Feeding Marine Polysaccharides to Alleviate the Negative Effects Associated with Weaning in Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Negative Biological Effects Associated with Weaning
3. Traditional and Alternative Dietary Interventions
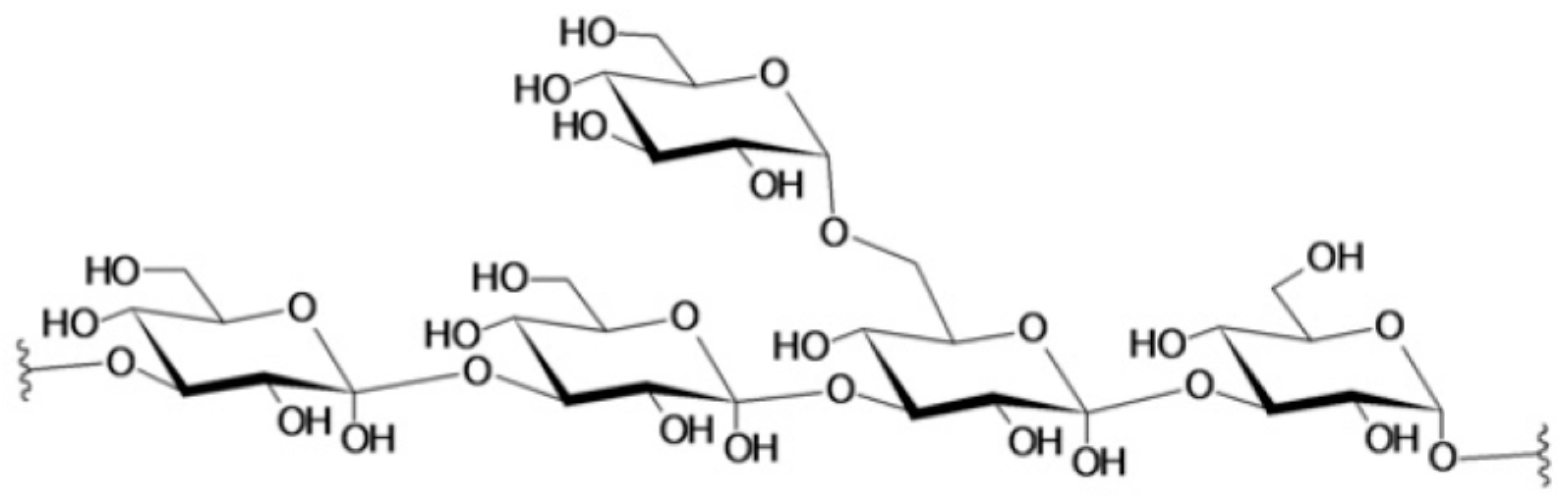
4. Marine Polysaccharides
| Pig Age | Dietary Supplement | Dose | Time and Duration of Supplementation | Effect on Growth Performance and Diarrhoea Scores | Effect on Parameters of GIT Functionality and Health | Ref. |
|---|---|---|---|---|---|---|
| Weaned pigs | ||||||
| 24-day-old | Laminarin (Laminaria spp.) Fucoidan (Laminaria spp.) Laminarin + Fucoidan | 300 mg/kg 240 mg/kg 300 mg/kg + 240 mg/kg | After weaning for 21 days | + ADG and G:F in pigs fed laminarin-supplemented diets + ADG in pigs fed with diet supplemented solely with fucoidan (interaction) − diarrhoea score in pigs fed laminarin-supplemented diets | − faecal E. coli in pigs fed laminarin-supplemented diets + faecal Lactobacillus spp. in pigs fed with diet supplemented solely with fucoidan (interaction) | [49] |
| 24-day-old | Laminarin (Laminaria spp.) Fucoidan (Laminaria spp.) Laminarin + Fucoidan | 150 or 300 mg/kg 240 mg/kg 150 or 300 mg/kg + 240 mg/kg | After weaning for 35 days | + ADG in pigs fed 300 mg/kg laminarin-supplemented diets + G:F in pigs fed with diet supplemented solely with 300 mg/kg laminarin or fucoidan (interaction) − FS in pigs fed 150 or 300 mg/kg laminarin-supplemented diets and in pigs fed with diet supplemented solely with fucoidan (interaction) | + faecal Lactobacillus spp. in pigs fed fucoidan-supplemented diets 0 faecal E. coli, Bifidobacterium spp. | [50] |
| 28-day-old | 65% laminarin-rich extract (Laminaria spp.) | 300 mg/kg | After weaning for 14 days | + ADG, ADFI 0 diarrhoea score | + VH in duodenum and jejunum and CD in jejunum − Enterobacteriaceae in caecum + Lactobacillus spp. in colon + butyrate in colon + gene expression of nutrient transporters in small intestine and colon − gene expression of tight junction proteins, mucins and immune markers in small intestine and colon | [51] |
| 35-day-old | Dried seaweed (Ocean Harvest Technology) containing laminarin, fucoidan, alginate, mannitol, fucoxanthin and rhamnose sulphate. | 1500 mg/kg | After weaning for 52 days | 0 ADG, ADFI, G:F 0 diarrhoea score | − VH in jejunum | [53] |
| 35-day-old | Dried sea weed (Ascophyllum nodosum) | 2.5 g/kg 5 g/kg 10 g/kg | After weaning for 28 days | − ADG | ND | [55] |
| Finisher pigs | Dried seaweed extract (Ascophyllum nodosum) containing laminarin, fucoidan, alginate, mannitol, fucoxanthin and rhamnose sulphate. | 3 g/kg 6 g/kg 9 g/kg | After weaning for 28 days | − ADG 0 ADFI, G:F | ND | [56] |
| 28-day-old | 65% laminarin-rich extract (Laminaria spp.) | 300 mg/kg | After weaning for 14 days | + ADG, ADFI 0 diarrhoea score | − abundance of OTUs assigned to Enterobacteriaceae + abundance of OTUs assigned to the genus Prevotella | [64] |
| 24-day-old | Laminarin (Laminaria spp.) Fucoidan (Laminaria spp.) Laminarin + Fucoidan | 300 mg/kg 240 mg/kg 300 mg/kg + 240 mg/kg | After weaning for 8 days | ND | − Enterobacteriaceae population in pigs offer fucoidan (interaction). − AEEC strains in pigs offer laminarin (interaction). + VH and VH:CD ratio in pigs offered laminarin or fucoidan (interaction). − IL-6, IL-17A and IL-1b mRNA expression in pigs offered laminarin | [65] |
| 24-day-old | Laminarin (Laminaria spp.) | After weaning for 8 days | + ADG and ADFI − diarrhoea score | ND | [66] | |
| 24-day-old | Laminarin (Laminaria spp.) | 0 mg/kg 240 mg/kg ZnO | After weaning for 32 days | + ADG and G:F, similar effect to ZnO | + digestibility of GE + the expression of glucose transporters in small intestine compared with the basal diet. | [67] |
| 24-day-old | 44% fucoidan-rich extract (Laminaria spp.) | 0 mg/kg 125 mg/kg 250 mg/kg | After weaning for 14 days | − diarrhoea score 0 ADG, ADFI and G:F | 0 effect on VH − abundance of Prevotella and Lachnospiraceae + the abundance of Helicobacter | [68] |
5. Laminarin
5.1. Antibacterial Activity
5.2. Prebiotic Activity
