Stages of Granulomatous Response Against Histozoic Metazoan Parasites in Mullets (Osteichthyes: Mugilidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Parasitological Analysis
2.3. Bacteriological Analysis
2.4. Histology and Immunohistochemistry
2.5. Image Analysis
2.6. Statistical Analysis
3. Results
3.1. Parasitological Findings
3.2. Bacteriological Findings
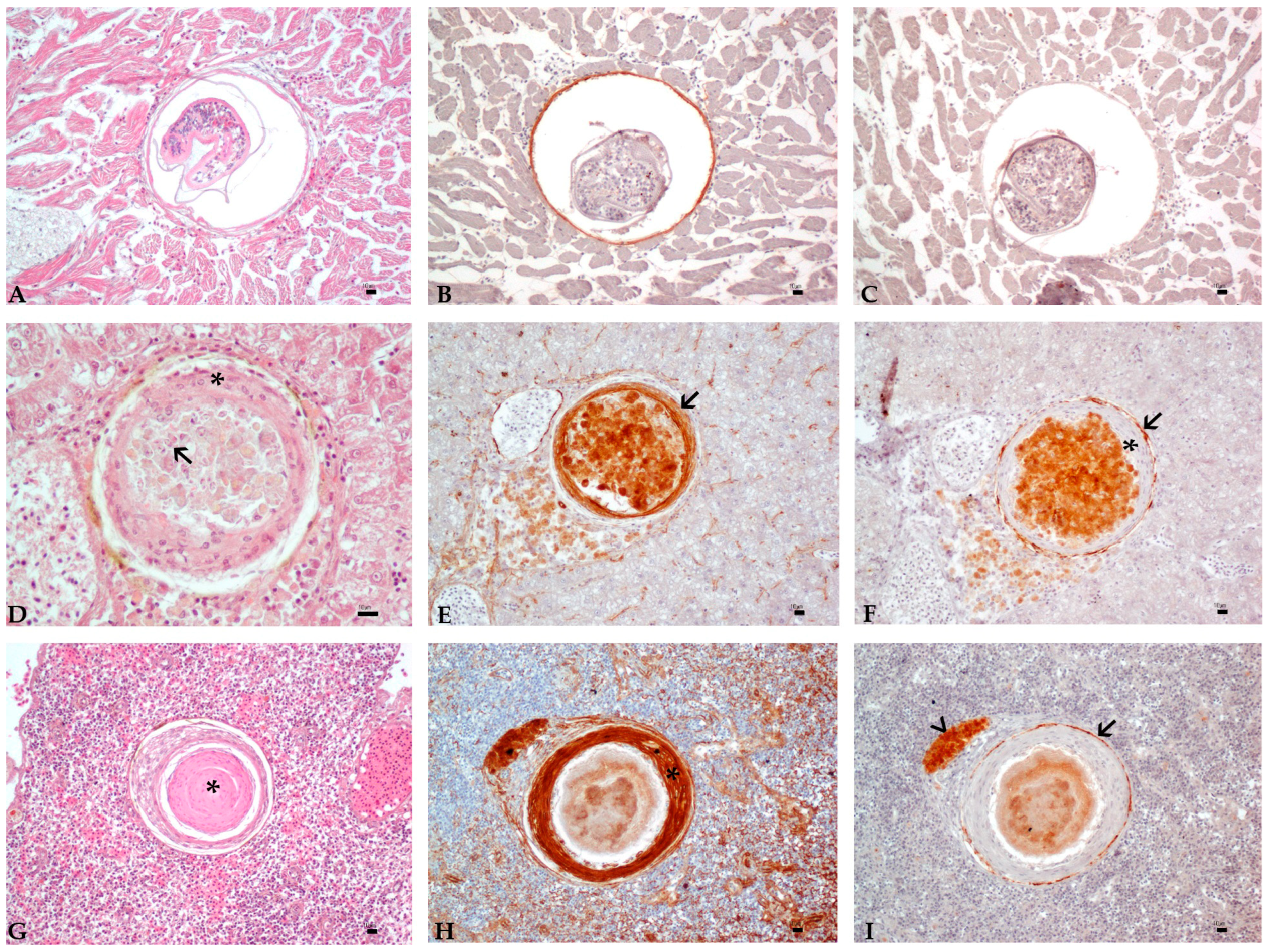
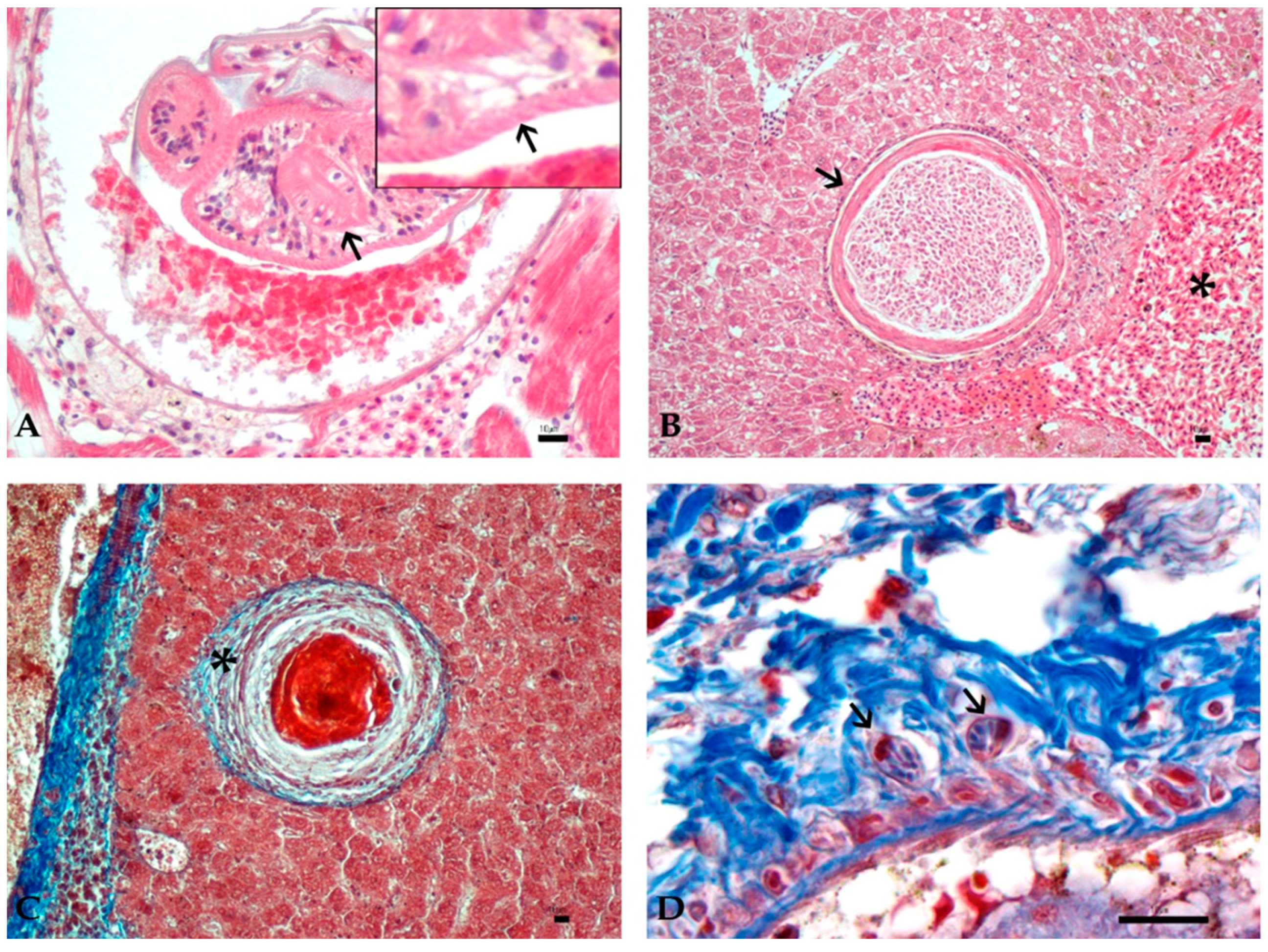
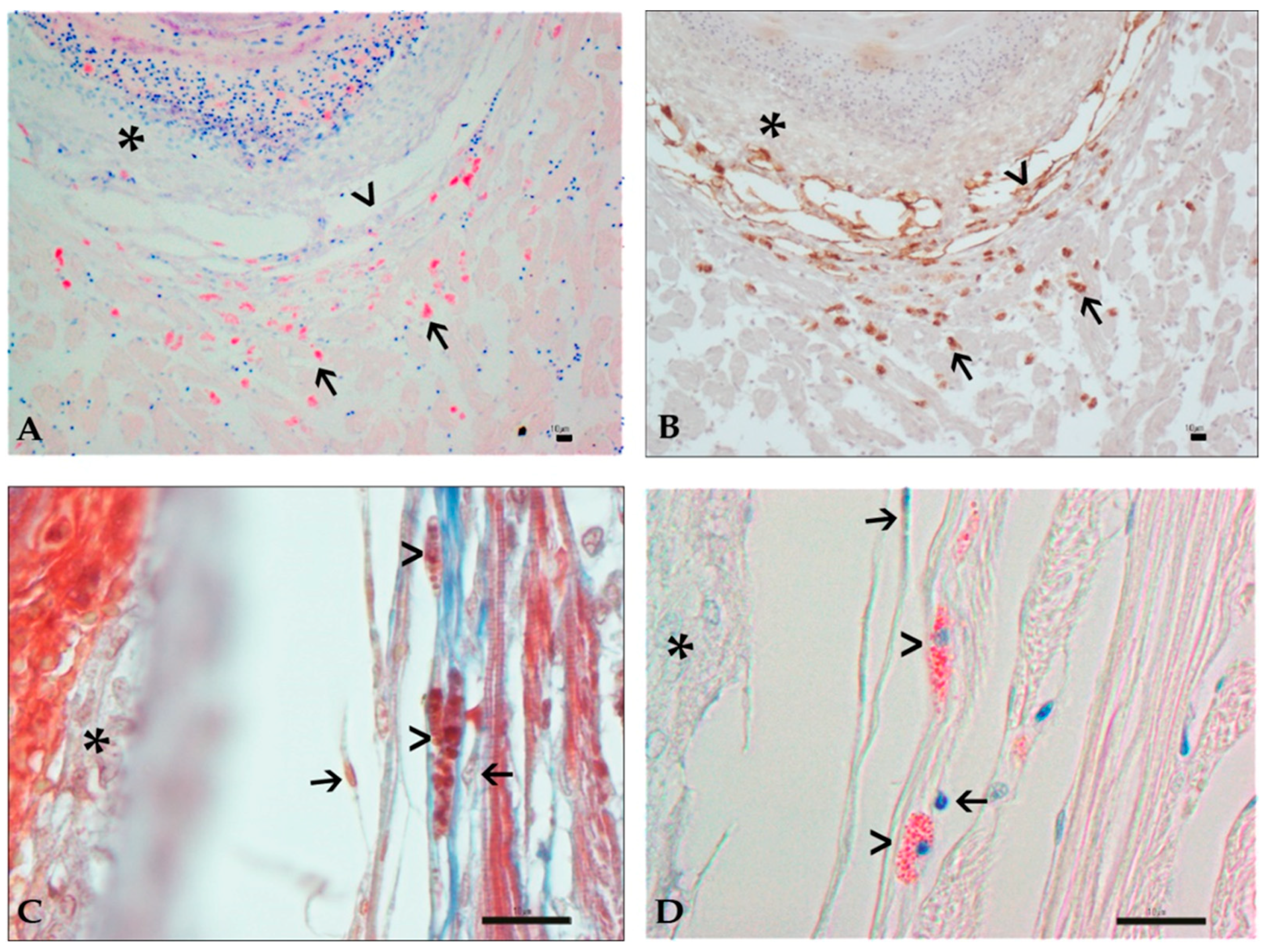
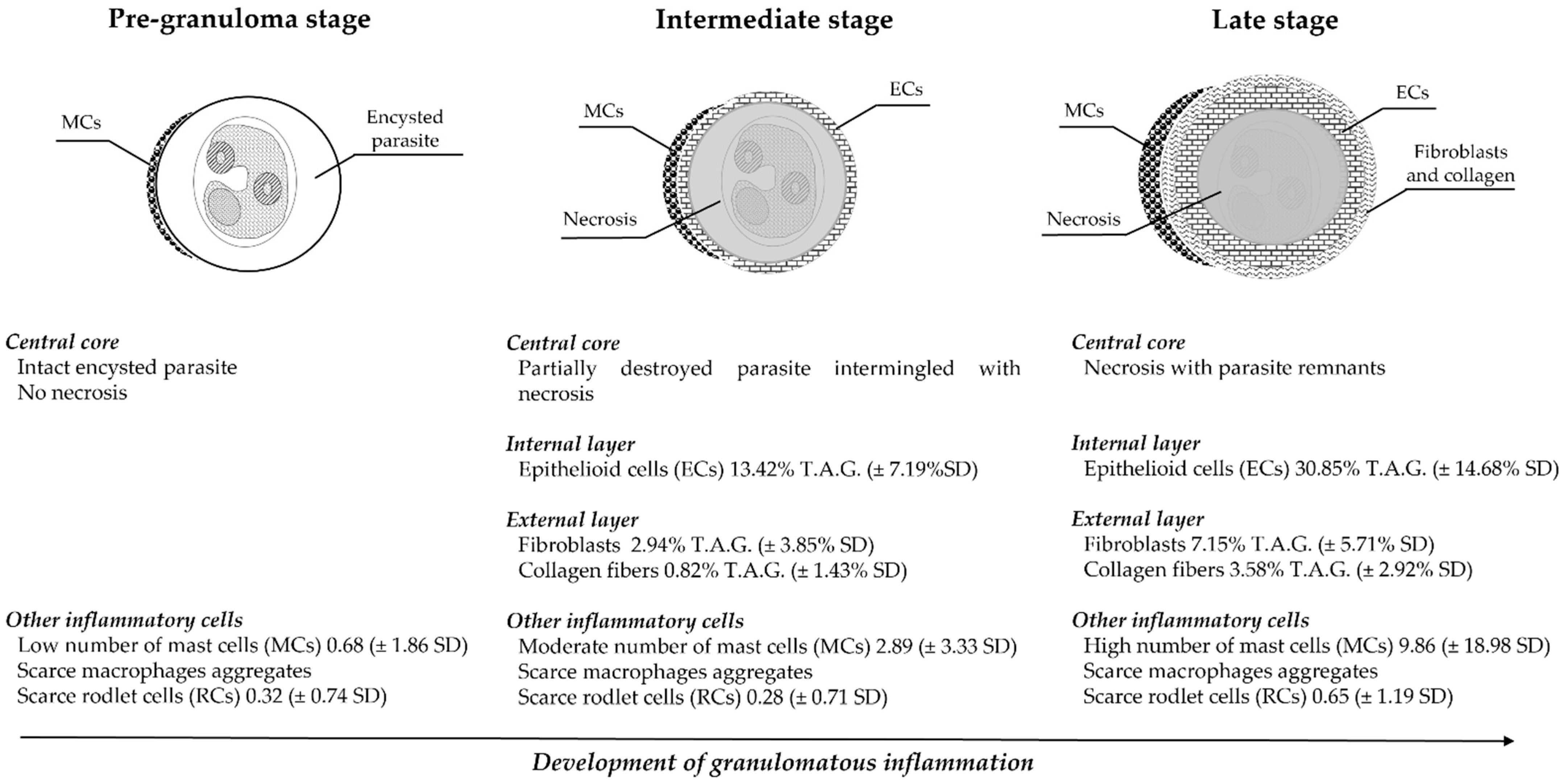
3.3. Histology and Immunohistochemistry Evaluation
3.4. Image and Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timur, M.; Roberts, R.J. Carrageenin granuloma in the plaice (Pleuronectes platessa); a histopathological study of chronic inflammation in a teleost fish. J. Comp. Pathol. 1977, 87, 89–96. [Google Scholar] [CrossRef]
- Noga, E.J.; Dykstra, M.J.; Wright, J.F. Chronic inflammatory cells with epithelial cell characteristics in teleost fishes. Vet. Pathol. 1989, 26, 429–437. [Google Scholar] [CrossRef]
- Olsen, A.B.; Mikalsen, J.; Rode, M.; Alfjorden, A.; Hoel, E.; Straum-Lie, K.; Haldorsen, R.; Colquhoun, D.J. A Novel systemic granulomatous inflammatory disease in farmed atlantic cod, Gadus morhua L., associated with a bacterium belonging to the genus Francisella. J. Fish Dis. 2006, 29, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, D.T.; Rhodes, M.W. Mycobacteriosis in fishes: A review. Vet. J. 2009, 180, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, D.J.; Duodu, S. Francisella infections in farmed and wild aquatic organisms. Vet. Res. 2011, 42, 47. [Google Scholar] [CrossRef] [PubMed]
- Avci, H.; Birincioğlu, S.; Epikmen, E.T.; Dereli, M. Comparative histopathological and immunohistochemical evaluations in juvenile sea bass (Dicentrarhus labrax) and gilthead sea bream (Sparus aurata) naturally infected with Photobacterium damselae subsp. piscicida. Revue Méd. Vét. 2013, 164, 72–79. [Google Scholar]
