Regional Differences in the Diets of Adélie and Emperor Penguins in the Ross Sea, Antarctica
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Sample Preparation and Stable Isotope Analysis
2.3. Statistical Analysis
3. Results
3.1. Isotopic Signatures of Penguin Chicks and Their Prey
3.2. Diet Proportions of Penguin Chicks
4. Discussion
4.1. Influence of Latitudinal Diet Differences for Adélie Penguin
4.2. Diet Preference of Emperor Penguin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michel, L.N.; Danis, B.; Dubois, P.; Eleaume, M.; Fournier, J.; Gallut, C.; Jane, P.; Lepoint, G. Increased sea ice cover alters food web structure in East Antarctica. Sci. Rep. 2019, 9, 8062. [Google Scholar] [CrossRef]
- Calizza, E.; Careddu, G.; Sporta Caputi, S.; Rossi, L.; Costantini, M.L. Time- and depth-wise trophic niche shifts in Antarctic benthos. PLoS ONE 2018, 13, e0194796. [Google Scholar] [CrossRef]
- Hopkins, T.L. Midwater food web in McMurdo Sound, Ross Sea, Antarctica. Mar. Biol. 1987, 96, 93–106. [Google Scholar] [CrossRef]
- Leonori, I.; De Felice, A.; Canduci, G.; Costantini, I.; Biagiotti, I.; Giuliani, G.; Budillon, G. Krill distribution in relation to environmental parameters in mesoscale structures in the Ross Sea. J. Mar. Syst. 2017, 166, 159–171. [Google Scholar] [CrossRef]
- Lee, D.B.; Choi, K.H.; Ha, H.K.; Yang, E.J.; Lee, S.H.; Lee, S.H.; Shin, H.C. Mesozooplankton distribution patterns and grazing impacts of copepods and Euphausia crystallorophias in the Amundsen Sea, West Antarctica, during austral summer. Polar Biol. 2013, 36, 1215–1230. [Google Scholar] [CrossRef]
- Taki, K.; Yabuki, T.; Noiri, Y.; Hayashi, T.; Naganobu, M. Horizontal and vertical distribution and demography of euphausiids in the Ross Sea and its adjacent waters in 2004/2005. Polar Biol. 2008, 31, 1343–1356. [Google Scholar] [CrossRef]