5.3. Immunomodulatory Activity
5.4. Effects of Laminarin-Rich Extracts on Pig GIT Functionality
6. Fucoidan
6.1. Antibacterial Activity
6.2. Prebiotic Activity
6.3. Immunomodulatory Activity
6.4. Effects of Fucoidan-Rich Extracts on Pig Performance and GIT Functionality
7. Laminarin and Fucoidan Interaction
8. Feeding Seaweed Extracts to the Pregnant and Lactating Sow
9. Chitin and Its Derivatives
9.1. Antibacterial Effects of Chitosan and COS
9.2. Effects of COS on Growth Performance
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- McCracken, B.A.; Spurlock, M.E.; Roos, M.A.; Zuckermann, F.A.; Gaskins, H.R. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J. Nutr. 1999, 129, 613–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, G.Z.; Pluske, J.R. The low feed intake in newly-weaned pigs: Problems and possible solutions. Asian-Australas J. Anim. Sci. 2007, 20, 440–452. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.A.; Verstegen, M.W.; Tamminga, S. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr. Res. Rev. 2001, 14, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.; Blikslager, A.T.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver. Physiol. 2010, 298, G352–G363. [Google Scholar] [CrossRef] [Green Version]
- Shannon, M.C.; Hill, G.M. Trace mineral supplementation for the intestinal health of young monogastric animals. Front. Vet. Sci. 2019, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Redondo, L.M.; Chacana, P.A.; Dominguez, J.E.; Fernandez Miyakawa, M.E. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 2014, 5, 118. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, T.; O’Doherty, J.V. Marine macroalgal extracts to maintain gut homeostasis in the weaning piglet. Domest. Anim. Endocrinol. 2016, 56, S84–S89. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Weaning—A challenge to gut physiologists. Livest. Sci. 2007, 108, 82–93. [Google Scholar] [CrossRef]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Vente-Spreeuwenberg, M.A.; Verdonk, J.M.; Verstegen, M.W.; Beynen, A.C. Villus height and gut development in weaned piglets receiving diets containing either glucose, lactose or starch. Br. J. Nutr. 2003, 90, 907–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedemann, M.S.; Højsgaard, S.; Jensen, B.B. Small intestinal morphology and activity of intestinal peptidases in piglets around weaning. J. Anim. Physiol. Anim. Nutr. 2003, 87, 32–41. [Google Scholar] [CrossRef]
- Boudry, G.l.; Péron, V.; Le Huërou-Luron, I.; Lallès, J.P.; Sève, B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 2004, 134, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Cera, K.R.; Mahan, D.C.; Cross, R.F.; Reinhart, G.A.; Whitmoyer, R.E. Effect of Age, Weaning and Postweaning Diet on Small Intestinal Growth and Jejunal Morphology in Young Swine. J. Anim. Sci. 1988, 66, 574–584. [Google Scholar] [CrossRef]
- Montagne, L.; Boudry, G.; Favier, C.; Le Huerou-Luron, I.; Lalles, J.P.; Seve, B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 2007, 97, 45–57. [Google Scholar] [CrossRef]
- Spreeuwenberg, M.A.M.; Verdonk, J.M.A.J.; Gaskins, H.R.; Verstegen, M.W.A. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J. Nutr. 2001, 131, 1520–1527. [Google Scholar] [CrossRef] [Green Version]
- Bomba, L.; Minuti, A.; Moisa, S.J.; Trevisi, E.; Eufemi, E.; Lizier, M.; Chegdani, F.; Lucchini, F.; Rzepus, M.; Prandini, A.; et al. Gut response induced by weaning in piglet features marked changes in immune and inflammatory response. Funct. Integr. Genom. 2014, 14, 657–671. [Google Scholar] [CrossRef]
- Hu, C.H.; Xiao, K.; Luan, Z.S.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Pié, S.; Lallès, J.P.; Blazy, F.; Laffitte, J.; Sève, B.; Oswald, I.P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Pajarillo, E.A.B.; Chae, J.-P.; Balolong, M.P.; Kim, H.B.; Kang, D.-K. Assessment of fecal bacterial diversity among healthy piglets during the weaning transition. J. Gen. Appl. Microbiol. 2014, 60, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Frese, S.A.; Parker, K.; Calvert, C.C.; Mills, D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 2015, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 2017, 8, 1688. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Han, Y.; Meng, F.; Zhao, J.; Zhou, Z.; Fan, H. Fecal microbiota succession of piglets from birth to post-weaning by 454 pyrosequencing analysis. Trans. Tianjin Univ. 2017, 23, 211–220. [Google Scholar] [CrossRef]