- Ortega, J.; Noguera, A.; García-Quirós, A.; Viana, D.; Selva, L.; de Juan, L.; Romero, B.; García-Parraga, D.; Crespo, J.L.; Corpa, J.M. Lesional patterns associated with mycobacteriosis in an atlantic horse mackerel, Trachurus trachurus (L.), aquarium population. J. Fish Dis. 2014, 37, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Varello, K.; Prearo, M.; Serracca, L.; Meloni, D.; Rossini, I.; Righetti, M.; Pezzolato, M.; Fioravanti, M.L.; Ercolini, C.; Bozzetta, E. Granulomatous lesions in a wild mullet population from the eastern ligurian sea (Italy): Mycobacteriosis vs. pseudotuberculosis. J. Fish Dis. 2014, 37, 553–558. [Google Scholar] [CrossRef]
- Essam, H.M.; Abdellrazeq, G.S.; Tayel, S.I.; Torky, H.A.; Fadel, A.H. Pathogenesis of Photobacterium damselae subspecies infections in sea bass and sea bream. Microb. Pathog. 2016, 99, 41–50. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Fernandes, C.E.; Galindo, G.M.; Castaldelli, G.; Manera, M.; DePasquale, J.A.; Lorenzoni, M.; Bertin, S.; Giari, L. Nematode infection in liver of the fish Gymnotus inaequilabiatus (Gymnotiformes: Gymnotidae) from the Pantanal region in Brazil: Pathobiology and inflammatory response. Parasites Vectors 2016, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Manera, M.; DePasquale, J.A.; Pironi, F.; Giari, L. Liver of the fish Gymnotus inaequilabiatus and nematode larvae infection: Histochemical features and expression of proliferative cell nuclear antigen. J. Fish Dis. 2017, 40, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Antuofermo, E.; Pais, A.; Polinas, M.; Cubeddu, T.; Righetti, M.; Sanna, M.A.; Prearo, M. Mycobacteriosis caused by Mycobacterium marinum in reared mullets: First evidence from Sardinia (Italy). J. Fish Dis. 2017, 40, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.T.; Yunis-Aguinaga, J.; Cueva-Quiroz, V.A.; Filho, J.R.E.; Mouriño, J.L.P.; Claudiano, G.S.; Moraes, F.R.; Moraes, J.R.E. Isolation and characterization of pathology in case of massive mortality by Photobacterium damselae subsp. piscicida in Rachycentron canadum. Biosci. J. Uberlândia 2020, 36, 1732–1741. [Google Scholar] [CrossRef]
- Iaria, C.; Saoca, C.; Guerrera, M.C.; Ciulli, S.; Brundo, M.V.; Piccione, G.; Lanteri, G. Occurrence of diseases in fish used for experimental research. Lab. Anim. 2019, 53, 619–662. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J.; Rodger, H.D. The pathophysiology and systemic pathology of teleosts. In Fish Pathology; Roberts, D.J., Ed.; W.B. Saunders: London, UK, 2001; pp. 55–132. [Google Scholar]
