Patterns and Drivers of Rodent Abundance across a South African Multi-Use Landscape
Abstract
:Simple Summary
Abstract
1. Introduction
- (i)
- An area-typology hypothesis, i.e., cumulative effect of management-induced changes to vegetation, grazing pressure, etc., creates area-specific differences in rodent abundance. Patchiness will also be tested to acknowledge in which area each group is more or less clumped, regarding their abundance values. Although the exact effect of area on rodent abundance is not fully predictable [37] (given the disturbance gradient) we expected the communal lands to have the lowest values of abundance and highest patchiness (i.e., more clumped), followed by mixed farms and the game reserve, with higher abundances and lower patchiness;
- (ii)
- (iii)
- (iv)
2. Materials and Methods
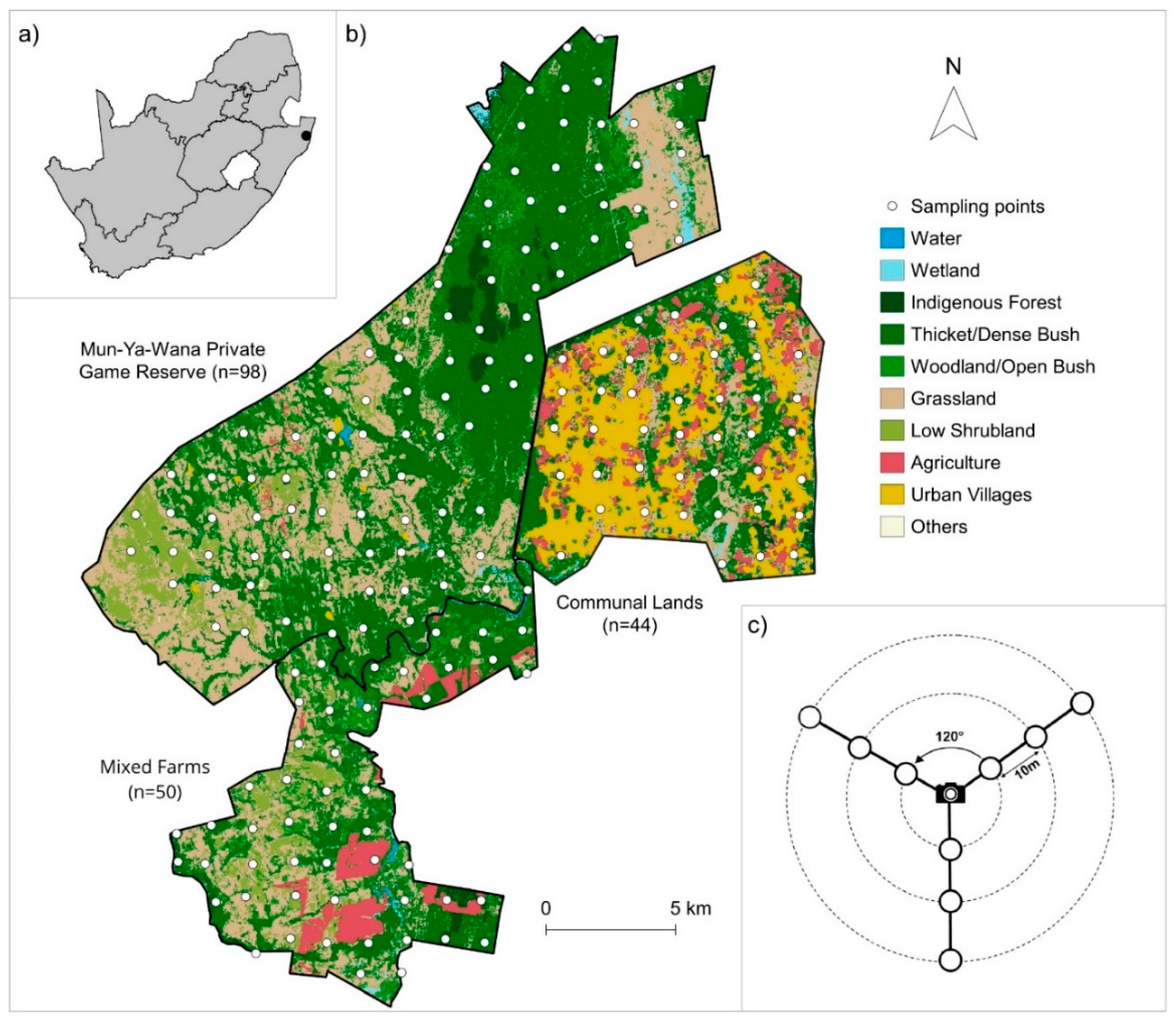
2.1. Study Area
