A Taxonomic Survey of Female Oviducal Glands in Chondrichthyes: A Comparative Overview of Microanatomy in the Two Reproductive Modes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Histological Procedures
2.3. Macroscopic and Microscopic Measurements and Statistical Analysis
3. Results
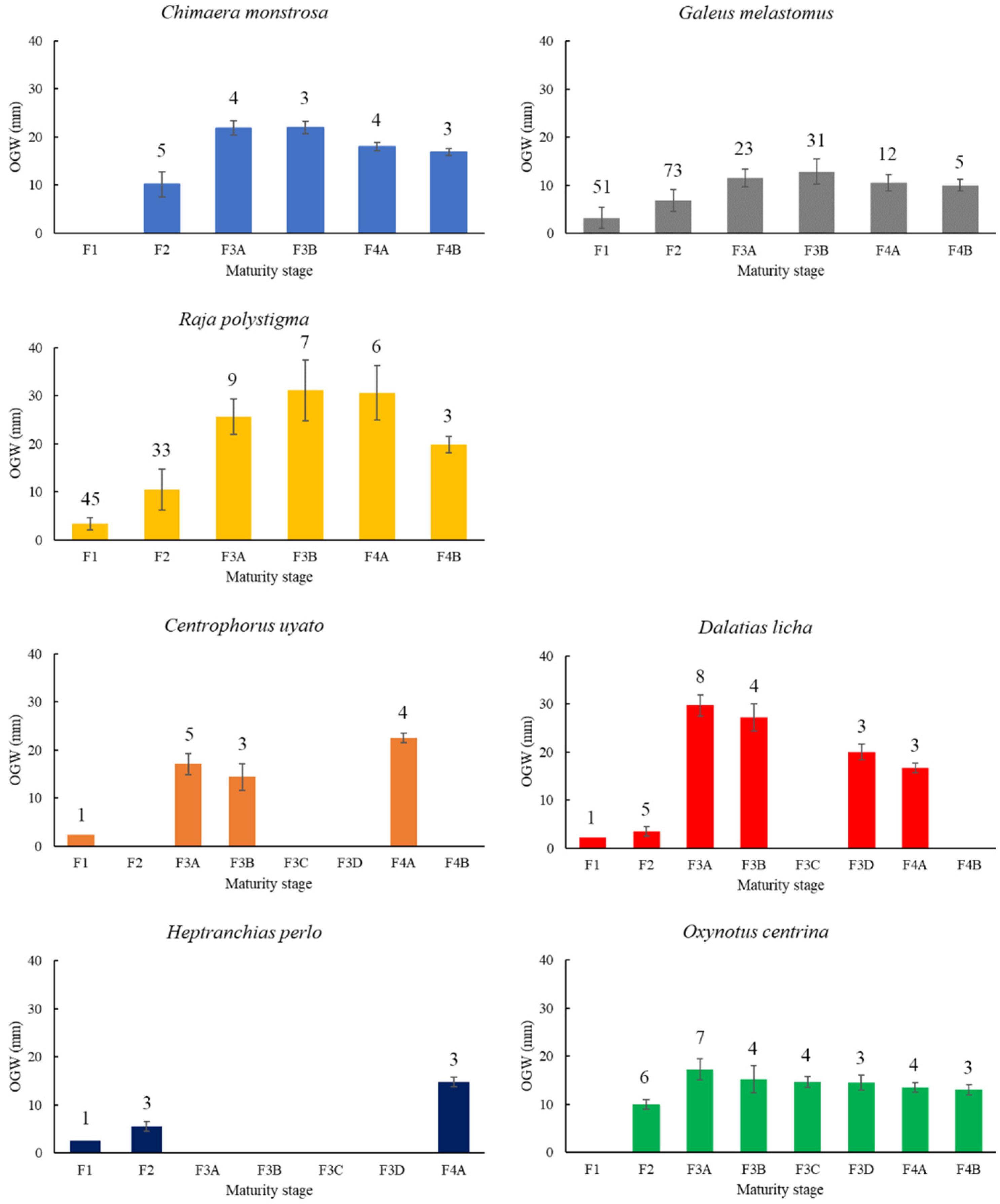
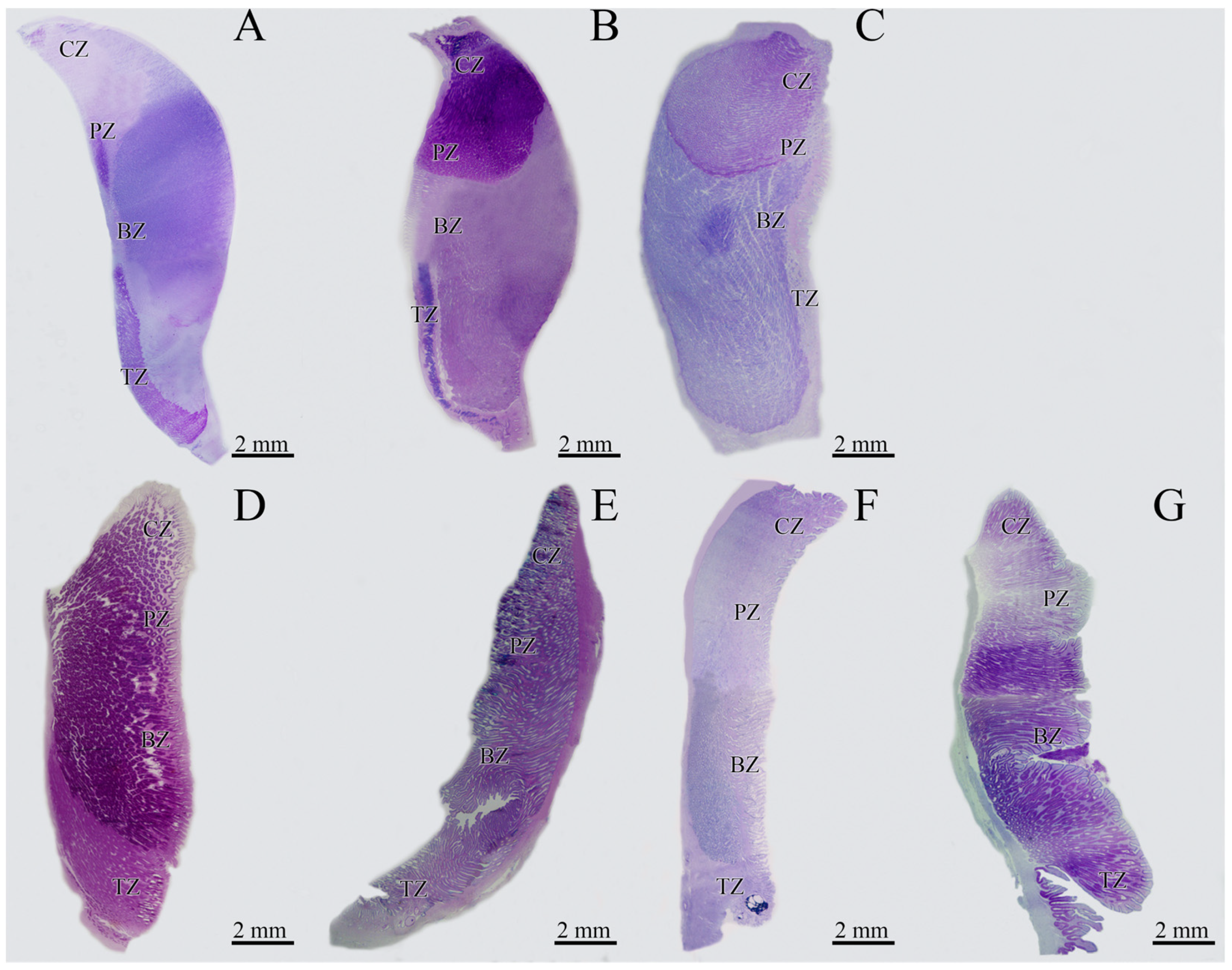
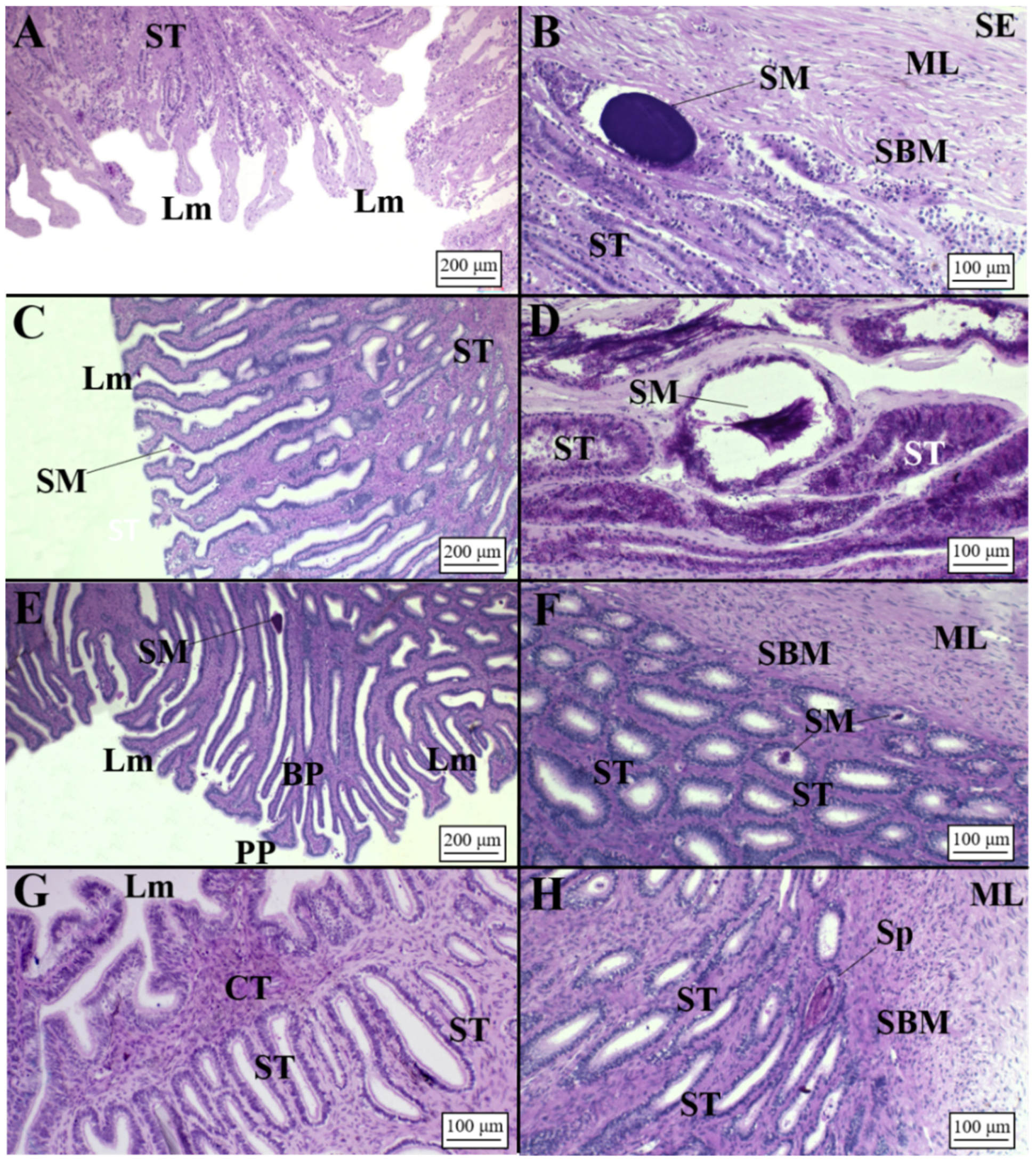
3.1. Macroscopic Development of the OG
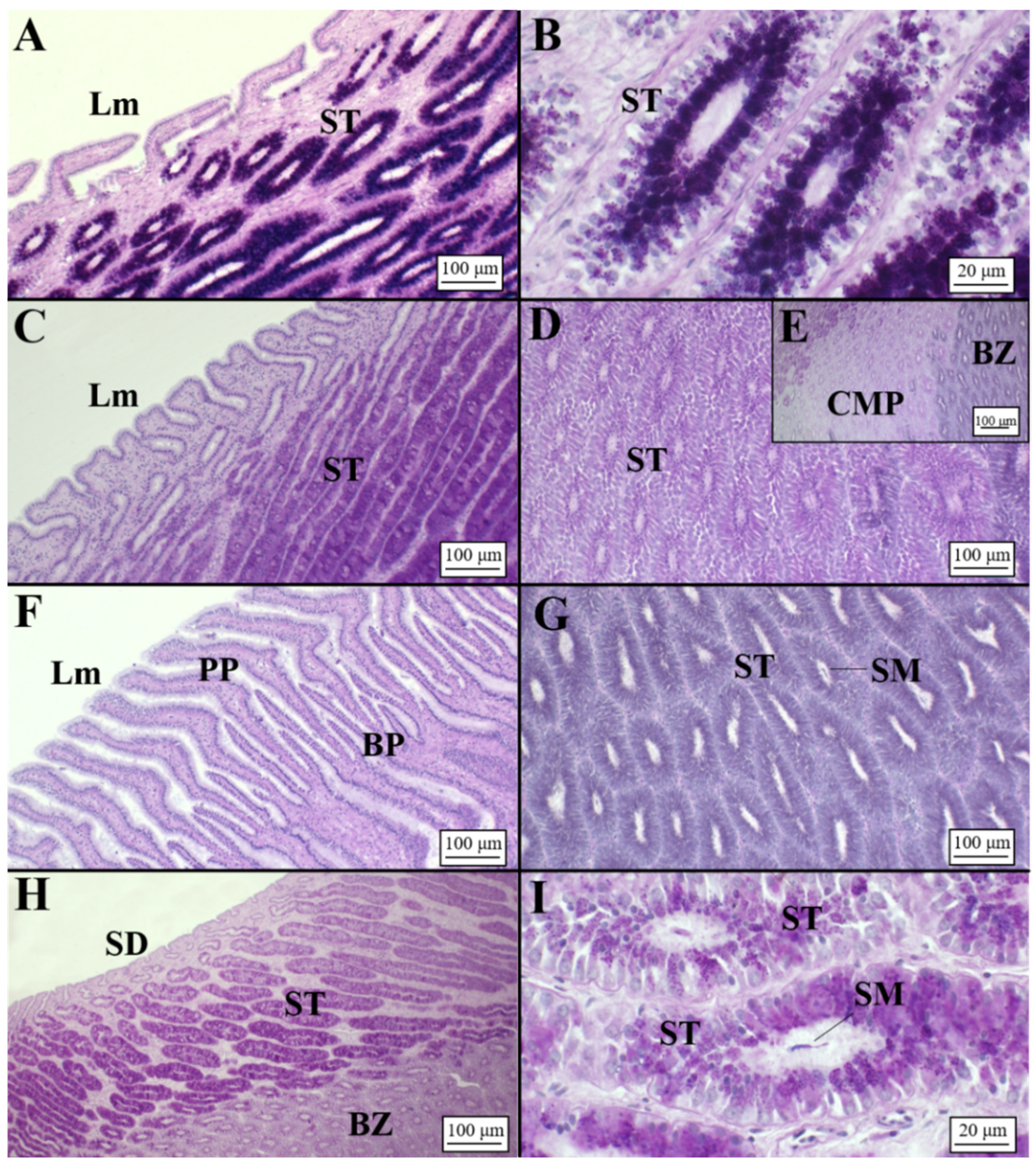
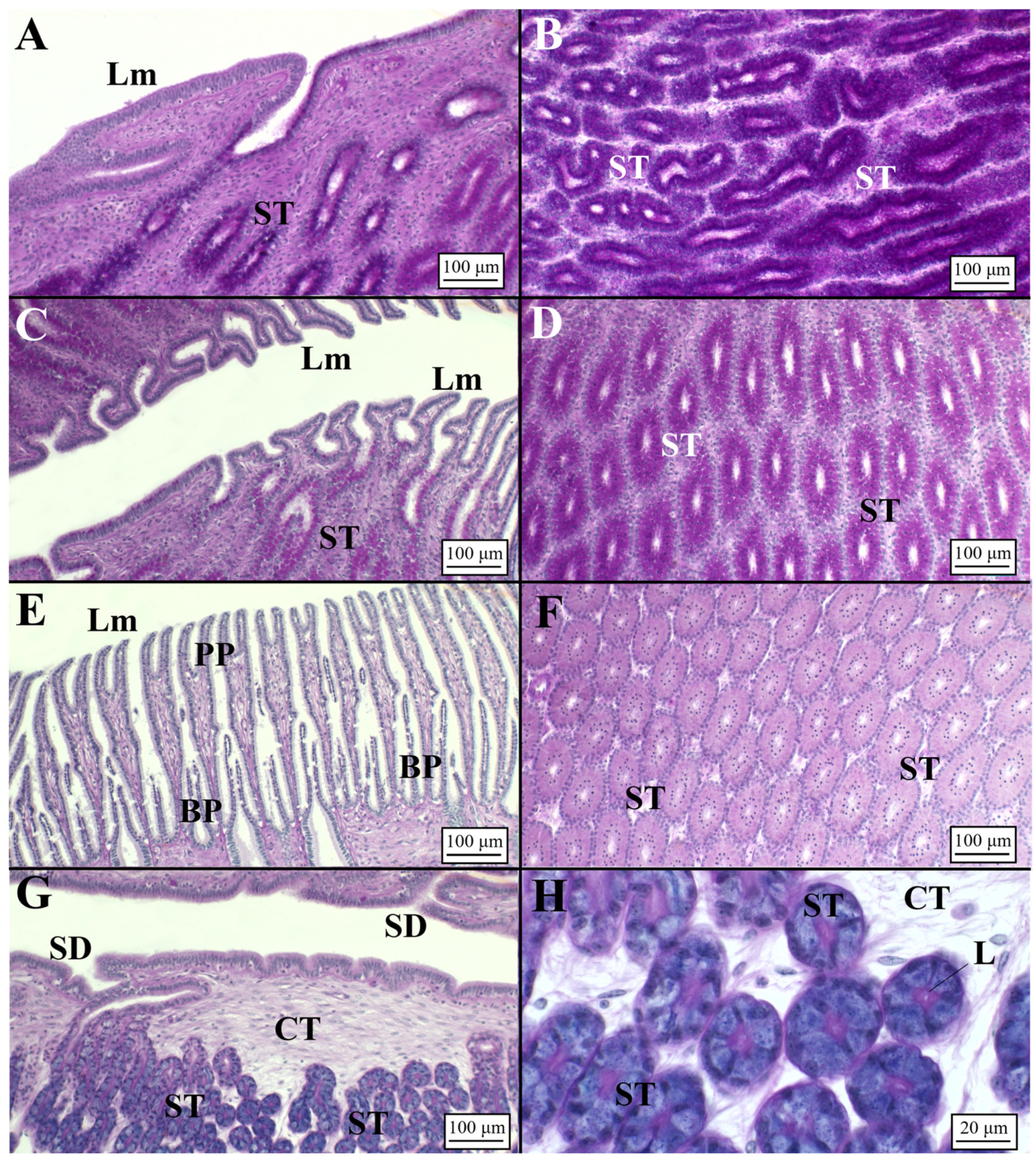
3.2. Microscopic Development of the OG
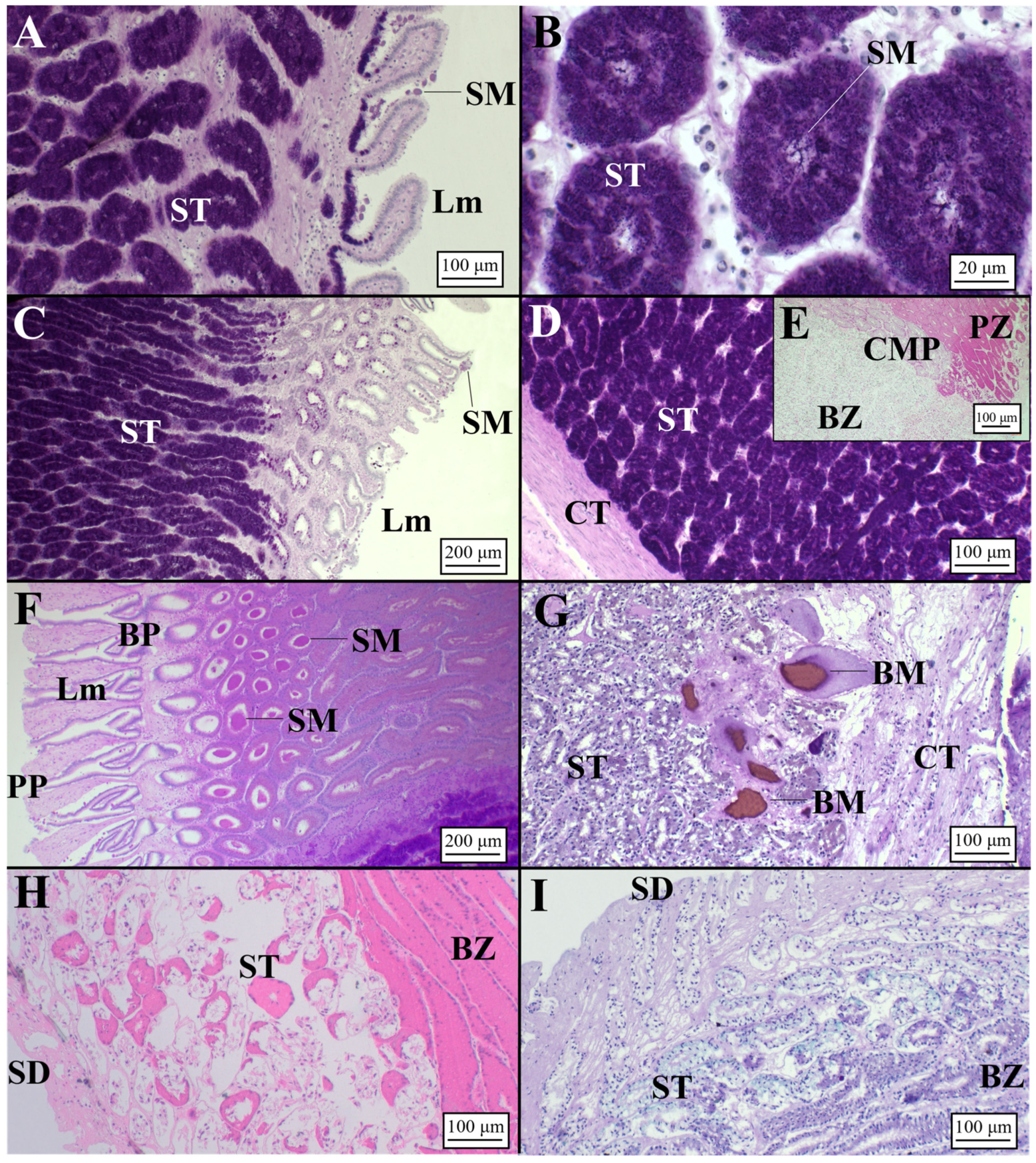
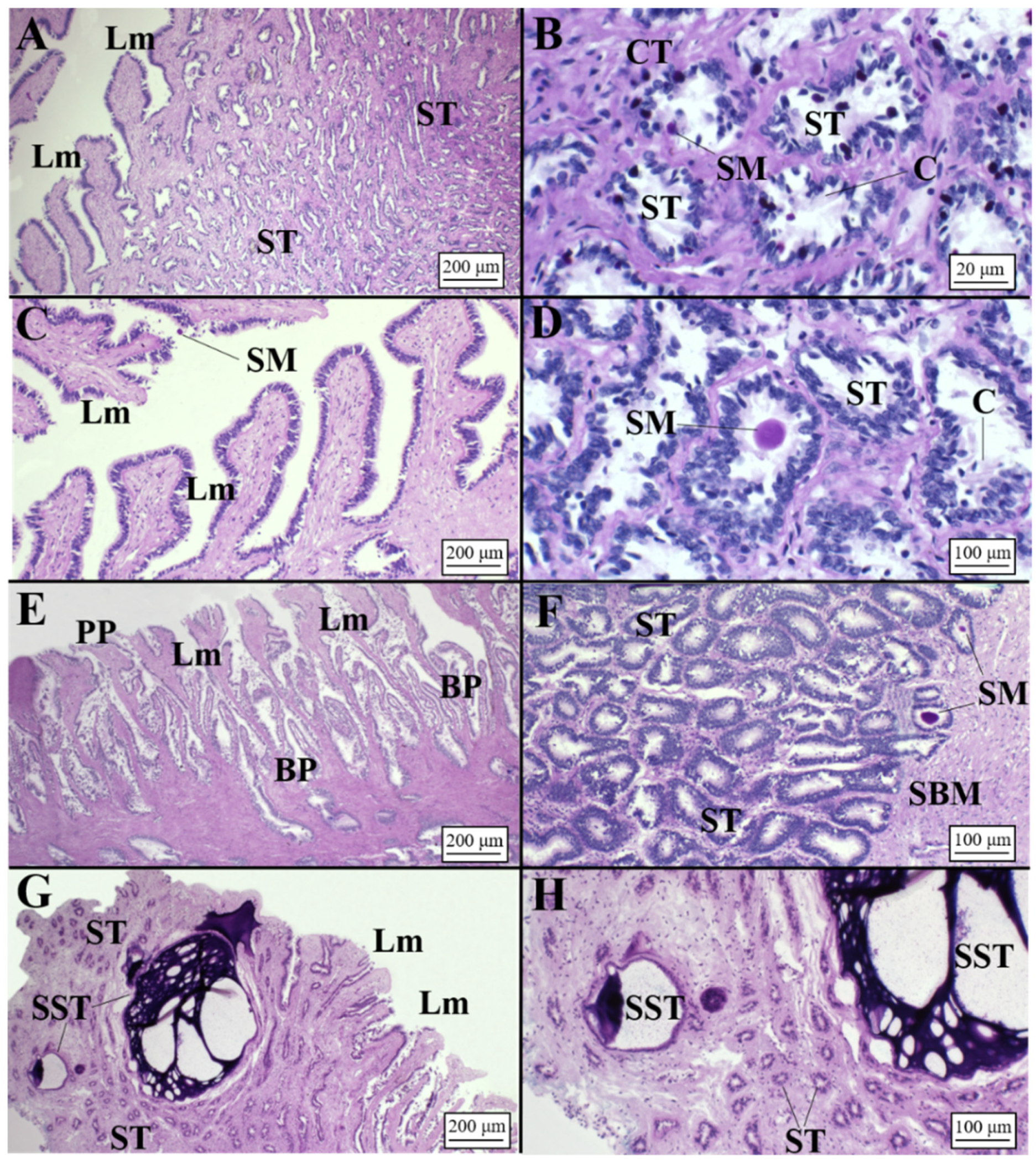
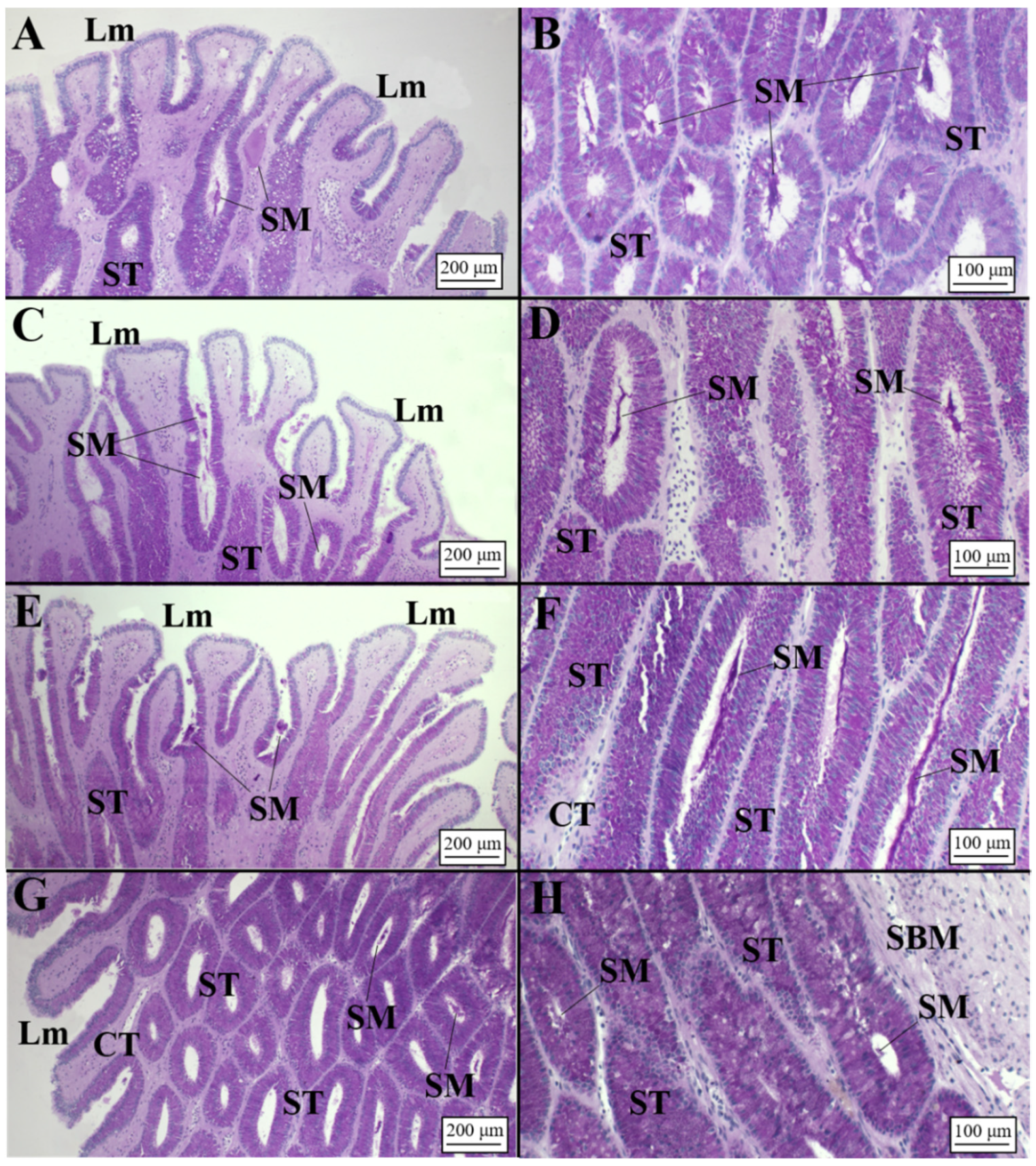
3.2.1. Oviparous Species
Chimaera monstrosa
Galeus melastomus
Raja polystigma
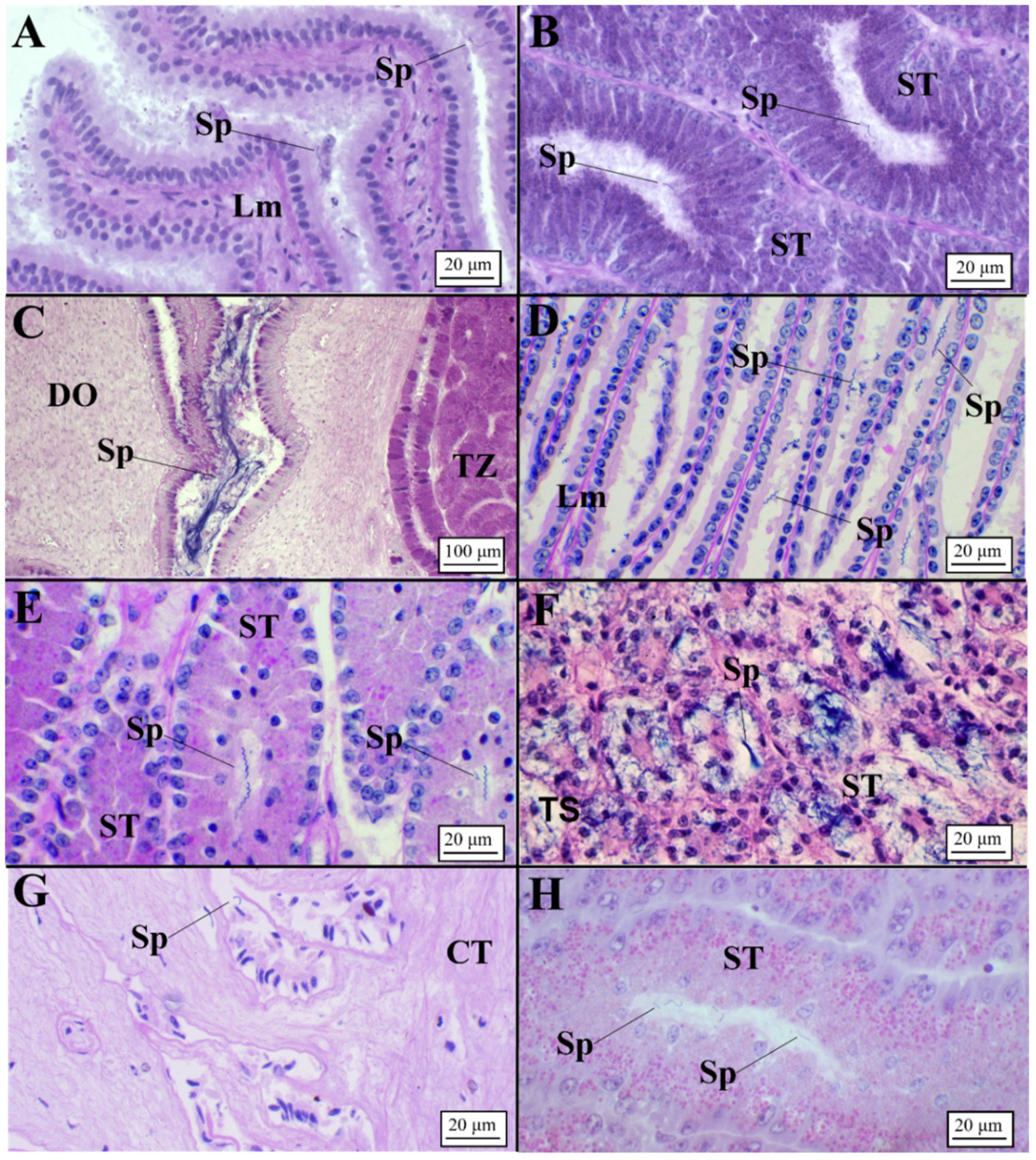
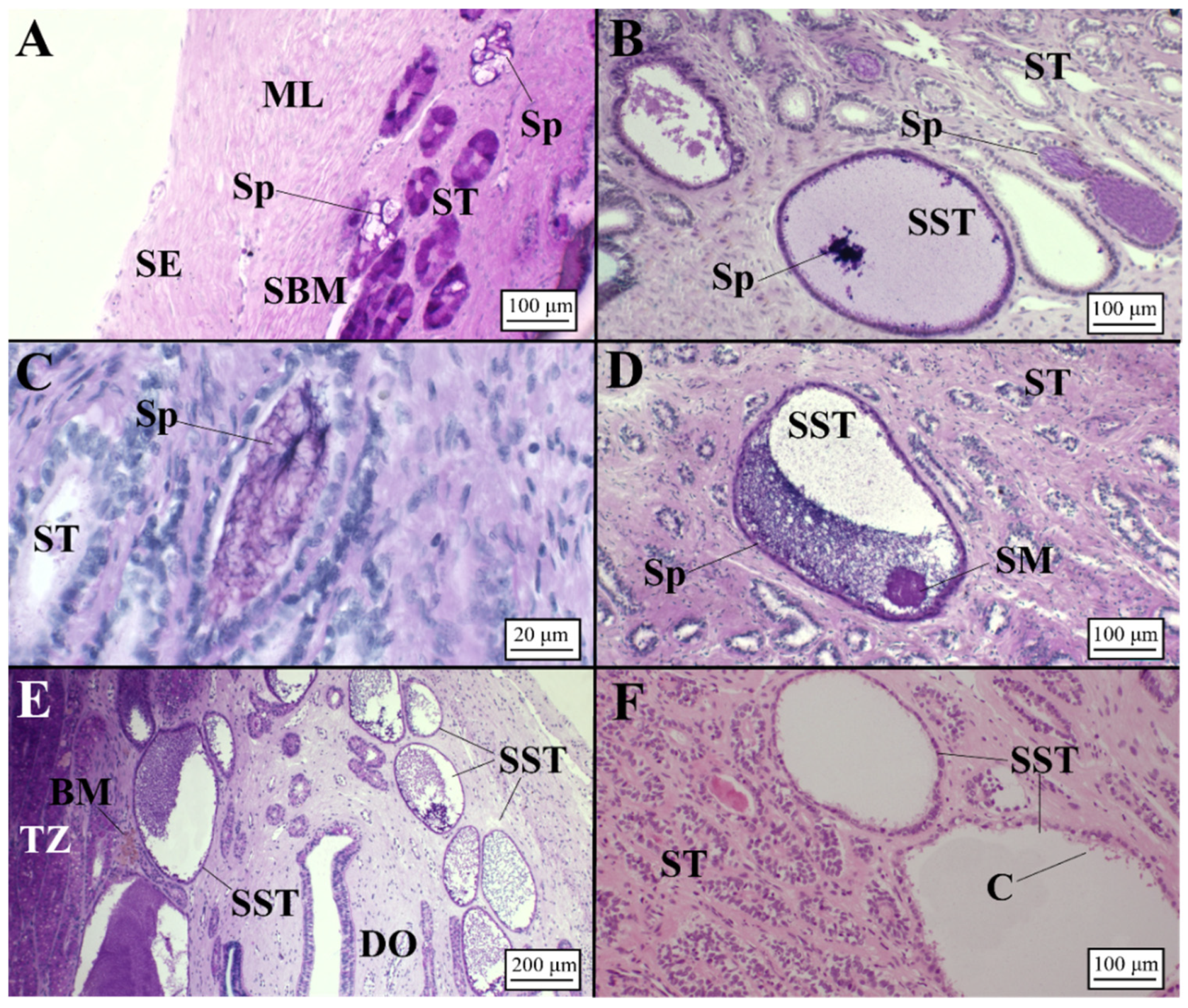
Presence of Sperm
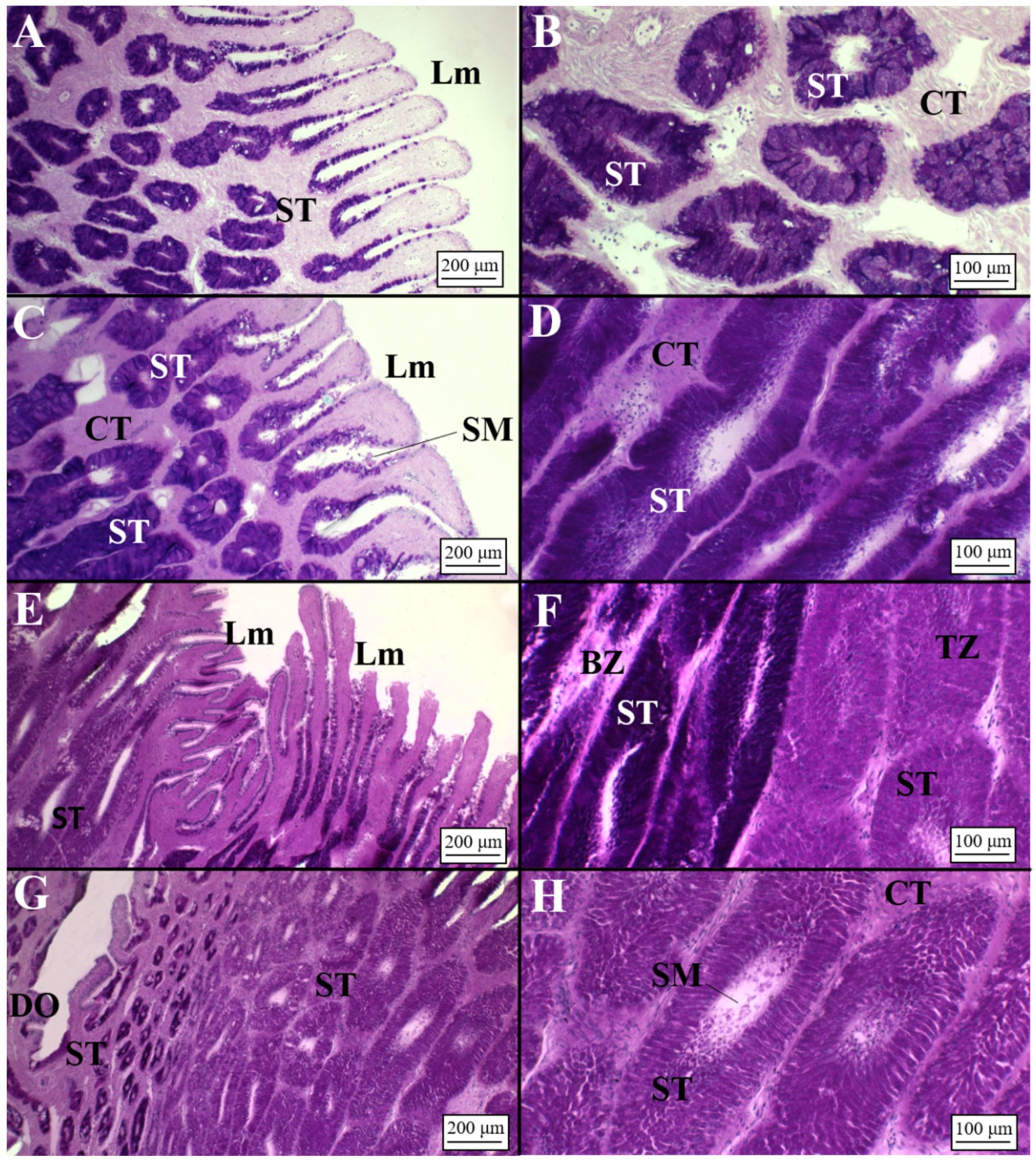
3.2.2. Viviparous Species
Centrophorus uyato
Dalatias licha
Heptranchias perlo
Oxynotus centrina
Presence of Sperm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conrath, C.L.; Musick, J.A. Reproductive Biology of Elasmobranchs. In Biology of Sharks and their Relatives, 2nd ed.; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 291–312. [Google Scholar]
- Grogan, D.E.; Lund, R.; Greenfest-Allen, E. The Origin and Relationships of Early Chondrichthyans. In Biology of Sharks and Their Relatives, 2nd ed.; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–29. [Google Scholar] [CrossRef]
- Awruch, C.A. Reproductive Strategies. In Physiology of Elasmobranch Fishes: Structure and Interaction with Environment; Shadwick, R.E., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 34A, Chapter 7; pp. 255–310. [Google Scholar]
- Nakaya, K.; White, W.T.; Ho, H.-C. Discovery of a new mode of oviparous reproduction in sharks and its evolutionary implications. Sci. Rep. 2020, 10, 12280. [Google Scholar] [CrossRef]
- Hamlett, W.C.; Koob, T.J. Female Reproductive System. In Sharks, Skates and Rays the Biology of Elasmobranch Fishes; Hamlett, W.C., Ed.; John Hopkins University Press: Baltimore, MD, USA, 1999; pp. 398–443. [Google Scholar]
- Carrier, J.C.; Pratt, H.L.; Castro, J.I. Elasmobranch Reproduction. In Biology of Sharks and their Relatives, 1st ed.; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 269–286. [Google Scholar]