- Lyver, P.O.B.; Barron, M.; Barton, K.J.; Ainley, D.G.; Pollard, A.; Gordon, S.; McNeill, S.; Ballard, G.; Wilson, P.R. Trends in the breeding population of Adélie penguins in the Ross Sea, 1981–2012: A coincidence of climate and resource extraction effects. PLoS ONE 2014, 9, e91188. [Google Scholar] [CrossRef][Green Version]
- Kooyman, G.L.; Ponganis, P.J. Rise and fall of Ross Sea emperor penguin colony populations: 2000 to 2012. Antarct. Sci. 2017, 29, 207–208. [Google Scholar] [CrossRef]
- Cherel, Y.; Kooyman, G.L. Food of emperor penguins (Aptenodytes forsteri) in the western Ross Sea, Antarctica. Mar. Biol. 1998, 130, 335–344. [Google Scholar] [CrossRef]
- Cherel, Y. Isotopic niches of emperor and Adélie penguins in Adélie Land, Antarctica. Mar. Biol. 2008, 154, 813–821. [Google Scholar] [CrossRef]
- Barber-Meyer, S.M.; Kooyman, G.L.; Ponganis, P.J. Estimating the relative abundance of emperor penguins at inaccessible colonies using satellite imagery. Polar Biol. 2007, 30, 1565–1570. [Google Scholar] [CrossRef]
- Huang, T.; Sun, L.; Long, N.; Wang, Y.; Huang, W. Penguin tissue as a proxy for relative krill abundance in East Antarctica during the Holocene. Sci. Rep. 2013, 3, 2807. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.B.; Hofmann, E.E.; Klinck, J.M.; Piñones, A.; Dinniman, M.S. Distributions of krill and Antarctic silverfish and correlations with environmental variables in the western Ross Sea, Antarctica. Mar. Ecol. Prog. Ser. 2017, 584, 45–65. [Google Scholar] [CrossRef]
- Sala, A.; Azzali, M.; Russo, A. Krill of the Ross Sea: Distribution, abundance and demography of Euphausia superba and Euphausia crystallorophias during the Italian Antarctic expedition (January–February 2000). Sci. Mar. 2002, 66, 123–133. [Google Scholar] [CrossRef]
- Brooks, C.M.; Caccavo, J.A.; Ashford, J.; Dunbar, R.; Goetz, K.; La Mesa, M.; Zane, L. Early life history connectivity of Antarctic silverfish (Pleuragramma antarctica) in the Ross Sea. Fish. Oceanogr. 2018, 27, 274–287. [Google Scholar] [CrossRef]
