Hartley, M.; Wood, A.; Yon, L. Facilitating the social behaviour of bull elephants in zoos. Int. Zoo Yearb. 2019, 53, 62–77. [Google Scholar] [CrossRef]
- Watters, J.V.; Powell, D.M. Measuring Animal Personality for Use in Population Management in Zoos: Suggested Methods and Rationale. Zoo Biol. 2012, 31, 1–12. [Google Scholar] [CrossRef]
- Ciardelli, L.E.; Weiss, A.; Powell, D.M.; Reiss, D. Personality dimensions of the captive California sea lion (Zalophus californianus). J. Comp. Psychol. 2017, 131, 50–58. [Google Scholar] [CrossRef][Green Version]
- Massen, J.J.M.; Koski, S.E. Chimps of a feather sit together: Chimpanzee friendships are based on homophily in personality. Evol. Hum. Behav. 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Makecha, R.; Fad, O.; Kuczaj, I.; Stan, A. The Role of Touch in the Social Interactions of Asian Elephants (Elephas maximus). J. Comp. Psychol. 2012, 25, 60–82. [Google Scholar]
- Williams, E.; Carter, A.; Hall, C.; Bremner-Harrison, S. Exploring the relationship between personality and social interactions in zoo-housed elephants: Incorporation of keeper expertise. Appl. Anim. Behav. Sci. 2019, 221, 104876. [Google Scholar] [CrossRef]
- Wielebnowski, N.C. Behavioral differences as predictors of breeding status in captive cheetahs. Zoo Biol. 1999, 18, 335–349. [Google Scholar] [CrossRef]
- Martin-Wintle, M.S.; Shepherdson, D.; Zhang, G.; Huang, Y.; Luo, B.; Swaisgood, R.R. Do opposites attract? Effects of personality matching in breeding pairs of captive giant pandas on reproductive success. Biol. Conserv. 2017, 207, 27–37. [Google Scholar] [CrossRef]
- Koski, S.E.; de Vries, H.; van de Kraats, A.; Sterck, E.H.M. Stability and change of social relationship quality in captive chimpanzees (Pan troglodytes). Int. J. Primatol. 2012, 33, 905–921. [Google Scholar] [CrossRef]
- Levengood, A.L.; Dudzinski, K.M. Is blood thicker than water? The role of kin and non-kin in non-mother-calf associations of captive bottlenose dolphins (Tursiops truncatus). Behav. Proc. 2016, 124, 52–59. [Google Scholar] [CrossRef]