- Jordan, R.P.; Graham, C.T.; Minto, C.; Henderson, A.C. Assessment of sperm storage across different reproductive modes in the elasmobranch fishes. Environ. Biol. Fish. 2021, 104, 27–39. [Google Scholar] [CrossRef]
- Prasad, R.R. The structure, phylogenetic significance, and function of the nidamental glands of some elasmobranchs of the Madras coast. Proc. Natl. Inst. Sci. India Part. B Biol. Sci. 1944, 11, 282–302. [Google Scholar]
- Colonello, J.H.; Christiansen, H.E.; Cousseau, M.B.; Macchi, G.J. Uterine dynamics of the southern eagle ray Myliobatis goodei (Chondrichthyes: Myliobatidae) from the southwest Atlantic Ocean. Ita. J. Zool. 2013, 80, 187–194. [Google Scholar] [CrossRef]
- Burgos-Vázquez, M.I.; Chávez-García, V.E.; Cruz-Escalona, V.H.; Navia, A.F.; Mejía-Falla, P.A. Reproductive strategy of the Pacific cownose ray Rhinoptera steindachneri in the southern Gulf of California. Mar. Freshwater Res. 2018, 70, 93–106. [Google Scholar] [CrossRef]
- Hamlett, W.C.; Hysell, M.K.; Jezior, M.; Rozycki, T.; Brunette, N.; Tumilty, K. Fundamental zonation in elasmobranch oviducal glands. In Proceedings of the 5th Indo-Pacific Fish Conference, Nouméa, Society of French Ichthyology, France, Paris, 3–8 November 1997; pp. 271–280. [Google Scholar]
- Metten, H. Studies on the reproduction of the dogfish. Philos. Trans. R. Soc. London Ser. B 1939, 230, 217–238. [Google Scholar]
- Knight, D.P.; Feng, D.; Stewart, M.; King, E. Changes in macromolecular organization in collagen assemblies during secretion in the nidamental gland and formation of the egg capsule wall in the dogfish Scyliorhinus canicula. Trans. R. Soc. Lond. Ser. B 1993, 341, 419–436. [Google Scholar]
- Serra-Pereira, B.; Afonso, F.; Farias, I.; Joyce, P.; Ellis, M.; Figueiredo, I.; Gordo, L.S. The development of the oviducal gland in the Rajid thornback ray, Raja clavata. Helgol. Mar. Res. 2011, 65, 399. [Google Scholar] [CrossRef]
- Marongiu, M.F.; Porcu, C.; Bellodi, A.; Cuccu, D.; Mulas, A.; Follesa, M.C. Oviducal gland microstructure of two Rajidae species, Raja miraletus and Dipturus oxyrinchus. J. Morphol. 2015, 76, 1392–1403. [Google Scholar] [CrossRef]
- Marongiu, M.F.; Porcu, C.; Bellodi, A.; Cannas, R.; Carbonara, P.; Cau, A.; Coluccia, E.; Moccia, D.; Mulas, A.; Pesci, P.; et al. Abundance, distribution and reproduction of the Data-Deficient species (Squalus blainville) around Sardinia Island (central western Mediterranean Sea) as a contribution to its conservation. Mar. Freshwater Res. 2020, 19372. [Google Scholar] [CrossRef]