- Guevarra, R.B.; Hong, S.H.; Cho, J.H.; Kim, B.R.; Shin, J.; Lee, J.H.; Kang, B.N.; Kim, Y.H.; Wattanaphansak, S.; Isaacson, R.E.; et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J. Anim. Sci. Biotechnol. 2018, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Konstantinov, S.R.; Awati, A.A.; Williams, B.A.; Miller, B.G.; Jones, P.; Stokes, C.R.; Akkermans, A.D.; Smidt, H.; de Vos, W.M. Post-natal development of the porcine microbiota composition and activities. Environ. Microbiol. 2006, 8, 1191–1199. [Google Scholar] [CrossRef]
- Pieper, R.; Janczyk, P.; Zeyner, A.; Smidt, H.; Guiard, V.; Souffrant, W.B. Ecophysiology of the developing total bacterial and lactobacillus communities in the terminal small intestine of weaning piglets. Microb. Ecol. 2008, 56, 474–483. [Google Scholar] [CrossRef]
- Urubschurov, V.; Janczyk, P.; Souffrant, W.B.; Freyer, G.; Zeyner, A. Establishment of intestinal microbiota with focus on yeasts of unweaned and weaned piglets kept under different farm conditions. FEMS Microbiol. Ecol. 2011, 77, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Dou, S.; Gadonna-Widehem, P.; Rome, V.; Hamoudi, D.; Rhazi, L.; Lakhal, L.; Larcher, T.; Bahi-Jaber, N.; Pinon-Quintana, A.; Guyonvarch, A.; et al. Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS ONE 2017, 12, e0169851. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Nagy, B.; Fekete, P.Z. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet. Res. 1999, 30, 259–284. [Google Scholar]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Bin, P.; Tang, Z.; Liu, S.; Chen, S.; Xia, Y.; Liu, J.; Wu, H.; Zhu, G. Intestinal microbiota mediates Enterotoxigenic Escherichia coli-induced diarrhea in piglets. BMC Vet. Res. 2018, 14, 385. [Google Scholar] [CrossRef] [Green Version]
- Kogan, G.; Kocher, A. Role of yeast cell wall polysaccharides in pig nutrition and health protection. Livest. Sci. 2007, 109, 161–165. [Google Scholar] [CrossRef]
- Zanello, G.; Meurens, F.; Serreau, D.; Chevaleyre, C.; Melo, S.; Berri, M.; D’Inca, R.; Auclair, E.; Salmon, H. Effects of dietary yeast strains on immunoglobulin in colostrum and milk of sows. Vet. Immunol. Immunopathol. 2013, 152, 20–27. [Google Scholar] [CrossRef]
- Castillo, M.; Martin-Orue, S.M.; Taylor-Pickard, J.A.; Perez, J.F.; Gasa, J. Use of mannanoligosaccharides and zinc chelate as growth promoters and diarrhea preventative in weaning pigs: Effects on microbiota and gut function. J. Anim. Sci. 2008, 86, 94–101. [Google Scholar] [CrossRef]
- Searle, L.E.J.; Cooley, W.A.; Jones, G.; Nunez, A.; Crudgington, B.; Weyer, U.; Dugdale, A.H.; Tzortzis, G.; Collins, J.W.; Woodward, M.J.; et al. Purified galactooligosaccharide, derived from a mixture produced by the enzymic activity of Bifidobacterium bifidum, reduces Salmonella enterica serovar Typhimurium adhesion and invasion in vitro and in vivo. J. Med. Microbiol. 2010, 59, 1428–1439. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Britti, M.S.; Bosi, P.; Oswald, I.; Mengheri, E. Alternatives to in-feed antibiotics in pigs: Evaluation of probiotics, zinc or organic acids as protective agents for the intestinal mucosa. A comparison of in vitro and in vivo results. Anim. Res. 2005, 54, 203–218. [Google Scholar] [CrossRef]
- Stensland, I.; Kim, J.C.; Bowring, B.; Collins, A.M.; Mansfield, J.P.; Pluske, J.R. A comparison of diets supplemented with a feed additive containing organic acids, cinnamaldehyde and a permeabilizing complex, or zinc oxide, on post-weaning diarrhoea, selected bacterial populations, blood measures and performance in weaned pigs experimentally infected with Enterotoxigenic, E. coli. Animals 2015, 5, 1147–1168. [Google Scholar] [CrossRef] [Green Version]