- Campos-Sánchez, J.C.; Esteban, M.Á. Review of inflammation in fish and value of the zebrafish model. J. Fish Dis. 2020. [Google Scholar] [CrossRef]
- Neumann, N.F.; Stafford, J.L.; Barreda, D.; Ainsworth, A.J.; Belosevic, M. Antimicrobial mechanisms of fish phagocytes and their role in host defense. Dev. Comp. Immunol. 2001, 25, 807–825. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Inflammation and repair. In Robbins and Cotran Pathologic Basis of Disease; Kumar, V., Abbas, A.K., Aster, J.C., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2015; pp. 69–111. [Google Scholar]
- Sosa, E.R.; Landsberg, J.H.; Stephenson, C.M.; Forstchen, A.B. Aphanomyces invadans and ulcerative mycosis in estuarine and freshwater fish in florida. J. Aquat. Anim. Health 2007, 19, 14–26. [Google Scholar] [CrossRef]
- Martınez-Lara, P.; Martınez-Porchas, M.; Gollas-Galvan, T.; Hernandez-Lopez, J.; Robles-Porchas, G.R. Granulomatosis in fish aquaculture: A mini review. Rev. Aquac. 2015, 1–10. [Google Scholar] [CrossRef]
- Gjurčević, E.; Kužir, S.; Žmak, L.; Obrovac, M.; Gudan Kurilj, A.; Savoca, S.; Pađen, A.; Matanović, K. A case of mycobacteriosis in farmed pikeperch (Sander lucioperca) cultured in a recirculating aquaculture system. Aquac. Res. 2020, 51, 4824–4827. [Google Scholar] [CrossRef]
- Nowak, B.; Clark, A.; Pankhurst, T.; Speare, D. Langhans’ giant cells in the gills of an Atlantic salmon. Aust. Vet. J. 2000, 78, 191–192. [Google Scholar] [CrossRef]
- Bakenhaster, M.D.; Lowerre-Barbieri, S.; Kiryu, Y.; Walters, S.; Fajer-Avila, E.J. Philometra floridensis (Nematoda: Philometridae) damages ovarian tissue without reducing host (Sciaenops ocellatus) fecundity. Dis. Aquat. Org. 2014, 108, 227–239. [Google Scholar] [CrossRef]
- Feist, S.W.; Longshaw, M. Histopathology of fish parasite infections—Importance for populations. J. Fish Biol. 2008, 73, 2143–2160. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Giari, L.; Simoni, E.; Shinn, A.P.; Manera, M.; Bosi, G. Histopathology, ultrastructure and immunohistochemistry of Coregonus lavaretus hearts naturally infected with Ichthyocotylurus erraticus (Trematoda). Dis. Aquat. Org. 2005, 66, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Groff, J.M.; Naydan, D.K.; Higgins, R.J.; Zinkl, J.G. Cytokeratin-filament expression in epithelial and non-epithelial tissues of the common carp (Cyprinus carpio). Cell Tissue Res. 1997, 287, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, D.T.; Vogelbein, W.K.; Ottinger, C.A. Ultrastructure of Mycobacterium marinum granuloma in striped bass Morone saxatilis. Dis. Aquat. Organ. 