- Hamlett, W.C.; Knight, D.P.; Koob, T.J.; Jezior, M.; Luong, T.; Rozycki, T.; Brunette, N.; Hysell, M.K. Survey of oviducal gland structure and function in elasmobranchs. J. Exp. Zool. 1998, 282, 399–420. [Google Scholar] [CrossRef]
- Storrie, M.T.; Walker, T.I.; Laurenson, L.J.; Hamlett, W.C. Microscopic organization of the sperm storage tubules in the oviducal gland of the female gummy shark (Mustelus antarcticus), with observations on sperm distribution and storage. J. Morphol. 2008, 269, 1308–1324. [Google Scholar] [CrossRef] [PubMed]
- Moura, T.; Serra-Pereira, B.; Gordo, L.S.; Figueiredo, I. Sperm storage in males and females of the deepwater Portuguese dogfish with notes on oviducal gland microscopic organization. J. Zool. 2011, 283, 210–219. [Google Scholar] [CrossRef]
- Porcu, C.; Marongiu, M.; Follesa, M.; Bellodi, A.; Mulas, A.; Pesci, P.; Cau, A. Reproductive aspects of the velvet belly lantern shark Etmopterus spinax (Condrichthyes: Etmopteridae), from the central western Mediterranean Sea. Notes on gametogenesis and oviducal gland microstructure. Mediterr. Mar. Sci. 2014, 15, 313–326. [Google Scholar] [CrossRef]
- Dutilloy, A.; Dunn, M.R. Observations of sperm storage in some deep-sea elasmobranchs. Deep-Sea Res. PT I 2020, 166, 103405. [Google Scholar] [CrossRef]
- Hamlett, W.C. Reproductive Biology and Phylogeny of Chondrichthyes. In Sharks, Batoids, and Chimaeras, 1st ed.; Hamlett, W.C., Ed.; CRC Press: Boca Raton, FL, USA, 2005; Volume 3, p. 576. [Google Scholar] [CrossRef]
- Galíndez, E.J.; Díaz-Andrade, M.C.; Avaca, M.S.; Estecondo, S. Morphological study of the oviductal gland in the smallnose fanskate Sympterygia bonapartii (Müller and Henle, 1841) (Chondrichthyes, Rajidae). Braz. J. Biol. 2010, 70, 325–333. [Google Scholar] [CrossRef]
- Moya, A.C.; Acuña, F.; Díaz Andrade, M.C.; Barbeito, C.G.; Galíndez, E.J. Glycan expression as a tool for a deeper understanding of a reproductive gland in a skate of economic importance. J. Fish. Biol. 2020, 98, 537–547. [Google Scholar] [CrossRef]
- Porcu, C.; Marongiu, M.F.; Bellodi, A.; Cannas, R.; Cau, A.; Melis, R.; Mulas, A.; Soldovilla, G.; Vacca, L.; Follesa, M.C. Morphological descriptions of the eggcases of skates (Rajidae) from the central–western Mediterranean, with notes on their distribution. Helgol. Mar. Res. 2017, 71, 10. [Google Scholar] [CrossRef]