- Valeriano, V.D.; Balolong, M.P.; Kang, D.K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef] [Green Version]
- Torrallardona, D. Spray Dried Animal Plasma as an Alternative to Antibiotics in Weanling Pigs—A Review. Asian-Aust. J. Anim. Sci. 2010, 23, 131–148. [Google Scholar] [CrossRef]
- Torres-Pitarch, A.; Hermans, D.; Manzanilla, E.G.; Bindelle, J.; Everaert, N.; Beckers, Y.; Torrallardona, D.; Bruggeman, G.; Gardiner, G.E.; Lawlor, P.G. Effect of feed enzymes on digestibility and growth in weaned pigs: A systematic review and meta-analysis. Anim. Feed Sci. Technol. 2017, 233, 145–159. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluske, J.R. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 2013, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Michel, G.; Tonon, T.; Scornet, D.; Cock, J.M.; Kloareg, B. Central and storage carbon metabolism of the brown alga Ectocarpus siliculosus: Insights into the origin and evolution of storage carbohydrates in Eukaryotes. New Phytol. 2010, 188, 67–81. [Google Scholar] [CrossRef]
- Wang, D.; Kim, D.H.; Kim, K.H. Effective production of fermentable sugars from brown macroalgae biomass. Appl. Microbiol. Biotechnol. 2016, 100, 9439–9450. [Google Scholar] [CrossRef]
- Overland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Corino, C.; Modina, S.C.; Di Giancamillo, A.; Chiapparini, S.; Rossi, R. Seaweeds in Pig Nutrition. Animals 2019, 9, 1126. [Google Scholar] [CrossRef] [Green Version]
- McDonnell, P.; Figat, S.; O’Doherty, J.V. The effect of dietary laminarin and fucoidan in the diet of the weanling piglet on performance, selected faecal microbial populations and volatile fatty acid concentrations. Animal 2010, 4, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; ’Doherty, J.V.O. Effect of supplementing varying inclusion levels of laminarin and fucoidan on growth performance, digestibility of diet components, selected faecal microbial populations and volatile fatty acid concentrations in weaned pigs. Anim. Feed Sci. Technol. 2013, 183, 151–159. [Google Scholar] [CrossRef]
- Rattigan, R.; Sweeney, T.; Maher, S.; Thornton, K.; Rajauria, G.; O’Doherty, J.V. Laminarin-rich extract improves growth performance, small intestinal morphology, gene expression of nutrient transporters and the large intestinal microbial composition of piglets during the critical post-weaning period. Br. J. Nutr. 2020, 123, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Rattigan, R.; Sweeney, T.; Maher, S.; Ryan, M.T.; Thornton, K.; O’Doherty, J.V. Effects of reducing dietary crude protein concentration and supplementation with either laminarin or zinc oxide on the growth performance and intestinal health of newly weaned pigs. Anim. Feed Sci. Technol. 2020, 270, 114693. [Google Scholar] [CrossRef]
- Satessa, G.D.; Kjeldsen, N.J.; Mansouryar, M.; Hansen, H.H.; Bache, J.K.; Nielsen, M.O. Effects of alternative feed additives to medicinal zinc oxide on productivity, diarrhoea incidence and gut development in weaned piglets. Animal 2020, 14, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Dierick, N.; Ovyn, A.; De Smet, S. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs. J. Sci. Food Agric. 2009, 89, 584–594. [Google Scholar] [CrossRef]
- Michiels, J.; Skrivanova, E.; Missotten, J.; Ovyn, A.; Mrazek, J.; De Smet, S.; Dierick, N. Intact brown seaweed (Ascophyllum nodosum) in diets of weaned piglets: Effects on performance, gut bacteria and morphology and plasma oxidative status. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1101–1111. [Google Scholar] [CrossRef]