- O’Driscoll, R.L.; Macaulay, G.J.; Gauthier, S.; Pinkerton, M.; Hanchet, S. Distribution, abundance and acoustic properties of Antarctic silverfish (Pleuragramma antarcticum) in the Ross Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2011, 58, 181–195. [Google Scholar] [CrossRef]
- Volkman, N.J.; Presler, P.; Trivelpiece, W. Diets of Pygoscelid Penguins at King George Island, Antarctica. Condor 1980, 82, 373. [Google Scholar] [CrossRef]
- Tierney, M.; Southwell, C.; Emmerson, L.M.; Hindell, M.A. Evaluating and using stable-isotope analysis to infer diet composition and foraging ecology of Adélie penguins Pygoscelis adeliae. Mar. Ecol. Prog. Ser. 2008, 355, 297–307. [Google Scholar] [CrossRef]
- Lyver, P.O.B.; MacLeod, C.J.; Ballard, G.; Karl, B.J.; Barton, K.J.; Adams, J.; Ainley, D.G.; Wilson, P.R. Intra-seasonal variation in foraging behavior among Adélie penguins (Pygocelis adeliae) breeding at Cape Hallett, Ross Sea, Antarctica. Polar Biol. 2011, 34, 49–67. [Google Scholar] [CrossRef]
- Colominas-Ciuró, R.; Santos, M.; Coria, N.; Barbosa, A. Sex-specific foraging strategies of Adélie penguins (Pygoscelis adeliae): Females forage further and on more krill than males in the Antarctic Peninsula. Polar Biol. 2018, 41, 2635–2641. [Google Scholar] [CrossRef]
- Nyssen, F.; Brey, T.; Lepoint, G.; Bouquegneau, J.M.; De Broyer, C.; Dauby, P. A stable isotope approach to the eastern Weddell Sea trophic web: Focus on benthic amphipods. Polar Biol. 2002, 25, 280–287. [Google Scholar] [CrossRef][Green Version]
- Vallet, C.; Beans, C.; Koubbi, P.; Courcot, L.; Hecq, J.H.; Goffart, A. Food preferences of larvae of Antarctic silverfish Pleuragramma antarcticum Boulenger, 1902 from Terre Adélie coastal waters during summer 2004. Polar Sci. 2011, 5, 239–251. [Google Scholar] [CrossRef]