2004, 62, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Manrique, W.G.; da Claudiano, G.S.; de Castro, M.P.; Petrillo, T.R.; Pereira Figueiredo, M.A.; de Belo, M.A.A.; Berdeal, M.I.Q.; de Moraes, J.E.R.; de Moraes, F.R. Expression of cellular components in granulomatous inflammatory response in Piaractus mesopotamicus model. PLoS ONE 2015, 10, e0121625. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Lui, A.; Boldrini, P.; Pironi, F.; Giari, L. The inflammatory response of fish to helminth parasites. Parasite 2008, 15, 426–433. [Google Scholar] [CrossRef]
- Skorobrechova, E.M.; Nikishin, V.P. Structure of capsule surrounding acanthocephalans Corynosoma strumosum in paratenic hosts of three species. Parasitol. Res. 2011, 108, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, M.; Sayyaf Dezfuli, B.; Castaldelli, G.; Lanzoni, M.; Giari, L. Histological and ultrastructural study of Myxobolus mugchelo (Parenzan, 1966) with initial histopathology survey of the Liza ramada host intestine. Parasitol. Res. 2017, 116, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Miteva, K.; Tschöpe, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 2014, 102, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Manera, M.; Giari, L. Ultrastructural assessment of granulomas in the liver of perch (Perca fluviatilis) infected by tapeworm. J. Comp. Pathol. 2015, 152, 97–102. [Google Scholar] [CrossRef]
- Hügle, T. Beyond Allergy: The role of mast cells in fibrosis. Swiss Med. Wkly. 2014, 144, w13999. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.; Dezfuli, B.S. Rodlet cells in teleosts: A new insight into their nature and functions. J. Fish Biol. 2004, 65, 597–619. [Google Scholar] [CrossRef]