- Videan, E.N.; Fritz, J. Effects of short- and long-term changes in spatial density on the social behavior of captive chimpanzees (Pan troglodytes). Appl. Anim. Behav. Sci. 2007, 102, 95–105. [Google Scholar] [CrossRef]
- Coe, J.C.; Scott, D.; Lukas, K.E. Facility design for bachelor gorilla groups. Zoo Biol. 2009, 28, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Brando, S.; Buchanan-Smith, H.M. The 24/7 approach to promoting optimal welfare for captive wild animals. Behav. Proc. 2017, 156, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Krebs, B.L.; Marrin, D.; Phelps, A.; Krol, L.; Watters, J.V. Managing aged animals in zoos to promote positive welfare: A review and future directions. Animals 2018, 8, 116. [Google Scholar] [CrossRef] [PubMed]
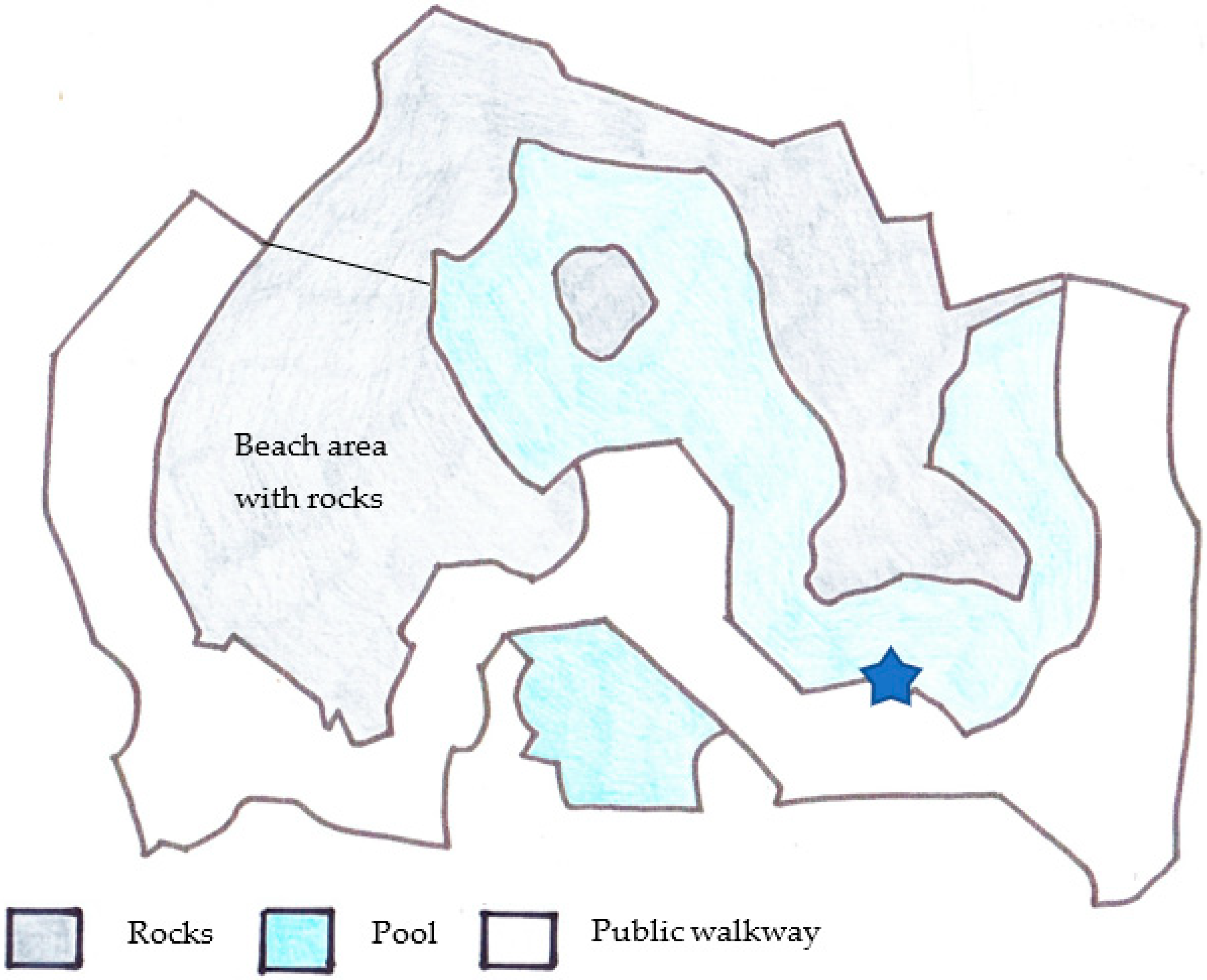
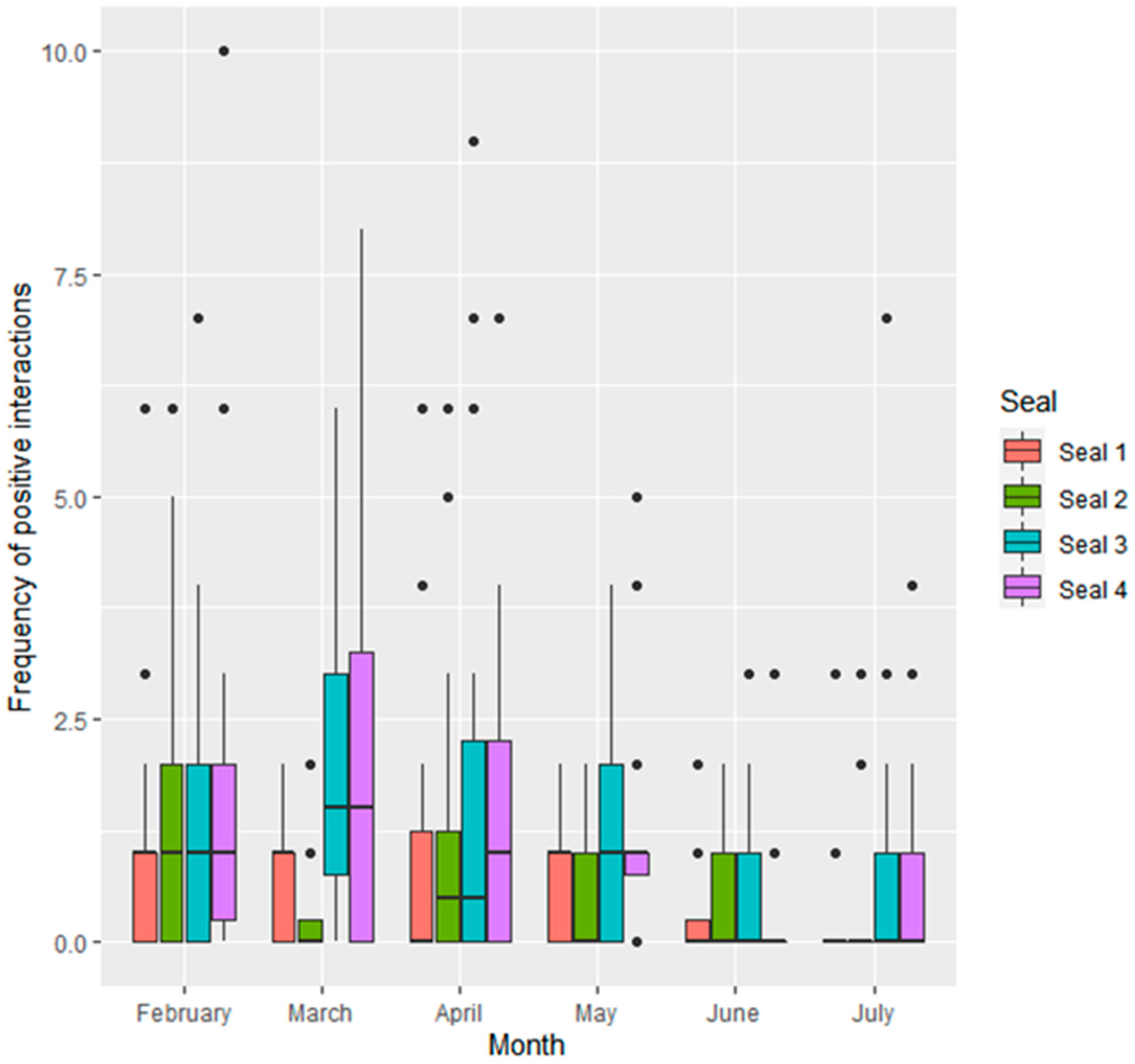
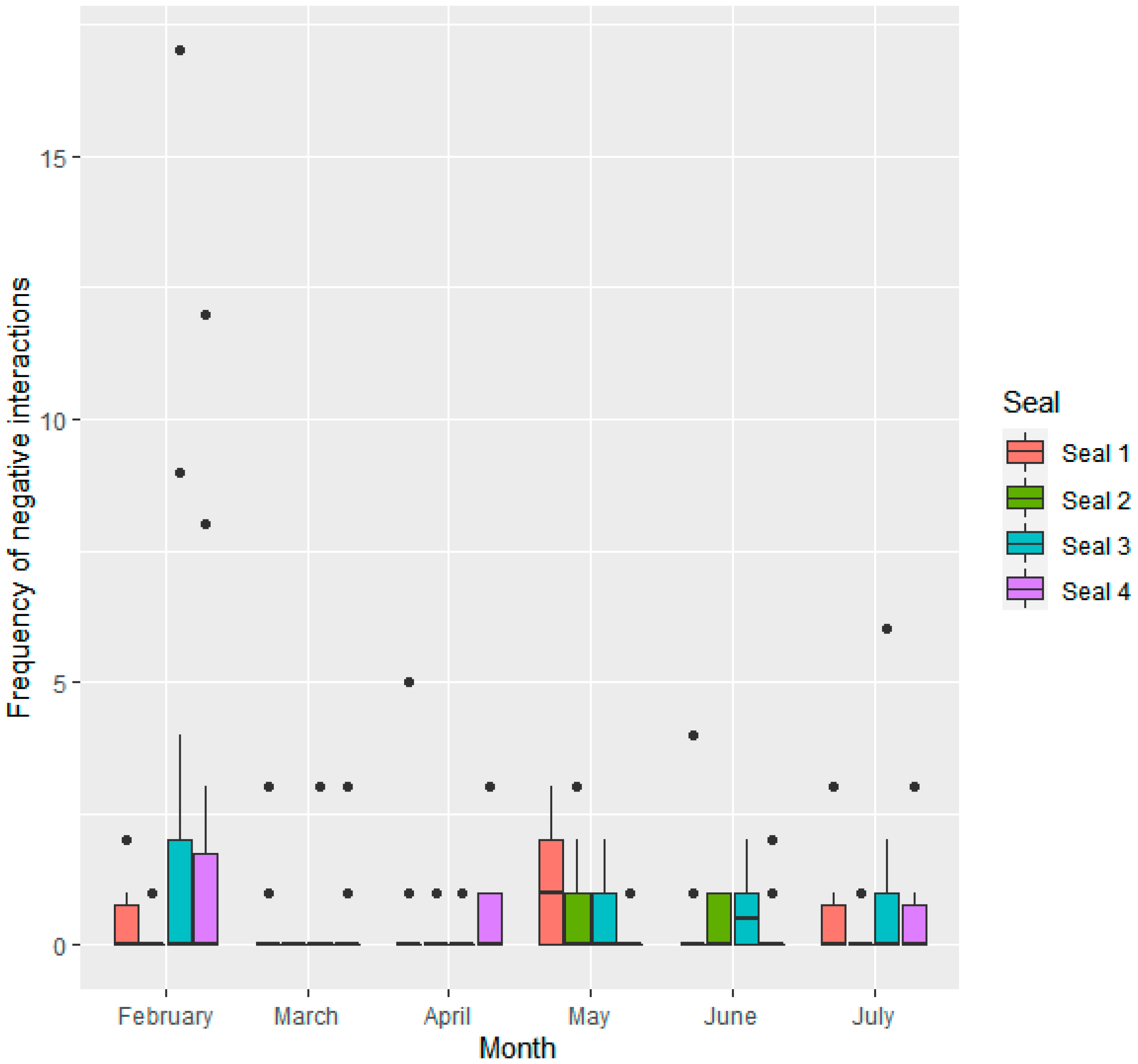
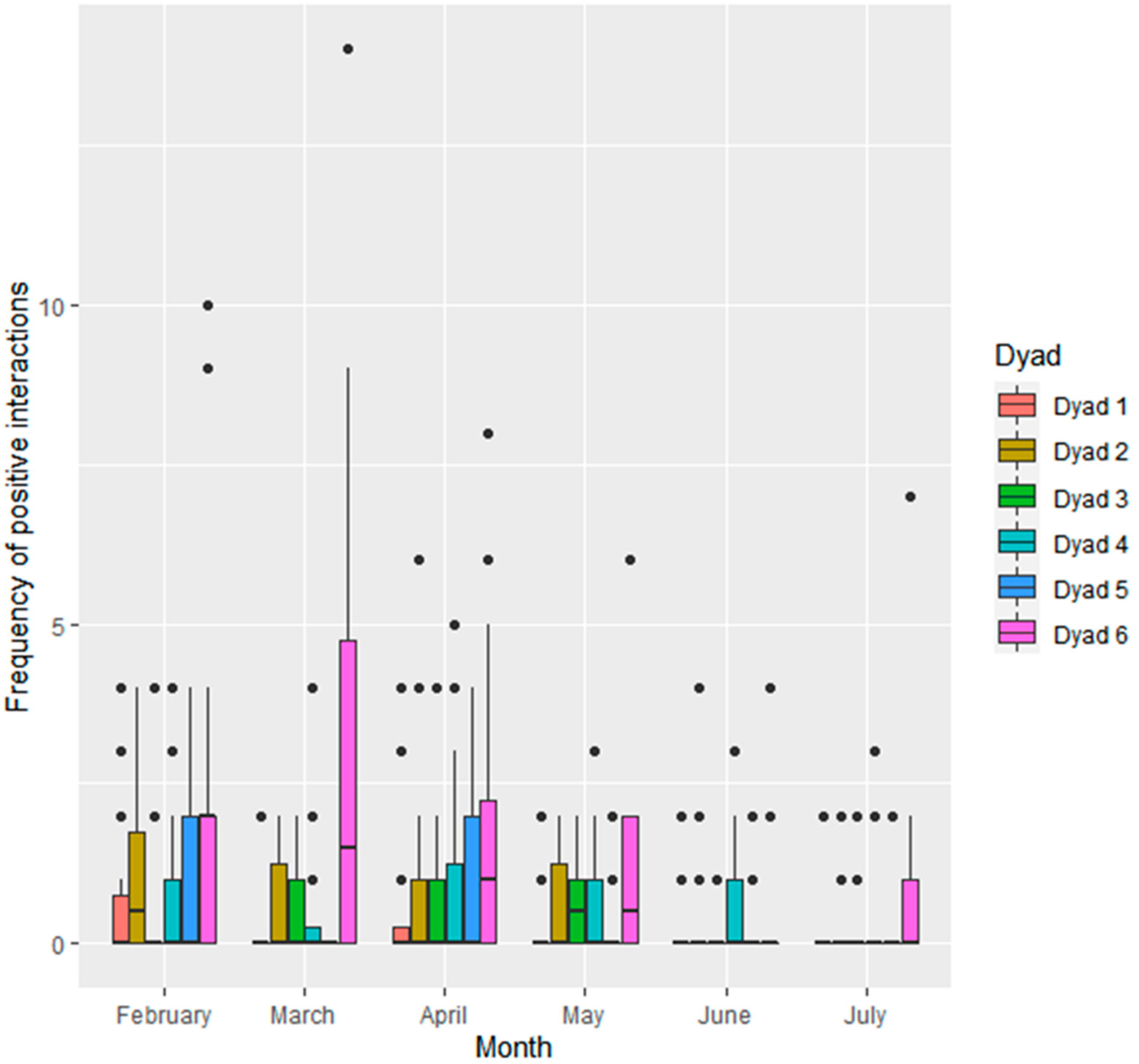
| Seal ID | Sex | Age at Start of Study | Stage of Maturity | Relationship to Others |
|---|---|---|---|---|
| S1 | M | 11 | Adult | Full brother to all |