- Orr, T.J.; Brennan, P.L.R. Sperm Storage and Delayed Fertilization. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 6, pp. 350–355. [Google Scholar]
- Holt, W.V.; Lloyd, R.E. Sperm storage in the vertebrate female reproductive tract: How does it work so well? Theriogenology 2010, 73, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Pratt, H.L. The storage of spermatozoa in the oviducal glands of western North Atlantic sharks. Environ. Biol. Fish. 1993, 38, 139–149. [Google Scholar] [CrossRef]
- Otero, M.D.; Serena, F.; Gerovasileiou, V.; Barone, M.; Bo, M.; Arcos, J.M.; Vulcano, A.; Xavier, J. Identification Guide of Vulnerable Species Incidentally Caught in Mediterranean Fisheries; IUCN: Malaga, Spain, 2019; 203p. [Google Scholar]
- Dulvy, N.K.; Allen, D.J.; Ralph, G.M.; Walls, R.H.L. The Conservation Status of Sharks, Rays and Chimaeras in the Mediterranean Sea; IUCN: Malaga, Spain, 2016. [Google Scholar]
- Cortés, E. Incorporating uncertainty into demographic modeling: Application to shark populations and their conservation. Conserv. Biol. 2002, 16, 1048–1062. [Google Scholar] [CrossRef]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Frisk, M.G.; Miller, T.J.; Fogarty, M.J. Estimation and analysis of biological parameters in elasmobranch fishes: A comparative life history study. Can. J. Fish. Aquat. Sci. 2001, 58, 969–981. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Massutí, E.; Mérigot, B.; Tserpes, G.; Jadaud, A.; Relini, G. The MEDITS trawl survey specifications in an ecosystem approach to fishery management. Sci. Mar. 2019, 83, 9–20. [Google Scholar] [CrossRef]
- Follesa, M.C.; Agus, B.; Bellodi, A.; Cannas, R.; Capezzuto, F.; Casciaro, L.; Cau, A.; Cuccu, D.; Donnaloia, M.; Fernandez-Arcaya, U. The MEDITS maturity scales as a useful tool for investigating the reproductive traits of key species in the Mediterranean Sea. Sci. Mar. 2019, 83S1, 235–256. [Google Scholar] [CrossRef]
- Cerri, P.S.; Sasso-Cerri, E. Staining methods applied to glycol methacrylate embedded tissue sections. Micron 2003, 34, 365–372. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999; 663p. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, F.J. TpsDig, Digitize Landmarks and Outlines, Version 2.16. In Department of Ecology and Evolution; State University of New York at Stony Brook: New York, NY, USA, 2005. [Google Scholar]