- Reite, O.B. The rodlet cells of teleostean fish: Their potential role in host defence in relation to the role of mast cells/eosinophilic granule cells. Fish Shellfish Immunol. 2005, 19, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Lui, A.; Pironi, F.; Manera, M.; Shinn, A.P.; Lorenzoni, M. Cell types and structures involved in tench, Tinca tinca (L.), defence mechanisms against a systemic digenean infection. J. Fish Dis. 2013, 36, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Paperna, I.; Overstreet, R.M. Parasites and diseases of mullets (Mugilidae). In Aquaculture of Grey Mullets; Oren, O.H., Ed.; Cambridge University Press: Cambridge, UK, 1981; pp. 1–19. [Google Scholar]
- Merella, P.; Garippa, G. Metazoan parasites of grey mullets (Teleostea: Mugilidae) from the Mistras Lagoon (Sardinia, western Mediterranean). Sci. Mar. 2001, 65, 201–206. [Google Scholar] [CrossRef]
- Turan, C. Biogeography and Distribution of Mugilidae in the Mediterranean and the Black Sea, and North-East Atlantic. In Biology, Ecology and Culture of Grey Mullets; Crosetti, D., Blader, S.J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 116–127. [Google Scholar]
- Rocha, S.; Casal, G.; Alves, Â.; Antunes, C.; Rodrigues, P.; Azevedo, C. Myxozoan biodiversity in mullets (Teleostei, Mugilidae) unravels hyperdiversification of Myxobolus (Cnidaria, Myxosporea). Parasitol. Res. 2019, 118, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Elsheikha, H.M.; Elshazly, A.M. Host-dependent variations in the seasonal prevalence and intensity of heterophyid encysted metacercariae (Digenea: Heterophyidea) in brackish water fish in Egypt. Vet. Parasitol. 2008, 153, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Faliex, E. Ultrastructural study of the host-parasite interface after infection of two species of teleosts by Labratrema minimus metacercariae (Trematoda, Bucephalidae). Dis. Aquat. Org. 1991, 10, 93–101. [Google Scholar] [CrossRef]
- Ben-Tuvia, A. Mugilidae. In Fishes of the North-Eastern Atlantic and the Mediterranean (FNAM); Whitehead, P.J.P., Bauchot, M.L., Hureau, J., Nielsen, C.J., Tortonese, E., Eds.; Unesco: Paris France, 1986; pp. 1197–1204. [Google Scholar]
- Bauchot, M.L. Poissons osseux. In Fiches FAO d’identification des espèces pour les besoins de la pêche. (Révision 1); Fischer, W., Bauchot, M.L., Schneider, M., Eds.; Méditerranée et Mer Noire; FAO: Roma, Italy, 1987; pp. 891–1422. [Google Scholar]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 6 May 2021).