- Gardiner, G.E.; Campbell, A.J.; O’Doherty, J.V.; Pierce, E.; Lynch, P.B.; Leonard, F.C.; Stanton, C.; Ross, R.P.; Lawlor, P.G. Effect of Ascophyllum nodosum extract on growth performance, digestibility, carcass characteristics and selected intestinal microflora populations of grower–finisher pigs. Anim. Feed Sci. Technol. 2008, 141, 259–273. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Perez, M.J.; Falque, E.; Dominguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Vaquero, M.; Rajauria, G.; Miranda, M.; Sweeney, T.; Lopez-Alonso, M.; O’Doherty, J. Seasonal variation of the proximate composition, mineral content, fatty acid profiles and other phytochemical constituents of selected brown macroalgae. Mar. Drugs 2021, 19, 204. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food. Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Vigors, S.; O’Doherty, J.V.; Rattigan, R.; McDonnell, M.J.; Rajauria, G.; Sweeney, T. Effect of a laminarin rich macroalgal extract on the caecal and colonic microbiota in the post-weaned pig. Mar. Drugs 2020, 18, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of dietary laminarin and fucoidan on selected microbiota, intestinal morphology and immune status of the newly weaned pig. Br. J. Nutr. 2013, 110, 1630–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouwhuis, M.A.; Sweeney, T.; Mukhopadhya, A.; Thornton, K.; McAlpine, P.O.; O’Doherty, J.V. Zinc methionine and laminarin have growth-enhancing properties in newly weaned pigs influencing both intestinal health and diarrhoea occurrence. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Heim, G.; Walsh, A.M.; Sweeney, T.; Doyle, D.N.; O’Shea, C.J.; Ryan, M.T.; O’Doherty, J.V. Effect of seaweed-derived laminarin and fucoidan and zinc oxide on gut morphology, nutrient transporters, nutrient digestibility, growth performance and selected microbial populations in weaned pigs. Br. J. Nutr. 2014, 111, 1577–1585. [Google Scholar] [CrossRef]
- Rattigan, R.; Sweeney, T.; Vigors, S.; Thornton, K.; Rajauria, G.; O’Doherty, A.J.V. The effect of increasing inclusion levels of a fucoidan-rich extract derived from Ascophyllum nodosum on growth performance and aspects of intestinal health of pigs post-weaning. Mar. Drugs 2019, 17, 680. [Google Scholar] [CrossRef] [Green Version]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Graiff, A.; Ruth, W.; Kragl, U.; Karsten, U. Chemical characterization and quantification of the brown algal storage compound laminarin—A new methodological approach. J. Appl. Phycol. 2016, 28, 533–543. [Google Scholar] [CrossRef]
- Adams, J.M.; Ross, A.B.; Anastasakis, K.; Hodgson, E.M.; Gallagher, J.A.; Jones, J.M.; Donnison, I.S. Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, Y.; Yi, L.; Dai, R.; Wang, Y.; Sun, M.; Shao, X.; Zhang, Z.; Yuan, S. Endo-beta-1,3-glucanase digestion combined with the HPAEC-PAD-MS/MS analysis reveals the structural differences between two laminarins with different bioactivities. Carbohydr. Polym. 2018, 194, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; O’Doherty, J.V.; Reilly, P.; Ryan, M.T.; Bahar, B.; Sweeney, T. The effects of laminarin derived from Laminaria digitata on measurements of gut health: Selected bacterial populations, intestinal fermentation, mucin gene expression and cytokine gene expression in the pig. Br. J. Nutr. 2011, 105, 669–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, T.; Collins, C.B.; Reilly, P.; Pierce, K.M.; Ryan, M.; O’Doherty, J.V. Effect of purified beta-glucans derived from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae on piglet performance, selected bacterial populations, volatile fatty acids and pro-inflammatory cytokines in the gastrointestinal tract of pigs. Br. J. Nutr. 2012, 108, 1226–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, P.; Dal Bello, F.; O’Doherty, J.; Arendt, E.K.; Sweeney, T.; Coffey, A. Analysis of bacterial community shifts in the gastrointestinal tract of pigs fed diets supplemented with beta-glucan from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae. Animal 2013, 7, 1079–1087. [Google Scholar] [CrossRef]
- Lynch, M.B.; Sweeney, T.; Callan, J.J.; O’Sullivan, J.T.; O’Doherty, J.V. The effect of dietary Laminaria-derived laminarin and fucoidan on nutrient digestibility, nitrogen utilisation, intestinal microflora and volatile fatty acid concentration in pigs. J. Sci. Food Agric. 2010, 90, 430–437. [Google Scholar] [CrossRef]