- Marongiu, M.F.; Porcu, C.; Bellodi, A.; Cannas, R.; Cau, A.; Cuccu, D.; Mulas, A.; Follesa, M.C. Temporal dynamics of demersal chondrichthyan species in the central western Mediterranean Sea: The case study in Sardinia Island. Fish. Res. 2017, 193, 81–94. [Google Scholar] [CrossRef]
- Follesa, M.C.; Marongiu, M.F.; Zupa, W.; Bellodi, A.; Cau, A.; Cannas, R.; Colloca, F.; Djurovic, M.; Isajlovic, I.; Jadaud, A.; et al. Spatial variability of Chondrichthyes in the northern Mediterranean. Sci. Mar. 2019, 83, 81–100. [Google Scholar] [CrossRef]
- Mancusi, C.; Massi, D.; Baino, R.; Cariani, A.; Crobe, V.; Ebert, D.A.; Ferrari, A.; Gordon, C.A.; Hoff, G.R.; Iglesias, S.P.; et al. An identification key for Chondrichthyes egg cases of the Mediterranean and Black Sea. Eur. Zool. 2021, 88, 436–448. [Google Scholar] [CrossRef]
- Smith, R.M.; Walker, T.I.; Hamlett, W.C. Microscopic organisation of the oviducal gland of the holocephalan elephant fish, Callorhynchus milii. Mar. Freshw. Res. 2004, 55, 155–164. [Google Scholar] [CrossRef]
- Cox, G.; Francis, M. Sharks and Rays of New Zealand; Canterbury University Press: Christchurch, New Zealand, 1997; 68p. [Google Scholar]
- Porcu, C.; Marongiu, M.F.; Olita, A.; Bellodi, A.; Cannas, R.; Carbonara, P.; Cau, A.; Mulas, A.; Pesci, P.; Follesa, M.C. The demersal bathyal fish assemblage of the Central-Western Mediterranean: Depth distribution, sexual maturation and reproduction. Deep-Sea Res. PT I 2020, 166, 103394. [Google Scholar] [CrossRef]
- Galíndez, E.J.; Estecondo, S. Histological remarks of the oviduct and the oviducal gland of Sympterygia acuta Garman, 1877. Braz. J. Biol. 2008, 68, 359–365. [Google Scholar] [CrossRef]
- Porcu, C.; Bellodi, A.; Cau, A.; Cannas, R.; Marongiu, M.F.; Mulas, A.; Follesa, M.C. Uncommon biological patterns of a little known endemic Mediterranean skate, Raja polystigma (Risso, 1810). Reg. Stud. Mar. Sci. 2020, 34, 101065. [Google Scholar] [CrossRef]
- Dean, B. Fishes, Living and Fossil. An Outline of Their Forms and Probable Relationships; Macmillan and Co.: New York, NY, USA, 1895. [Google Scholar]
- Dean, B. Chimaeroid Fishes and Their Development; Carnegie Institution of Washington: Washington, DC, USA, 1906. [Google Scholar]
- Awruch, C.A. Reproductive endocrinology in chondrichthyans: The present and the future. Gen. Comp. Endocrinol. 2013, 192, 60–70. [Google Scholar] [CrossRef]