- Michel, L.N.; David, B.; Dubois, P.; Lepoint, G.; De Ridder, C. Trophic plasticity of Antarctic echinoids under contrasted environmental conditions. Polar Biol. 2016, 39, 913–923. [Google Scholar] [CrossRef]
- Jia, Z.; Swadling, K.M.; Meiners, K.M.; Kawaguchi, S.; Virtue, P. The zooplankton food web under East Antarctic pack ice—A stable isotope study. Deep. Res. Part II Top. Stud. Oceanogr. 2016, 131, 189–202. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Casselman, J.M.; Rasmussen, J.B. Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 1999, 401, 464–467. [Google Scholar] [CrossRef]
- Fukumori, K.; Oi, M.; Doi, H.; Takahashi, D.; Okuda, N.; Miller, T.W.; Kuwae, M.; Miyasaka, H.; Genkai-Kato, M.; Koizumi, Y.; et al. Bivalve tissue as a carbon and nitrogen isotope baseline indicator in coastal ecosystems. Estuar. Coast. Shelf Sci. 2008, 79, 45–50. [Google Scholar] [CrossRef]
- Gillies, C.L.; Stark, J.S.; Johnstone, G.J.; Smith, S.D.A. Carbon flow and trophic structure of an Antarctic coastal benthic community as determined by δ 13C and δ 15N. Estuar. Coast. Shelf Sci. 2012, 97, 44–57. [Google Scholar] [CrossRef]
- Park, H.J.; Yeon, I.; Han, E.; Lee, Y.J.; Hanchet, S.M.; Baeck, G.W.; Kwon, Y.; Choi, S.G.; Lee, D.W.; Kang, C.K. Diet study of Antarctic toothfish caught in the east Antarctic based on stomach content, fatty acid and stable isotope analyses. CCAMLR Sci. 2015, 22, 29–44. [Google Scholar]
- Pilcher, N.; Gaw, S.; Eisert, R.; Horton, T.W.; Gormley, A.M.; Cole, T.L.; Lyver, P.O.B. Latitudinal, sex and inter-specific differences in mercury and other trace metal concentrations in Adélie and Emperor penguins in the Ross Sea, Antarctica. Mar. Pollut. Bull. 2020, 154, 111047. [Google Scholar] [CrossRef]
- Pinkerton, M.H.; Forman, J.; Bury, S.J.; Brown, J.; Horn, P.; O’Driscoll, R.L. Diet and trophic niche of Antarctic silverfish Pleuragramma antarcticum in the Ross Sea, Antarctica. J. Fish Biol. 2013, 82, 141–164. [Google Scholar] [CrossRef]