- Rattigan, R.; O’Doherty, J.V.; Vigors, S.; Ryan, M.T.; Sebastiano, R.S.; Callanan, J.J.; Thornton, K.; Rajauria, G.; Margassery, L.M.; Dobson, A.D.W.; et al. The effects of the marine-derived polysaccharides laminarin and chitosan on aspects of colonic health in pigs challenged with dextran sodium sulphate. Mar. Drugs 2020, 18, 262. [Google Scholar] [CrossRef]
- O’Shea, C.J.; O’Doherty, J.V.; Callanan, J.J.; Doyle, D.; Thornton, K.; Sweeney, T. The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and Enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. J. Nutr. Sci. 2016, 5, e15. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.T.; O’Shea, C.J.; Collins, C.B.; O’Doherty, J.V.; Sweeney, T. Effects of dietary supplementation with Laminaria hyperborea, Laminaria digitata, and Saccharomyces cerevisiae on the IL-17 pathway in the porcine colon. J. Anim. Sci. 2012, 90, 263–265. [Google Scholar] [CrossRef] [Green Version]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef] [Green Version]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal. Res. 2017, 22, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Bruhn, A.; Janicek, T.; Manns, D.; Nielsen, M.M.; Balsby, T.J.S.; Meyer, A.S.; Rasmussen, M.B.; Hou, X.; Saake, B.; Goke, C.; et al. Crude fucoidan content in two North Atlantic kelp species, Saccharina latissima and Laminaria digitata-seasonal variation and impact of environmental factors. J. Appl. Phycol. 2017, 29, 3121–3137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Vaquero, M.; Rajauria, G.; Tiwari, B.; Sweeney, T.; O’Doherty, J. Extraction and yield optimisation of fucose, glucans and associated antioxidant activities from Laminaria digitata by applying response surface methodology to high intensity ultrasound-assisted extraction. Mar. Drugs 2018, 16, 257. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Vaquero, M.; O’Doherty, J.V.; Tiwari, B.K.; Sweeney, T.; Rajauria, G. Enhancing the extraction of polysaccharides and antioxidants from macroalgae using sequential hydrothermal-assisted extraction followed by ultrasound and thermal technologies. Mar. Drugs 2019, 17, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marudhupandi, T.; Kumar, T.T.A. Antibacterial effect of fucoidan from Sargassum wightii against the chosen human bacterial pathogens. Int. Curr. Pharm. J. 2013, 2, 156–158. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Liu, Y.; Cao, M.J.; Liu, G.M.; Chen, Q.; Sun, L.; Chen, H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, C.H.; Lee, C.H. Antibacterial and antioxidant capacities and attenuation of lipid accumulation in 3T3-L1 adipocytes by low-molecular-weight fucoidans prepared from compressional-puffing-pretreated Sargassum crassifolium. Mar. Drugs 2018, 16, 24. [Google Scholar] [CrossRef] [Green Version]
- Saravana, P.S.; Cho, Y.N.; Patil, M.P.; Cho, Y.J.; Kim, G.D.; Park, Y.B.; Woo, H.C.; Chun, B.S. Hydrothermal degradation of seaweed polysaccharide: Characterization and biological activities. Food Chem. 2018, 268, 179–187. [Google Scholar] [CrossRef]
- Ashayerizadeh, O.; Dastar, B.; Pourashouri, P. Study of antioxidant and antibacterial activities of depolymerized fucoidans extracted from Sargassum tenerrimum. Int. J. Biol. Macromol. 2020, 151, 1259–1266. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Rajasekar, P.; Anjali, R.; Sathiyaraj, G.; Marudhupandi, T.; Selvam, S.; Prabhu, N.M.; You, S. Antibacterial efficacy of a fucoidan fraction (Fu-F2) extracted from Sargassum polycystum. Int. J. Biol. Macromol. 2019, 125, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Mou, H.; Luo, B.; Jiang, X. Inhibition of adhesion of intestinal pathogens (Escherichia coli, Vibrio cholerae, Campylobacter jejuni, and Salmonella Typhimurium) by common oligosaccharides. Foodborne Pathog. Dis. 2015, 12, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Bouwhuis, M.A.; McDonnell, M.J.; Sweeney, T.; Mukhopadhya, A.; O’Shea, C.J.; O’Doherty, J.V. Seaweed extracts and galacto-oligosaccharides improve intestinal health in pigs following Salmonella Typhimurium challenge. Animal 2017, 11, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Zaporozhets, T.S.; Besednova, N.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Makarenkova, I.D.; Kryzhanovsky, S.P.; Melnikov, V.G. The prebiotic potential of polysaccharides and extracts of seaweeds. Russ. J. Mar. Biol. 2014, 40, 1–9. [Google Scholar] [CrossRef]