- Hamlett, W.C.; Fishelson, L.; Baranes, A.; Hysell, C.K.; Sever, D.M. Ultrastructural analysis of sperm storage and morphology of the oviducal gland in the Oman shark, Iago omanensis (Triakidae). Mar. Freshwater Res. 2002, 53, 601–613. [Google Scholar] [CrossRef]
- Bernal, M.A.; Sinai, N.L.; Rocha, C.; Gaither, M.R.; Dunker, F.; Rocha, L.A. Long-term sperm storage in the brownbanded bamboo shark Chiloscyllium punctatum. J. Fish. Biol. 2015, 86, 1171–1176. [Google Scholar] [CrossRef]
- Kimley, A.P. The determinants of sexual segregation in the scalloped hammerhead shark, Sphyrna lewini. Environ. Biol. Fish. 1987, 18, 27–40. [Google Scholar] [CrossRef]
- Economakis, A.E.; Lobel, P.S. Aggregation behavior of the grey reef shark, Carcharhinus amblyrhynchos, at Johnston Atoll, Central Pacific Ocean. Environ. Biol. Fish. 1998, 51, 129–139. [Google Scholar] [CrossRef]
- Finucci, B.; Dunn, M.R.; Jones, E.G. Aggregations and associations in deep-sea chondrichthyans. ICES J. Mar. Sci. 2018, 75, 1613–1626. [Google Scholar] [CrossRef]
- Figueiredo, I.; Moura, T.; Neves, A.; Gordo, L.S. Reproductive strategy of leafscale gulper shark Centrophorus squamosus and the Portuguese dogfish Centroscymnus coelolepis on the Portuguese continental slope. J. Fish. Biol. 2008, 73, 206–225. [Google Scholar] [CrossRef]
- Pratt, H.; Carrier, J.C. A Review of Elasmobranch Reproductive Behavior with a Case Study on the Nurse Shark, Ginglymostoma cirratum. Environ. Biol. Fish. 2001, 60, 157–188. [Google Scholar] [CrossRef]
- Sims, D.W. Differences in Habitat Selection and Reproductive Strategies of Male and Female Sharks. In Sexual Segregation in Vertebrates: Ecology of the Two Sexes; Ruckstuhl, K.E., Neuhaus, P., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 127–147. [Google Scholar]
- Finucci, B.; Duffy, C.A.J.; Francis, M.P.; Gibson, C.; Kyne, P.M. The extinction risk of New Zealand chondrichthyans. Aquat. Conserv. 2019, 29, 783–797. [Google Scholar] [CrossRef]
| Reproduction Mode | Order | Family | Species | Depth Range (m) | Size Range (cm) | IUCN Status |
|---|---|---|---|---|---|---|
| Oviparity | Carcharhiniformes | Pentanchidae | Galeus melastomus (Rafinesque, 1810) | 100–730 | 6.9–89.7 † | LC |
| Chimaeriformes | Chimaeridae | Chimaera monstrosa (Linnaeus, 1758) | 320–1132 | 3.6–27.8 * | NT | |