- Vasil, C.A.; Polito, M.J.; Patterson, W.P.; Emslie, S.D. Wanted: Dead or alive? Isotopic analysis (δ 13C and δ 15N) of Pygoscelis penguin chick tissues supports opportunistic sampling. Rapid Commun. Mass Spectrom. 2012, 26, 487–493. [Google Scholar] [CrossRef]
- Bunn, S.E.; Loneragan, N.R.; Kempster, M.A. Effects of acid washing on stable isotope ratios of C and N in penaeid shrimp and seagrass: Implications for food-web studies using multiple stable isotopes. Limnol. Oceanogr. 1995, 40, 622–625. [Google Scholar] [CrossRef]
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source partitioning using stable isotopes: Coping with too much variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef]
- Polito, M.J.; Trivelpiece, W.Z.; Reiss, C.S.; Trivelpiece, S.G.; Hinke, J.T.; Patterson, W.P.; Emslie, S.D. Intraspecific variation in a dominant prey species can bias marine predator dietary estimates derived from stable isotope analysis. Limnol. Oceanogr. Methods 2019, 17, 292–303. [Google Scholar] [CrossRef]
- Kohlbach, D.; Lange, B.A.; Graeve, M.; Vortkamp, M.; Flores, H. Varying dependency of Antarctic euphausiids on ice algae- and phytoplankton-derived carbon sources during summer. Mar. Biol. 2019, 166, 79. [Google Scholar] [CrossRef]
- Beaulieu, M.; Thierry, A.M.; Raclot, T.; Le Maho, Y.; Ropert-Coudert, Y.; Gachot-Neveu, H.; Ancel, A. Sex-specific parental strategies according to the sex of offspring in the Adélie penguin. Behav. Ecol. 2009, 20, 878–883. [Google Scholar] [CrossRef][Green Version]
- Juáres, M.A.; Casaux, R.; Corbalán, A.; Blanco, G.; Pereira, G.A.; Perchivale, P.J.; Coria, N.R.; Santos, M.M. Diet of Adélie penguins (Pygoscelis adeliae) at Stranger Point (25 de Mayo/King George Island, Antarctica) over a 13-year period (2003–2015). Polar Biol. 2018, 41, 303–311. [Google Scholar] [CrossRef]