- Lom, J.; Dyková, I. Myxozoan genera: Definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol. 2006, 53, 1–36. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Elshazly, A.M. Preliminary observations on infection of brackish and fresh water fish by heterophyid encysted metacercariae in Egypt. Parasitol. Res. 2008, 103, 971–977. [Google Scholar] [CrossRef]
- Pearson, J. Family Heterophyidae Leiper, 1909. In Keys to the Trematoda; Bray, R.A., Gibson, D.I., Jones, A., Eds.; CAB International and The Natural History Museum: Wallingford, UK, 2008; Volume 3, pp. 113–141. [Google Scholar]
- Yurakhno, V.M.; Ovcharenko, M.O. Study of Myxosporea (Myxozoa), infecting worldwide mullets with description of a new species. Parasitol. Res. 2014, 113, 3661–3674. [Google Scholar] [CrossRef][Green Version]
- Masala, S.; Piras, M.C.; Sanna, D.; Chai, J.-Y.; Jung, B.-K.; Sohn, W.-M.; Garippa, G.; Merella, P. Epidemiological and molecular data on Heterophyid trematode metacercariae found in the muscle of grey mullets (Osteichthyes: Mugilidae) from Sardinia (Western Mediterranean Sea). Parasitol. Res. 2016, 115, 3409–3417. [Google Scholar] [CrossRef]
- Du Moulin, G.; Stottmeier, K.D. Use of Cetylpyridinium Chloride in the decontamination of water for culture of mycobacteria. Appl. Environ. Microbiol. 1978, 36, 771–773. [Google Scholar] [CrossRef]
- Nowak, B.; Elliott, D.G.; Bruno, D.W. The identification of parasites in fish tissue sections. Bull. Eur. Assoc. Fish Pathol. 2002, 22, 173–177. [Google Scholar]
- Bruno, D.W.; Nowak, B.; Elliott, D.G. Guide to the Identification of fish protozoan and metazoan parasites in stained tissue sections. Dis. Aquat. Organ. 2006, 70, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Antuofermo, E.; Cocco, R.; Borzacchiello, G.; Burrai, G.P.; Meloni, F.; Bonelli, P.; Pirino, S.; Cossu-Rocca, P.; Bosincu, L. Bilateral ovarian malignant mixed mullerian tumor in a dog. Vet. Pathol. 2009, 46, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Polinas, M.; Burrai, G.P.; Vitiello, V.; Falchi, L.; Zedda, M.T.; Masala, G.; Marras, V.; Satta, G.; Alberti, A.; Antuofermo, E. Histological and immunohistochemical features of trichoblastoma in a sarda breed sheep. Animals 2020, 10, 2039. [Google Scholar] [CrossRef]
- Marino, F.; Licata, L.; Albano, M.; Ieni, A.; Di Caro, G.; Macrì, B. Angioleiomyoma in a conger (Conger conger). Dis. Aquat. Org. 2016, 119, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Mulero, I.; Sepulcre, M.P.; Meseguer, J.; García-Ayala, A.; Mulero, V. Histamine is stored in mast cells of most evolutionarily advanced fish and regulates the fish inflammatory response. Proc. Natl. Acad. Sci. USA 2007, 104. [Google Scholar] [CrossRef] [PubMed]
- Nieddu, A.M.; Lochi, P.G. Heterophydi e ospiti intermedi in Sardegna. Rivista di Parassitol. 1988, 5, 261–267. [Google Scholar]
- Culurgioni, J.; Sabatini, A.; De Murtas, R.; Mattiucci, S.; Figus, V. Helminth parasites of fish and shellfish from the Santa Gilla lagoon in southern Sardinia, Italy. J. Helminthol. 2014, 88, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Ghobashy, M.A.; Soliman, M.F.M.; Hassan, E.A. Responses of the mullet, Liza auratus and the cichlid, Oreochromis niloticus from Lake Manzala (Egypt) to heterophyd infection. Int. J. Zool. Res. 2010, 6, 13–23. [Google Scholar] [CrossRef]
- Martorelli, S.R.; Lino, A.; Marcotegui, P.; Montes, M.M.; Alda, P.; Panei, C.J. Morphological and molecular identification of the fish-borne metacercaria of Ascocotyle (Phagicola) Longa Ransom, 1920 in Mugil Liza from Argentina. Vet. Parasitol. 2012, 190, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, T. Parasites of juvenile golden grey mullet Liza aurata Risso, 1810 in Sarikum Lagoon Lake at Sinop, Turkey. Acta Parasitol. 2013, 58, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Kotb, H.L.; Mahdy, O.A.; Shaheed, I.B. Parasitological and histopathological study of digenetic trematodes in mullets from Lake Qarun, Egypt. Glob. Vet. 2014, 13, 202–208. [Google Scholar] [CrossRef]
- Chai, J.-Y.; Sohn, W.-M.; Na, B.-K.; Jeoung, H.-G.; Sinuon, M.; Socheat, D. Stellantchasmus falcatus (Digenea: Heterophyidae) in Cambodia: Discovery of metacercariae in mullets and recovery of adult flukes in an experimental hamster. Korean J. Parasitol. 2016, 54, 537–541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diamanka, A.; Fall, M.; Diebakate, C.; Faye, N.; Toguebaye, B.S. Identification of Myxobolus episquamalis (Myxozoa, Myxobolidae) in flathead mullet Mugil cephalus (Pisces, Teleostei, Mugilidae) from the coast of Senegal (eastern tropical Atlantic Ocean). Acta Adriat. 2008, 49, 19–23. [Google Scholar]
- Kim, W.-S.; Kim, J.-H.; Jang, M.-S.; Jung, S.-J.; Oh, M.-J. Infection of wild mullet (Mugil Cephalus) with Myxobolus episquamalis in Korea. Parasitol. Res. 2013, 112, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, M. Microparasites of worldwide mullets. Ann. Parasitol. 2015, 61, 229–239. [Google Scholar] [CrossRef]
- Yurakhno, V.M.; Thi, H.V. First data on Bivalvulida myxosporean of Nha Trang Bay mullets (Vietnam). Mar. Biol. J. 2019, 4, 82–88. [Google Scholar] [CrossRef]