| Rajiformes | Rajidae | Raja polystigma (Regan, 1923) | 33–600 | 15.6–59.3 † | LC | |
| Viviparity (yolk-sac) | Hexanchiformes | Hexanchidae | Heptranchias perlo (Bonnaterre, 1788) | 336–600 | 54.5–113.3 † | DD |
| Squaliformes | Centrophoridae | Centrophorus uyato (Rafinesque, 1810) | 445–600 | 38.7–106.5 † | CR | |
| Dalatidae | Dalatias licha (Bonnaterre, 1788) | 300–800 | 26.2–108.6 † | VU | ||
| Oxynotidae | Oxynotus centrina (Linnaeus, 1758) | 175–600 | 25.6–78.2 † | CR |
| Species | Club | Papillary | Baffle | Terminal | ||||
|---|---|---|---|---|---|---|---|---|
| mm2 | % | mm2 | % | mm2 | % | mm2 | % | |
| C. monstrosa | 12.8–13.9 | 13.2–13.6 | 13.0–14.2 | 13.3–13.8 | 63.0–67.2 | 63.1–66.4 | 8.5–9.3 | 8.2–9.0 |
| G. melastomus | 10.1–11.2 | 18.0–19.5 | 4.9–6.3 | 9.9–10.8 | 34.7–35.9 | 60.1–65.0 | 4.1–4.9 | 7.8–8.5 |
| R. polystigma | 7.2–8.4 | 15.0–16.2 | 7.3–8.2 | 15.0–16.2 | 28.6–34.3 | 61.1–66.7 | 1.9–2.3 | 4.2–4.8 |
| C. uyato | 18.2–21.9 | 20.6–220 | 23.5 | 23.2 | 38.2 | 37.7 | 18.1 | 17.9 |
| D. licha | 3.8–5.1 | 10.6–11.9 | 9.6–10.7 | 24.5–26.0 | 21.5–22.9 | 55.1–56.9 | 1.9–2.9 | 5.8–6.9 |
| H. perlo | 14.9 | 27.8 | 12.4 | 23.1 | 20.7 | 38.5 | 5.6 | 10.6 |
| O. centrina | 9.9–11.0 | 13.2–15.1 | 8.6–10.4 | 12.2–14.0 | 35.4–36.9 | 48.5–50.8 | 14.4–15.7 | 20.4–21.9 |
| Species | Club | Papillary | Baffle | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± S.D. | Min–Max | N | Mean ± S.D. | Min–Max | N | Mean ± S.D. | Min–Max | |
| C. monstrosa | 37 | 94.3 ± 45.5 | 56.3–235.4 | 33 | 119.6 ± 19.9 | 89.5–169.5 | 34 | 407.7 ± 88.0 | 264.5–537.8 |
| G. melastomus | 15 | 177.7 ± 34.4 | 128.9–212.7 | 8 | 108.8 ± 22.3 | 63.3–141.2 | 28 | 506.6 ± 29.5 | 476.3–550.9 |
| R. polistygma | 14 | 296.9 ± 165.3 | 101.4–786.9 | 12 | 252.8 ± 59.7 | 158.5–373.1 | 22 | 584.3 ± 105.5 | 399.4–792.4 |
| C. uyato | 31 | 857.9 ± 69.0 | 725.7–982.2 | 14 | 828.8 ± 207.6 | 503.7–1407.1 | 43 | 304.6 ± 151.7 | 136.8–572.4 |
| D. licha | 17 | 1555.5 ± 233.2 | 1046.6–1701.0 | 26 | 1804.4 ± 38.2 | 1756.2–1892.3 | 56 | 1013.0 ± 452.4 | 191.4–1962.8 |
| H. perlo | 19 | 547.7 ± 174.5 | 193.2–803.0 | 21 | 442.6 ± 88.9 | 307.8–575.6 | 22 | 982.8 ± 327.9 | 534.7–1459.2 |
| O. centrina | 16 | 532.4 ± 92.5 | 368.5–617.4 | 16 | 695.8 ± 180.4 | 519.0–1167.4 | 44 | 888.0 ± 200.7 | 600.0–1239.6 |
| Species | Club | Papillary | Baffle | Terminal | ||||
|---|---|---|---|---|---|---|---|---|