- Giraldo, C.; Cherel, Y.; Vallet, C.; Mayzaud, P.; Tavernier, E.; Moteki, M.; Hosie, G.; Koubbi, P. Ontogenic changes in the feeding ecology of the early life stages of the Antarctic silverfish (Pleuragramma antarcticum) documented by stable isotopes and diet analysis in the Dumont d’Urville Sea (East Antarctica). Polar Sci. 2011, 5, 252–263. [Google Scholar] [CrossRef]
- Hart, K.M.; Hyrenbach, K.D. Distribution patterns and biomasses of Antarctic krill (Euphausia superba) and ice krill (E. crystallorophias) with referece to Antarctic minke whales in the Ross Sea in 2005 using Kaiyo Maru -JARPA joint survey data. Endanger. Species Res. 2010, 10, 9–20. [Google Scholar] [CrossRef]
- Barber-Meyer, S.M.; Kooyman, G.L.; Ponganis, P.J. Trends in western Ross Sea emperor penguin chick abundances and their relationships to climate. Antarct. Sci. 2008, 20, 3–11. [Google Scholar] [CrossRef]
- Ainley, D.G.; Wilson, P.R.; Barton, K.J.; Ballard, G.; Nur, N.; Karl, B. Diet and foraging effort of Adelie penguins in relation to pack-ice conditions in the southern Ross Sea. Polar Biol. 1998, 20, 311–319. [Google Scholar] [CrossRef]
- Tierney, M.; Nichols, P.D.; Wheatley, K.E.; Hindell, M.A. Blood fatty acids indicate inter- and intra-annual variation in the diet of Adélie penguins: Comparison with stomach content and stable isotope analysis. J. Exp. Mar. Biol. Ecol. 2008, 367, 65–74. [Google Scholar] [CrossRef]
- Polito, M.; Emslie, S.D.; Walker, W. A 1000-year record of Adélie penguin diets in the southern Ross Sea. Antarct. Sci. 2002, 14, 327–332. [Google Scholar] [CrossRef][Green Version]
- Tierney, M.; Emmerson, L.; Hindell, M. Temporal variation in Adélie penguin diet at Béchervaise Island, east Antarctica and its relationship to reproductive performance. Mar. Biol. 2009, 156, 1633–1645. [Google Scholar] [CrossRef]
- Yang, L.; Sun, L.; Emslie, S.D.; Xie, Z.; Huang, T.; Gao, Y.; Yang, W.; Chu, Z.; Wang, Y. Oceanographic mechanisms and penguin population increases during the Little Ice Age in the southern Ross Sea, Antarctica. Earth Planet. Sci. Lett. 2018, 481, 136–142. [Google Scholar] [CrossRef]