- Bahri, S.; Marques, A. Myxosporean parasites of the genus Myxobolus from Mugil cephalus in Ichkeul lagoon, Tunisia: Description of two new species. Dis. Aquat. Org. 1996, 27, 115–122. [Google Scholar] [CrossRef]
- Bahri, S.; Andree, K.B.; Hedrick, R.P. Morphological and phylogenetic studies of marine Myxobolus spp. from mullet in Ichkeul Lake, Tunisia. J. Eukaryot. Microbiol. 2003, 50, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Maíllo-Bellón, P.A.; Marques, A.; Graciaroyo, M.P. Myxosporean infection of Grey Mullet in the Ebro Delta: Identification and ultrastructure of Myxobolus ichkeulensis Bahri & Marques, 1996 Infecting the Gills of Mugil cephalus L. Acta Protozool. 2011, 50, 67–71. [Google Scholar]
- Al-Bassel, D.A.H.; Hussein, A.N.A. A survey on parasites infecting mullets from Egypt and Libya. Egypt. Acad. J. Biol. Sci. 2012, 4, 9–19. [Google Scholar] [CrossRef]
- Yemmen, C.; Ktari, M.H.; Bahri, S. Parasitofauna of some mugilid and soleid fish species from Tunisian lagoons. Acta Adriat. 2012, 52, 173–182. [Google Scholar]
- Özer, A.; Kirca, D.Y. Parasite fauna of golden grey mullet Liza aurata (Risso, 1810) collected from Lower Kýzýlýrmak Delta in Samsun, Turkey. Helminthologia 2013, 50, 269–280. [Google Scholar] [CrossRef]
- Molnár, K. Site preference of myxozoans in the kidneys of hungarian fishes. Dis. Aquat. Org. 2007, 78, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Pellitero, P. Fish Immunity and Parasite Infections: From Innate Immunity to Immunoprophylactic Prospects. Vet. Immunol. Immunopathol. 2008, 126, 171–198. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A. Living off a fish: A trade-off between parasites and the immune system. Fish Shellfish Immunol. 2008, 25, 358–372. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A. Fish immune response to myxozoan parasites. Parasite 2008, 15, 420–425. [Google Scholar] [CrossRef]
- Huizinga, H.W.; Nadakavukaren, M.J. Cellular responses of goldfish, Carassius auratus (L.), to metacercariae of Ribeiroia marini (Faust & Hoffman, 1934). J. Fish Dis. 1997, 20, 401–408. [Google Scholar]
- Longshaw, M.; Frear, P.A.; Feist, S.W. Descriptions, development and pathogenicity of myxozoan (Myxozoa: Myxosporea) parasites of juvenile cyprinids (Pisces: Cyprinidae). J. Fish Dis. 2005, 28, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Zilberg, D.; Jones, J.B.; Burger, M.A.A.; Nicholls, P.K.; Nolan, D.; Crockford, M.; Stephens, F. New Pathological condition in cultured mulloway Argyrosomus japonicus: Histopathological, ultrastructural and molecular studies. Dis. Aquat. Org. 2012, 100, 219–230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dezfuli, B.S.; Bo, T.; Lorenzoni, M.; Shinn, A.P.; Giari, L. Fine structure and cellular responses at the host-parasite interface in a range of fish-helminth systems. Vet. Parasitol. 2015, 208, 272–279. [Google Scholar] [CrossRef]
- Moreau, E.; Chauvin, A. Immunity against helminths: Interactions with the host and the intercurrent infections. J. Biomed. Biotechnol. 2010, 2010, 428593. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Bosi, G.; DePasquale, J.A.; Manera, M.; Giari, L. Fish innate immunity against intestinal helminths. Fish Shellfish Immunol. 2016, 50, 274–287. [Google Scholar] [CrossRef]
- Motran, C.C.; Silvane, L.; Chiapello, L.S.; Theumer, M.G.; Ambrosio, L.F.; Volpini, X.; Celias, D.P.; Cervi, L. Helminth infections: Recognition and modulation of the immune response by innate immune cells. Front. Immunol. 2018, 9, 664. [Google Scholar] [CrossRef] [PubMed]
- Dumbo, J.C.; Avenant-Oldewage, A. Histopathological changes induced by the digenean intestinal parasite Masenia Nkomatiensis Dumbo, Dos Santos, & Avenant-Oldewage, 2019 of the catfish Clarias Gariepinus (Burchell) from Incomati basin, Mozambique. J. Fish Dis. 2019, 42, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Simoni, E.; Rossi, R.; Manera, M. Rodlet cells and other inflammatory cells of Phoxinus phoxinus infected with Raphidascaris acus (Nematoda). Dis. Aquat. Org. 2000, 43, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Iaria, C.; Ieni, A.; Corti, I.; Puleio, R.; Brachelente, C.; Mazzullo, G.; Lanteri, G. immunohistochemical study of four fish tumors. J. Aquat. Anim. Health 2019, 31, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Pironi, F.; Simoni, E.; Shinn, A.P.; Giari, L. Selected pathological, immunohistochemical and ultrastructural changes associated with an infection by Diphyllobothrium dendriticum (Nitzsch, 1824) (Cestoda) plerocercoids in Coregonus lavaretus (L.) (Coregonidae). J. Fish Dis. 2007, 30, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, A.; Mladineo, I. Ultrastructure of Didymocystis semiglobularis (Didymozoidae, Digenea) cysts in the gills of pacific bluefin tuna (Thunnus Orientalis). Parasitol. Res. 2008, 103, 641–647. [Google Scholar] [CrossRef]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult zebrafish as a model system for cutaneous wound-healing research. J. Invest Dermatol. 2013, 133, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Pincha, N.; Hajam, E.Y.; Badarinath, K.; Batta, S.P.R.; Masudi, T.; Dey, R.; Andreasen, P.; Kawakami, T.; Samuel, R.; George, R.; et al. PAI1 mediates fibroblast-mast cell interactions in skin fibrosis. J. Clin. Investig. 2018, 128, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Sfacteria, A.; Brines, M.; Blank, U. The mast cell plays a central role in the immune system of teleost fish. Mol. Immunol. 2015, 63, 3–8. [Google Scholar] [CrossRef]