| S2 | M | 9 | Adult | Half-brother to all |
| S3 | M | 8 | Adult | Full brother to all |
| S4 | M | 7 | Adult | Full brother to all |
| Interaction Category | Behaviour | Description |
|---|---|---|
| Positive | Nose–nose | Touching another’s face with their nose |
| Nose–body | Touching another’s body (not the face/nose) with their nose | |
| Mouth–mouth | Gentle touching of another’s mouth with their mouth | |
| Play | Individuals jump, slide and porpoise round the enclosure. This may centre around enrichment | |
| Negative | Bite | Individuals use teeth to touch another individual |
| Display | Individuals have wide mouths and square each other up. May sway necks side to side opposite another individual | |
| Displacement | An individual approaches another causing them to move out of the way (by more than 1 body length). May result in approaching individual taking their place |
| Focal Seal | Nearest Neighbour (Mean % ± SD) | Alone | |||
|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||
| S1 | 21 ± 17 | 21 ± 17 | 19 ± 15 | 46 ± 24 | |
| S2 | 20 ± 17 | 34 ± 21 | 23 ± 17 | 32 ± 17 | |
| S3 | 18 ± 17 | 30 ± 21 | 42 ± 22 | 21 ± 16 | |
| S4 | 18 ± 14 | 21 ± 15 | 44 ± 23 | 27 ± 17 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmett, C.; Digby, M.; Pope, J.; Williams, E. Social Behaviour in Zoo Bachelor Groups: A Case Study of Related South American Fur Seals. Animals 2021, 11, 2682. https://doi.org/10.3390/ani11092682
Emmett C, Digby M, Pope J, Williams E. Social Behaviour in Zoo Bachelor Groups: A Case Study of Related South American Fur Seals. Animals. 2021; 11(9):2682. https://doi.org/10.3390/ani11092682
Chicago/Turabian StyleEmmett, Christa, Mathilda Digby, Jemma Pope, and Ellen Williams. 2021. "Social Behaviour in Zoo Bachelor Groups: A Case Study of Related South American Fur Seals" Animals 11, no. 9: 2682. https://doi.org/10.3390/ani11092682
APA StyleEmmett, C., Digby, M., Pope, J., & Williams, E. (2021). Social Behaviour in Zoo Bachelor Groups: A Case Study of Related South American Fur Seals. Animals, 11(9), 2682. https://doi.org/10.3390/ani11092682