- Burns, J.M.; Trumble, S.J.; Castellini, M.A.; Testa, J.W. The diet of Weddell seals in McMurdo Sound, Antarctica as determined from scat collections and stable isotope analysis. Polar Biol. 1998, 19, 272–282. [Google Scholar] [CrossRef]
- Burns, J.M.; Kooyman, G.L. Habitat use by weddell seals and emperor penguins foraging in the ross sea, antarctica. Am. Zool. 2001, 41, 90–98. [Google Scholar] [CrossRef][Green Version]
- Klages, N. Food and feeding ecology of emperor penguins in the eastern Weddell Sea. Polar Biol. 1989, 9, 385–390. [Google Scholar] [CrossRef]
- Golubev, S. Seabirds in Conditions of Local Chronic Oil Pollution, Davis Sea, Antarctica. Birds 2021, 2, 275–284. [Google Scholar] [CrossRef]
- Huang, T.; Sun, L.; Wang, Y.; Chu, Z.; Qin, X.; Yang, L. Transport of nutrients and contaminants from ocean to island by emperor penguins from Amanda Bay, East Antarctic. Sci. Total Environ. 2014, 468–469, 578–583. [Google Scholar] [CrossRef]
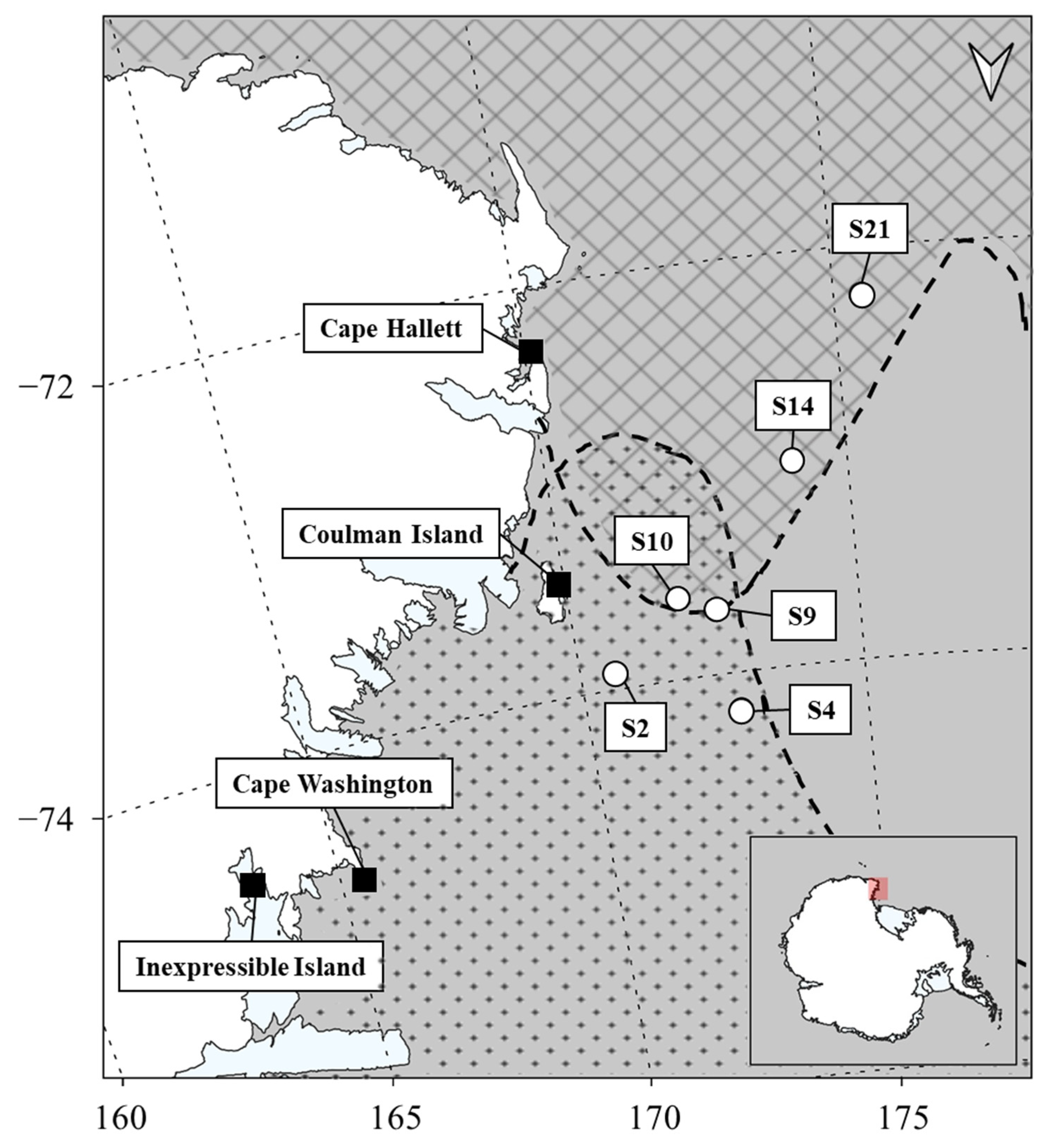
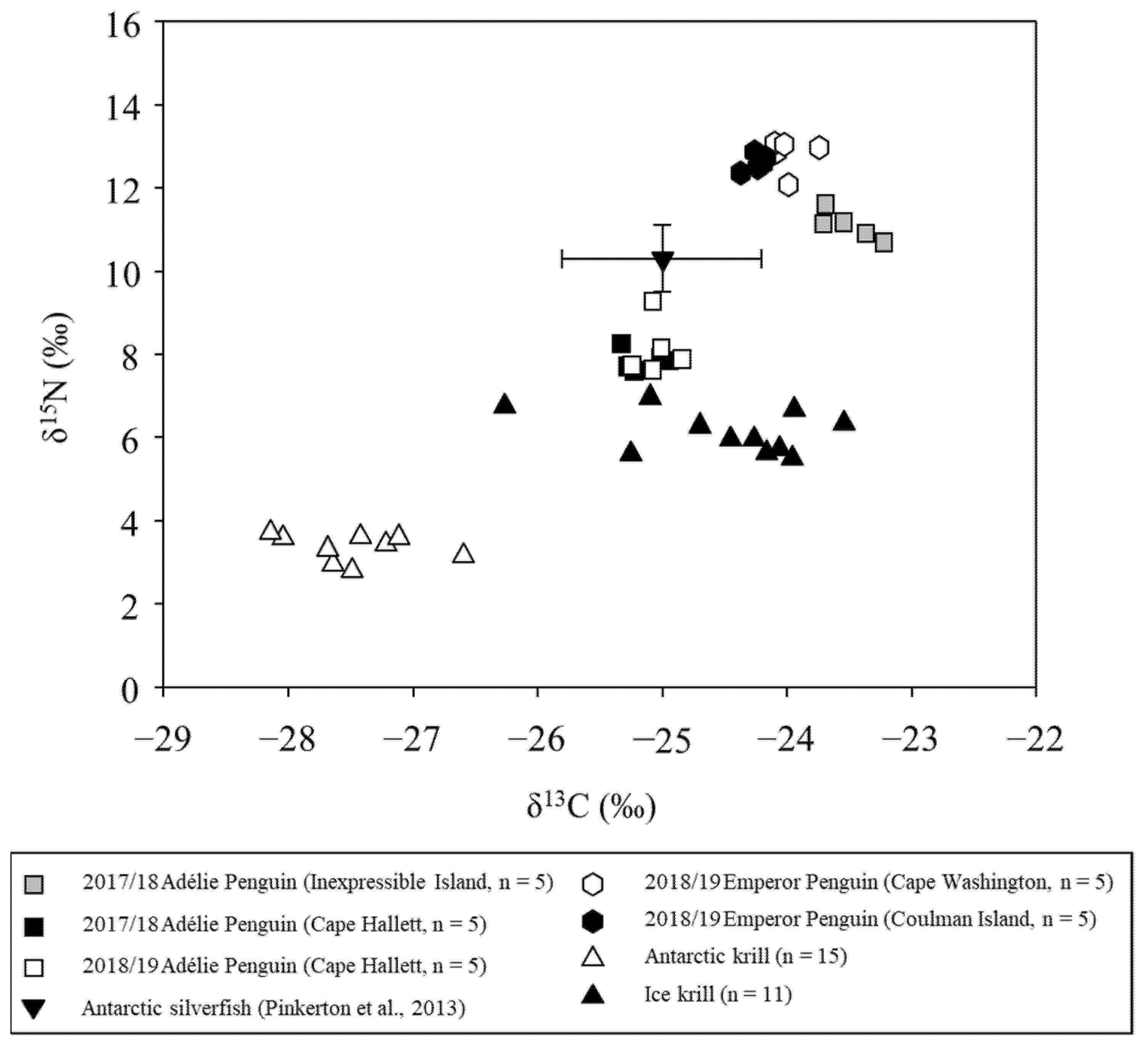
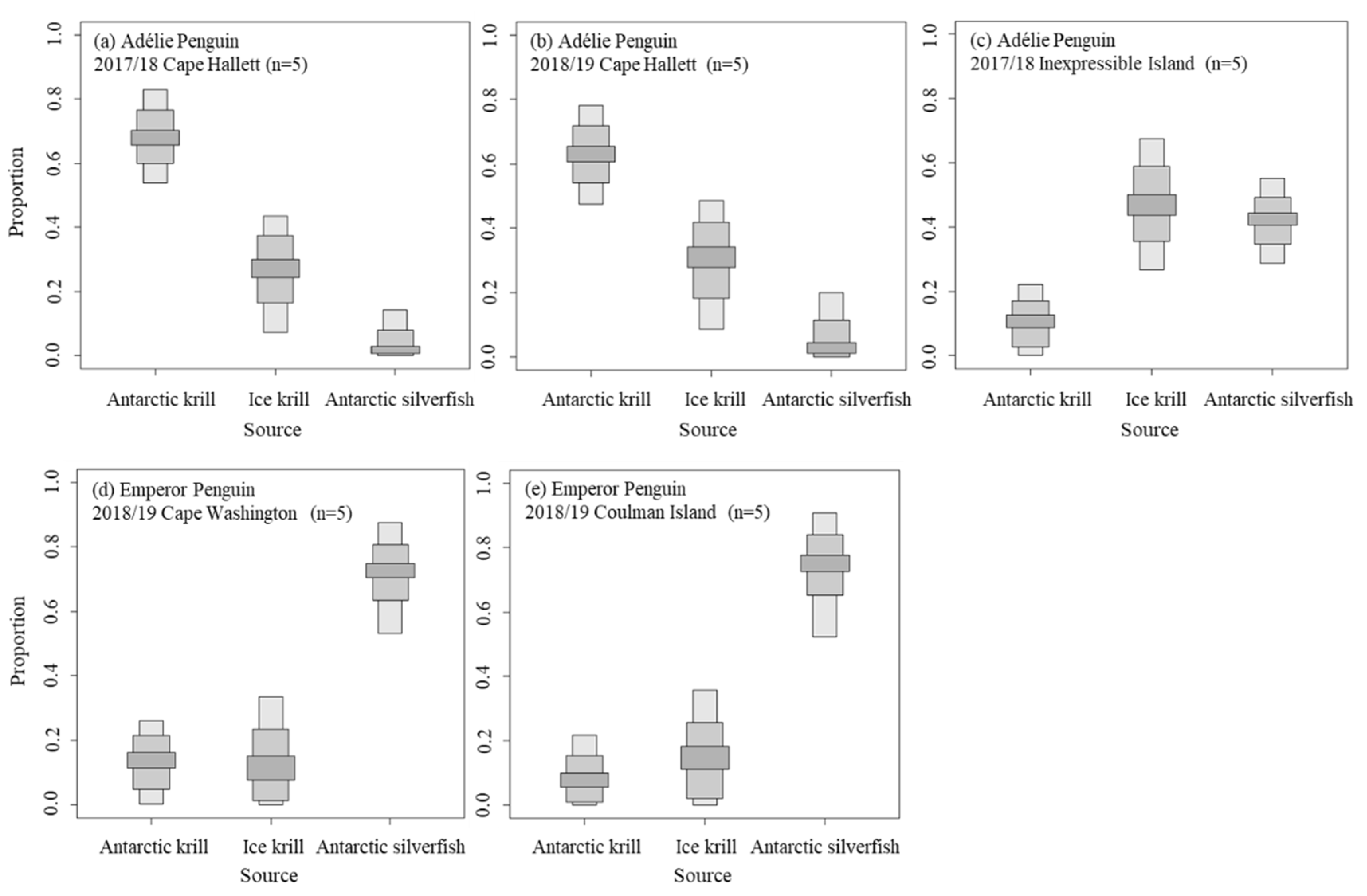
| Common Name | N | Analyzed Tissue | δ13C (‰) | δ15N (‰) | Sampling Date (yy/mm) | Sampling Area | References |
|---|---|---|---|---|---|---|---|
| Ice krill | 11 | Whole body | −24.52 ± 0.77 | 6.12 ± 0.51 | 19/01 | S2, S4, S9 | This study |
| Antarctic krill | 15 | Whole body | −27.41 ± 0.57 | 3.65 ± 0.58 | 19/01 | S10, S14, S21 | This study |
| Adélie Penguin | 5 | Down | −25.16 ± 0.17 | 7.86 ± 0.25 | 17/12 (guard–créche stage) | Cape Hallett | This study |
| 5 | Down | −25.05 ± 0.14 | 8.13 ± 0.67 | 18/12 (guard–créche stage) | Cape Hallett | This study | |
| 5 | Down | −23.50 ± 0.21 | 11.10 ± 0.34 | 18/01 (créche stage) | Inexpressible Island | This study | |
| Emperor Penguin | 5 | Down | −23.99 ± 0.15 | 12.81 ± 0.42 | 18/11 (créche stage) | Cape Washington | This study |