- Hwang, P.A.; Phan, N.N.; Lu, W.J.; Ngoc Hieu, B.T.; Lin, Y.C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okolie, C.L.; Mason, B.; Mohan, A.; Pitts, N.; Udenigwe, C.C. The comparative influence of novel extraction technologies on in vitro prebiotic-inducing chemical properties of fucoidan extracts from Ascophyllum nodosum. Food Hydrocoll. 2019, 90, 462–471. [Google Scholar] [CrossRef]
- Kong, Q.; Dong, S.; Gao, J.; Jiang, C. In vitro fermentation of sulfated polysaccharides from E. prolifera and L. japonica by human fecal microbiota. Int. J. Biol. Macromol. 2016, 91, 867–871. [Google Scholar] [CrossRef]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.O. Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.O.; Park, H.Y.; Xu, Q.; Park, J.I.; Zvyagintseva, T.; Stonik, V.A.; Kwak, J.Y. Ligand of scavenger receptor class A indirectly induces maturation of human blood dendritic cells via production of tumor necrosis factor-alpha. Blood 2009, 113, 5839–5847. [Google Scholar] [CrossRef] [Green Version]
- Makarenkova, I.D.; Logunov, D.Y.; Tukhvatulin, A.I.; Semenova, I.B.; Besednova, N.N.; Zvyagintseva, T.N. Interactions between sulfated polysaccharides from sea brown algae and Toll-Like receptors on HEK293 eukaryotic cells in vitro. Bull. Exp. Biol. Med. 2012, 154, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Chien, S.Y.; Chan, Y.L.; Lu, M.K.; Wu, C.H.; Kong, Z.L.; Wu, C.J. Inhibition of Lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J. Agric. Food Chem. 2011, 59, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ko, C.I.; Ahn, G.; You, S.; Kim, J.S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.J.; Xu, J.; Gao, X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, Á.R.; Gadicke, P.; Andrades, S.M.; Cubillos, R. Supplementing nursery pig feed with seaweed extracts increases final body weight of pigs. Austral. J. Vet. Sci. 2018, 50, 83–87. [Google Scholar] [CrossRef]
- Lynch, M.B.; Sweeney, T.; Callan, J.J.; O’Sullivan, J.T.; O’Doherty, J.V. The effect of dietary Laminaria derived laminarin and fucoidan on intestinal microflora and volatile fatty acid concentration in pigs. Livest. Sci. 2010, 133, 157–160. [Google Scholar] [CrossRef]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Molecular characteristics and antioxidant activity of laminarin extracted from the seaweed species Laminaria hyperborea, using hydrothermal-assisted extraction and a multi-step purification procedure. Food Hydrocoll. 2021, 112, 106332. [Google Scholar] [CrossRef]
- Bauer, E.; Williams, B.A.; Smidt, H.; Verstegen, M.W.; Mosenthin, R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr. Issues Intest. Microbiol. 2006, 7, 35–51. [Google Scholar]
- Heim, G.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of maternal supplementation with seaweed extracts on growth performance and aspects of gastrointestinal health of newly weaned piglets after challenge with enterotoxigenic Escherichia coli K88. Br. J. Nutr. 2014, 112, 1955–1965. [Google Scholar] [CrossRef] [Green Version]
- Demecková, V.; Kelly, D.; Coutts, A.G.P.; Brooks, P.H.; Campbell, A. The effect of fermented liquid feeding on the faecal microbiology and colostrum quality of farrowing sows. Int. J. Food Microbiol. 2002, 79, 85–97. [Google Scholar] [CrossRef]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; O’Doherty, J.V. Effect of maternal seaweed extract supplementation on suckling piglet growth, humoral immunity, selected microflora, and immune response after an ex vivo lipopolysaccharide challenge. J. Anim. Sci. 2012, 90, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Perez, P.F.; Dore, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J.; Donnet-Hughes, A. Bacterial imprinting of the neonatal immune system: Lessons from maternal cells? Pediatrics 2007, 119, e724–e732. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; Lynch, B.P.; O’Doherty, J.V. Effect of maternal fish oil and seaweed extract supplementation on colostrum and milk composition, humoral immune response, and performance of suckled piglets. J. Anim. Sci. 2010, 88, 2988–2997. [Google Scholar] [CrossRef] [Green Version]