| Min–Max | Mean ± S.D. | Min–Max | Mean ± S.D. | Min–Max | Mean ± S.D. | Min–Max | Mean ± S.D. | |
| C. monstrosa | 71.0–354.5 | 193.6 ± 67.8 | 82.3–566.6 | 228.5 ± 86.3 | 103.8–443.6 | 191.5 ± 60.3 | 89.0–614.2 | 267.9 ± 115.5 |
| G. melastomus | 65.1–332.4 | 132.0 ± 82.7 | 126.1–353.9 | 175.4 ± 46.4 | 174.0–429.1 | 262.4 ± 89.0 | 39.3–140.8 | 73.9 ± 22.1 |
| R. polistygma | 64.9–408.2 | 179.1 ± 75.1 | 64.4–483.0 | 169.9 ± 85.1 | 101.8–768.4 | 223.8 ± 106.9 | 52.0–428.6 | 119.0 ± 84.4 |
| C. uyato | 136.9–509.8 | 279.8 ± 70.8 | 140.7–1022.5 | 353.0 ± 122.9 | 190.8–1027.3 | 513.5 ± 179.8 | 84.2–1141.9 | 174.5 ± 166.2 |
| D. licha | 58.1–568.8 | 194.7 ± 108.7 | 89.4–683.8 | 269.2 ± 137.3 | 59.7–539.7 | 198.7 ± 84.7 | 63.0–434.1 | 176.7 ± 88.8 |
| H. perlo | 43.1–496.5 | 161.7 | 58.0–335.5 | 117.5 ± 41.7 | 60.2–343.1 | 128.9 ± 60.4 | 30.9–198.1 | 71.59 ± 36.0 |
| O. centrina | 173.3–1133.6 | 432.9 ± 195.9 | 222.3–971.0 | 444.0 ± 177.9 | 143.6–1230.9 | 494.6 ± 264.4 | 281.9–869.6 | 461.8 ± 130.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marongiu, M.F.; Porcu, C.; Pascale, N.; Bellodi, A.; Cau, A.; Mulas, A.; Pesci, P.; Porceddu, R.; Follesa, M.C. A Taxonomic Survey of Female Oviducal Glands in Chondrichthyes: A Comparative Overview of Microanatomy in the Two Reproductive Modes. Animals 2021, 11, 2653. https://doi.org/10.3390/ani11092653
Marongiu MF, Porcu C, Pascale N, Bellodi A, Cau A, Mulas A, Pesci P, Porceddu R, Follesa MC. A Taxonomic Survey of Female Oviducal Glands in Chondrichthyes: A Comparative Overview of Microanatomy in the Two Reproductive Modes. Animals. 2021; 11(9):2653. https://doi.org/10.3390/ani11092653
Chicago/Turabian StyleMarongiu, Martina Francesca, Cristina Porcu, Noemi Pascale, Andrea Bellodi, Alessandro Cau, Antonello Mulas, Paola Pesci, Riccardo Porceddu, and Maria Cristina Follesa. 2021. "A Taxonomic Survey of Female Oviducal Glands in Chondrichthyes: A Comparative Overview of Microanatomy in the Two Reproductive Modes" Animals 11, no. 9: 2653. https://doi.org/10.3390/ani11092653
APA StyleMarongiu, M. F., Porcu, C., Pascale, N., Bellodi, A., Cau, A., Mulas, A., Pesci, P., Porceddu, R., & Follesa, M. C. (2021). A Taxonomic Survey of Female Oviducal Glands in Chondrichthyes: A Comparative Overview of Microanatomy in the Two Reproductive Modes. Animals, 11(9), 2653. https://doi.org/10.3390/ani11092653