- Prendergast, C.T.; Sanin, D.E.; Mountford, A.P. CD4 T-cell hyporesponsiveness induced by schistosome larvae is not dependent upon eosinophils but may involve connective tissue mast cells. Parasite Immunol. 2016, 38, 81–92. [Google Scholar] [CrossRef]
- Reite, O.B.; Evensen, O. Inflammatory cells of teleostean fish: A review focusing on mast cells/eosinophilic granule cells and rodlet cells. Fish Shellfish Immunol. 2006, 20, 192–208. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Castaldelli, G.; Giari, L. Histopathological and ultrastructural assessment of two mugilid species infected with myxozoans and helminths. J. Fish Dis. 2018, 41, 299–307. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Giari, L. Mast cells in the gills and intestines of naturally infected fish: Evidence of migration and degranulation. J. Fish Dis. 2008, 31, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Agius, C.; Roberts, R.J. Melano-macrophage centres and their role in fish pathology. J. Fish Dis. 2003, 26, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Passantino, L.; Zupa, R.; Pousis, C.; Mylonas, C.C.; Hala, E.; Jirillo, E.; Corriero, A. Increased melanomacrophage centres in the liver of reproductively dysfunctional female greater amberjack Seriola dumerili (Risso, 1810). J. Fish Dis. 2020, 43, 503–514. [Google Scholar] [CrossRef] [PubMed]

| Summer Sampling | Autumn Sampling | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CH | ST | MI | MA | CH | ST | MI | MA | Total | |
| Mugil cephalus | 0 | 6 | 0 | 0 | 0 | 5 | 0 | 0 | 11 |
| Chelon labrosus | 16 | 10 | 0 | 0 | 0 | 1 | 0 | 8 | 35 |
| Chelon auratus | 1 | 13 | 0 | 29 | 0 | 23 | 0 | 17 | 83 |
| Chelon ramada | 13 | 1 | 30 | 0 | 30 | 1 | 30 | 5 | 110 |
| Total | 30 | 30 | 30 | 29 | 30 | 30 | 30 | 30 | 239 |
| TM | MP | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Mugil cephalus | 36.36% (4/11) | 36.36% (4/11) | 9.09% (1/11) | 9.09% (1/11) | 54.54% (6/11) | 0 | 0 | 0 |
| Chelon labrosus | 45.71% (16/35) | 5.71% (2/35) | 0 | 0 | 17.14% (6/35) | 8.57% (3/35) | 5.71% (2/35) | 0 |
| Chelon auratus | 45.78% (38/83) | 20.48% (17/83) | 2.40% (2/83) | 1.20% (1/83) | 43.37% (36/83) | 7.22% (6/83) | 1.20% (1/83) | 0 |
| Chelon ramada | 25.45% (28/110) | 18.18% (20/110) | 2.72% (3/110) | 0 | 44.54% (49/110) | 12.72% (14/110) | 0 | 0 |
| Total | 35.98% (86/239) | 17.99% (43/239) | 2.51% (6/239) | 0.83% (2/239) | 40.58% (97/239) | 9.62% (23/239) | 1.25% (3/239) | 0 |
| Heart | Liver | Spleen | Kidney | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TM | MP | TM | MP | TM | MP | TM | MP | TM | MP | |
| Mugil cephalus | 6/11 (54.5%) | - | 4/11 (36.3%) | 3/11 (27.2%) | 4/11 (36.3%) | 3/11 (27.2%) | 5/11 (45.4%) | 4/11 (36.3%) | p > 0.05 | p > 0.05 |
| Chelon labrosus | 2/35 (5.7%) | 1/35 (2.8%) | 5/35 (14.2%) | 8/35 (22.8%) | 2/35 (5.7%) | 11/35 (31.4%) | 4/35 (11.4%) | 7/35 (20%) | p > 0.05 | p < 0.05 |
| Chelon auratus | 12/83 (14.4%) | - | 10/83 (12%) | 16/83 (19.2%) | 2/83 (2.4%) | 14/83 (16.8%) | 26/83 (31.3%) | 17/83 (20.4%) | p < 0.05 | p < 0.05 |
| Chelon ramada | 14/110 (12.7%) | 2/110 (1.8%) | 9/110 (8.1%) | 13/110 (11.8%) | 5/110 (4.5%) | 17/110 (15.4%) | 12/110 (10.9%) | 63/110 (57.2%) | p > 0.05 | p < 0.05 |
| Total | 34/239 (14.2%) | 3/239 (1.2%) | 28/239 (11.7%) | 40/239 (16.7%) | 13/239 (5.4%) | 45/239 (18.8%) | 47/239 (19.6%) | 91/239 (38.1%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polinas, M.; Padrós, F.; Merella, P.; Prearo, M.; Sanna, M.A.; Marino, F.; Burrai, G.P.; Antuofermo, E. Stages of Granulomatous Response Against Histozoic Metazoan Parasites in Mullets (Osteichthyes: Mugilidae). Animals 2021, 11, 1501. https://doi.org/10.3390/ani11061501
Polinas M, Padrós F, Merella P, Prearo M, Sanna MA, Marino F, Burrai GP, Antuofermo E. Stages of Granulomatous Response Against Histozoic Metazoan Parasites in Mullets (Osteichthyes: Mugilidae). Animals. 2021; 11(6):1501. https://doi.org/10.3390/ani11061501
Chicago/Turabian StylePolinas, Marta, Francesc Padrós, Paolo Merella, Marino Prearo, Marina Antonella Sanna, Fabio Marino, Giovanni Pietro Burrai, and Elisabetta Antuofermo. 2021. "Stages of Granulomatous Response Against Histozoic Metazoan Parasites in Mullets (Osteichthyes: Mugilidae)" Animals 11, no. 6: 1501. https://doi.org/10.3390/ani11061501
APA StylePolinas, M., Padrós, F., Merella, P., Prearo, M., Sanna, M. A., Marino, F., Burrai, G. P., & Antuofermo, E. (2021). Stages of Granulomatous Response Against Histozoic Metazoan Parasites in Mullets (Osteichthyes: Mugilidae). Animals, 11(6), 1501. https://doi.org/10.3390/ani11061501