- Farmer, C.; Quesnel, H. Nutritional, hormonal, and environmental effects on colostrum in sows. J. Anim. Sci. 2009, 87, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Bouwhuis, M.A.; Sweeney, T.; Mukhopadhya, A.; McDonnell, M.J.; O’Doherty, J.V. Maternal laminarin supplementation decreases Salmonella Typhimurium shedding and improves intestinal health in piglets following an experimental challenge with S. Typhimurium post-weaning. Anim. Feed Sci. Technol. 2017, 223, 156–168. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.-G.; Park, H.-J.; Liu, C.-G.; Liu, C.-S.; Meng, X.-H.; Yu, L.-J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Chung, Y.C.; Chen, C.Y. Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef]
- Koide, S.S. Chitin-chitosan: Properties, benefits and risks. Nutr. Res. 1998, 18, 1091–1101. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Park, P.-J.; Kim, S.-K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym. 2001, 44, 71–76. [Google Scholar] [CrossRef]
- Walsh, A.M.; Sweeney, T.; Bahar, B.; Flynn, B.; O’Doherty, J.V. The effects of supplementing varying molecular weights of chitooligosaccharide on performance, selected microbial populations and nutrient digestibility in the weaned pig. Animal 2013, 7, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Piao, X.S.; Kim, S.W.; Wang, L.; Shen, Y.B.; Lee, H.S.; Li, S.Y. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and Lactobacillus in weaning pigs. J. Anim. Sci. 2008, 86, 2609–2618. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Piao, X.S.; Thacker, P.A.; Zeng, Z.K.; Li, P.F.; Wang, D.; Kim, S.W. Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J. Anim. Sci. 2010, 88, 3871–3879. [Google Scholar] [CrossRef] [Green Version]
- Rhoades, J.; Gibson, G.; Formentin, K.; Beer, M.; Rastall, R. Inhibition of the adhesion of enteropathogenic Escherichia coli strains to HT-29 cells in culture by chito-oligosaccharides. Carbohydr. Polym. 2006, 64, 57–59. [Google Scholar] [CrossRef]
- Podolsky, D.K. Oligosaccharide structures of human colonic mucin. J. Biol. Chem. 1985, 260, 8262–8271. [Google Scholar] [CrossRef]
- Lee, H.-W.; Park, Y.-S.; Jung, J.-S.; Shin, W.-S. Chitosan oligosaccharides, dp 2–8, have prebiotic effect on the Bifidobacterium bifidium and Lactobacillus sp. Anaerobe 2002, 8, 319–324. [Google Scholar] [CrossRef]
- Mikkelsen, L.L.; Jakobsen, M.; Jensen, B.B. Effects of dietary oligosaccharides on microbial diversity and fructo-oligosaccharide degrading bacteria in faeces of piglets post-weaning. Anim. Feed Sci. Technol. 2003, 109, 133–150. [Google Scholar] [CrossRef]
- Xiao, D.; Tang, Z.; Yin, Y.; Zhang, B.; Hu, X.; Feng, Z.; Wang, J. Effects of dietary administering chitosan on growth performance, jejunal morphology, jejunal mucosal sIgA, occludin, claudin-1 and TLR4 expression in weaned piglets challenged by enterotoxigenic Escherichia coli. Int. Immunopharmacol. 2013, 17, 670–676. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Doherty, J.V.; Venardou, B.; Rattigan, R.; Sweeney, T. Feeding Marine Polysaccharides to Alleviate the Negative Effects Associated with Weaning in Pigs. Animals 2021, 11, 2644. https://doi.org/10.3390/ani11092644
O’Doherty JV, Venardou B, Rattigan R, Sweeney T. Feeding Marine Polysaccharides to Alleviate the Negative Effects Associated with Weaning in Pigs. Animals. 2021; 11(9):2644. https://doi.org/10.3390/ani11092644
Chicago/Turabian StyleO’Doherty, John V., Brigkita Venardou, Ruth Rattigan, and Torres Sweeney. 2021. "Feeding Marine Polysaccharides to Alleviate the Negative Effects Associated with Weaning in Pigs" Animals 11, no. 9: 2644. https://doi.org/10.3390/ani11092644
APA StyleO’Doherty, J. V., Venardou, B., Rattigan, R., & Sweeney, T. (2021). Feeding Marine Polysaccharides to Alleviate the Negative Effects Associated with Weaning in Pigs. Animals, 11(9), 2644. https://doi.org/10.3390/ani11092644