| 5 | Down | −24.24 ± 0.08 | 12.60 ± 0.20 | 18/11 (créche stage) | Coulman Island | This study | |
| Antarctic silverfish | 140 | Whole body | −25.00 ± 0.80 | 10.30 ± 0.80 | 08/02–03 | Western Ross Sea | [30] |
| Year | Species | Breeding Site | Diet Proportion (%) | ||
|---|---|---|---|---|---|
| Antarctic Krill | Ice Krill | Antarctic Silverfish | |||
| 2017/18 | Adélie Penguin | Cape Hallett | 68 (66–70) | 27 (24–30) | 2 (0.7–2.8) |
| 2018/19 | Adélie Penguin | Cape Hallett | 63 (60–65) | 31 (28–34) | 3 (1.2–4.5) |
| 2017/18 | Adélie Penguin | Inexpressible Island | 11 (8.6–13) | 47 (44–50) | 43 (41–44) |
| 2018/19 | Emperor Penguin | Cape Washington | 8 (5.6–9.9) | 15 (11–18) | 76 (73–78) |
| 2018/19 | Emperor Penguin | Coulman Island | 14 (11–16) | 11 (7.6–15) | 73 (70–75) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-Y.; Gal, J.-K.; Lee, B.-Y.; Son, W.-J.; Jung, J.-W.; La, H.-S.; Shin, K.-H.; Kim, J.-H.; Ha, S.-Y. Regional Differences in the Diets of Adélie and Emperor Penguins in the Ross Sea, Antarctica. Animals 2021, 11, 2681. https://doi.org/10.3390/ani11092681
Hong S-Y, Gal J-K, Lee B-Y, Son W-J, Jung J-W, La H-S, Shin K-H, Kim J-H, Ha S-Y. Regional Differences in the Diets of Adélie and Emperor Penguins in the Ross Sea, Antarctica. Animals. 2021; 11(9):2681. https://doi.org/10.3390/ani11092681
Chicago/Turabian StyleHong, Seo-Yeon, Jong-Ku Gal, Bo-Yeon Lee, Wu-Ju Son, Jin-Woo Jung, Hyung-Sul La, Kyung-Hoon Shin, Jeong-Hoon Kim, and Sun-Yong Ha. 2021. "Regional Differences in the Diets of Adélie and Emperor Penguins in the Ross Sea, Antarctica" Animals 11, no. 9: 2681. https://doi.org/10.3390/ani11092681
APA StyleHong, S.-Y., Gal, J.-K., Lee, B.-Y., Son, W.-J., Jung, J.-W., La, H.-S., Shin, K.-H., Kim, J.-H., & Ha, S.-Y. (2021). Regional Differences in the Diets of Adélie and Emperor Penguins in the Ross Sea, Antarctica. Animals, 11(9), 2681. https://doi.org/10.3390/ani11092681