2.2. Rodent Sampling
2.3. Environmental Variables
2.4. Data Analyses/Modelling
2.4.1. Spatial Patterns of Rodent Relative Abundance Across Areas and Size-Based Groups
2.4.2. Influence of Environmental Variables on Rodent Relative Abundance
3. Results
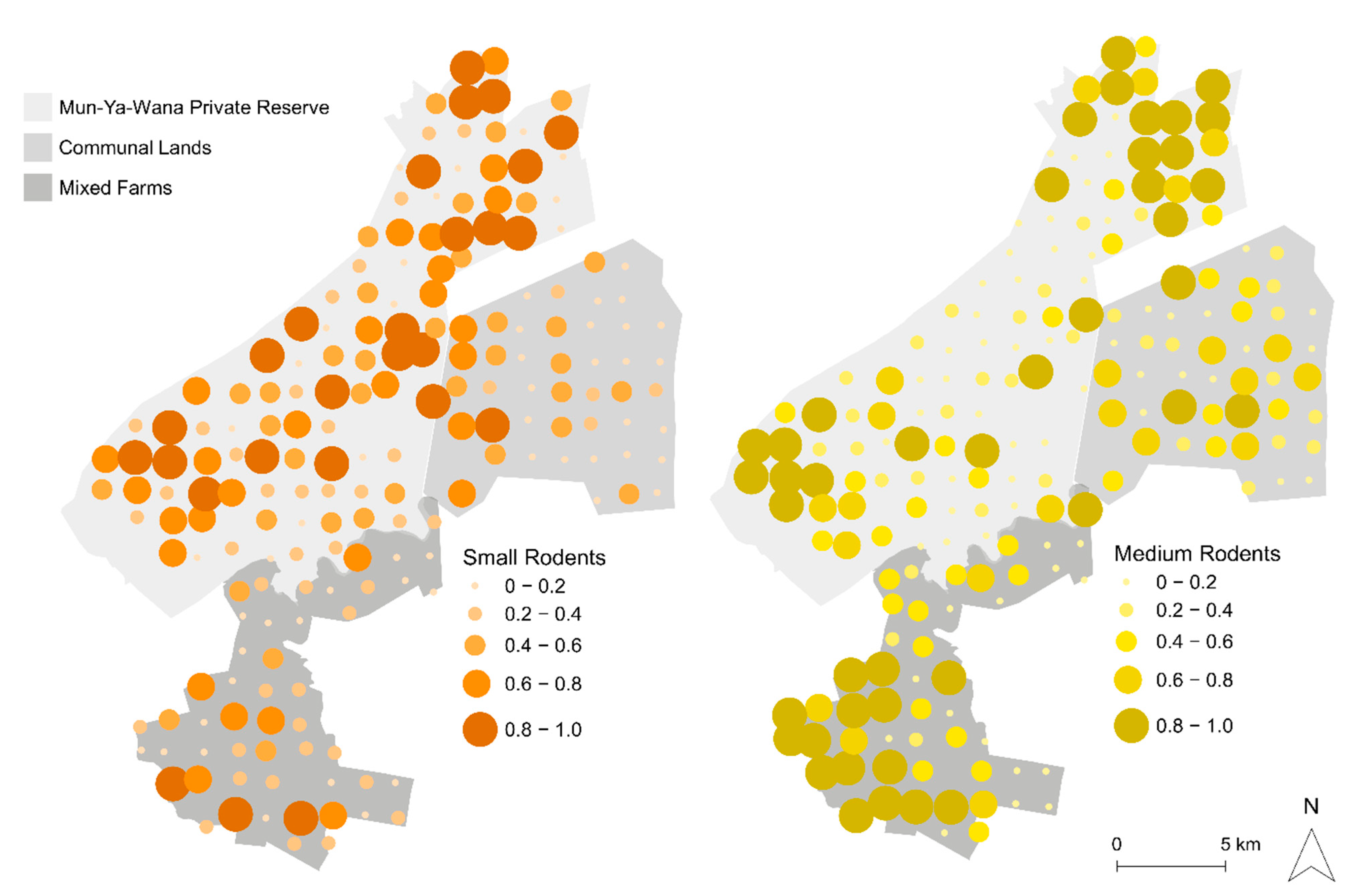
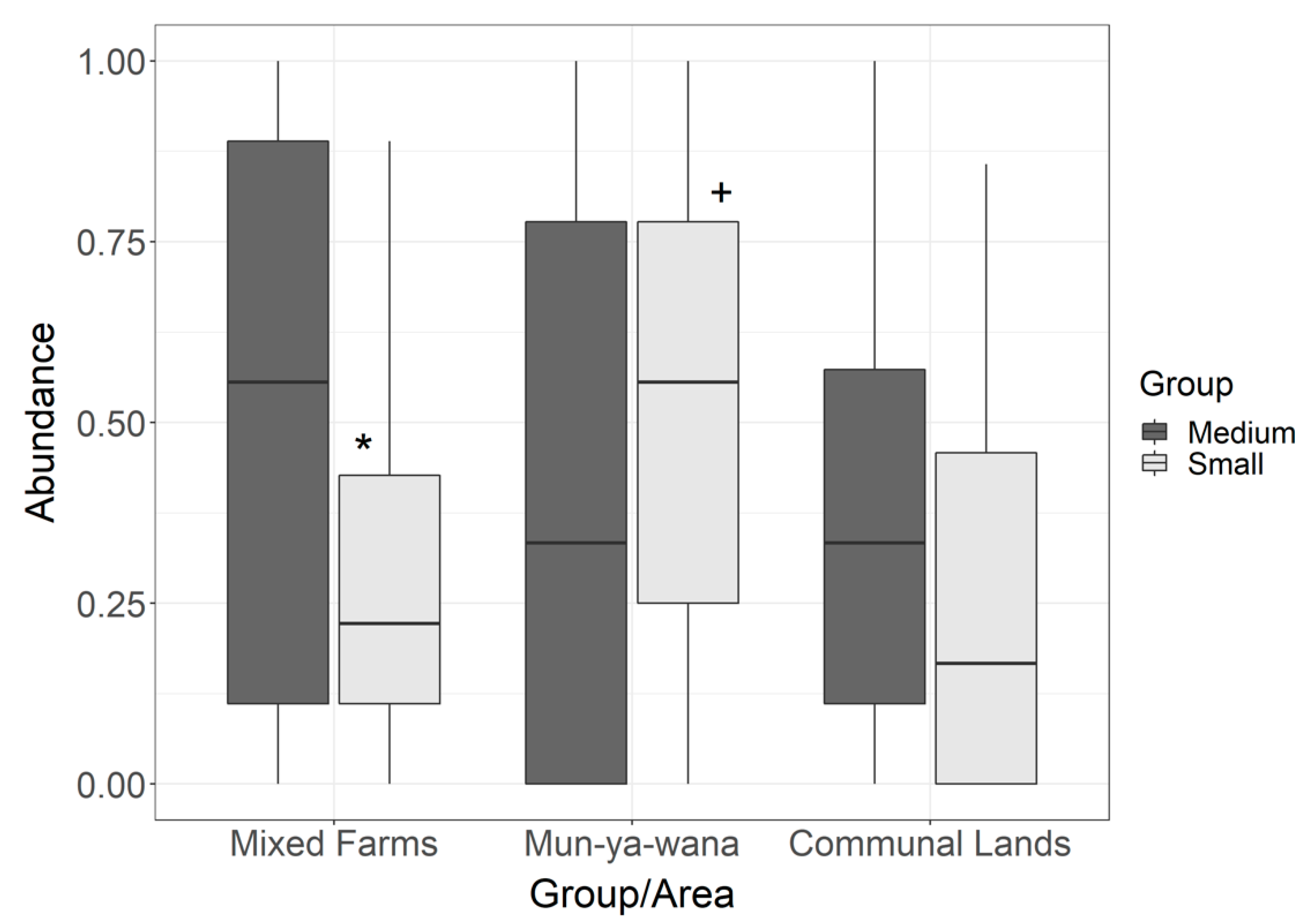
3.1. Spatial Patterns of Rodent Abundance Across Areas and Size-Based Groups
Rodent Patchiness
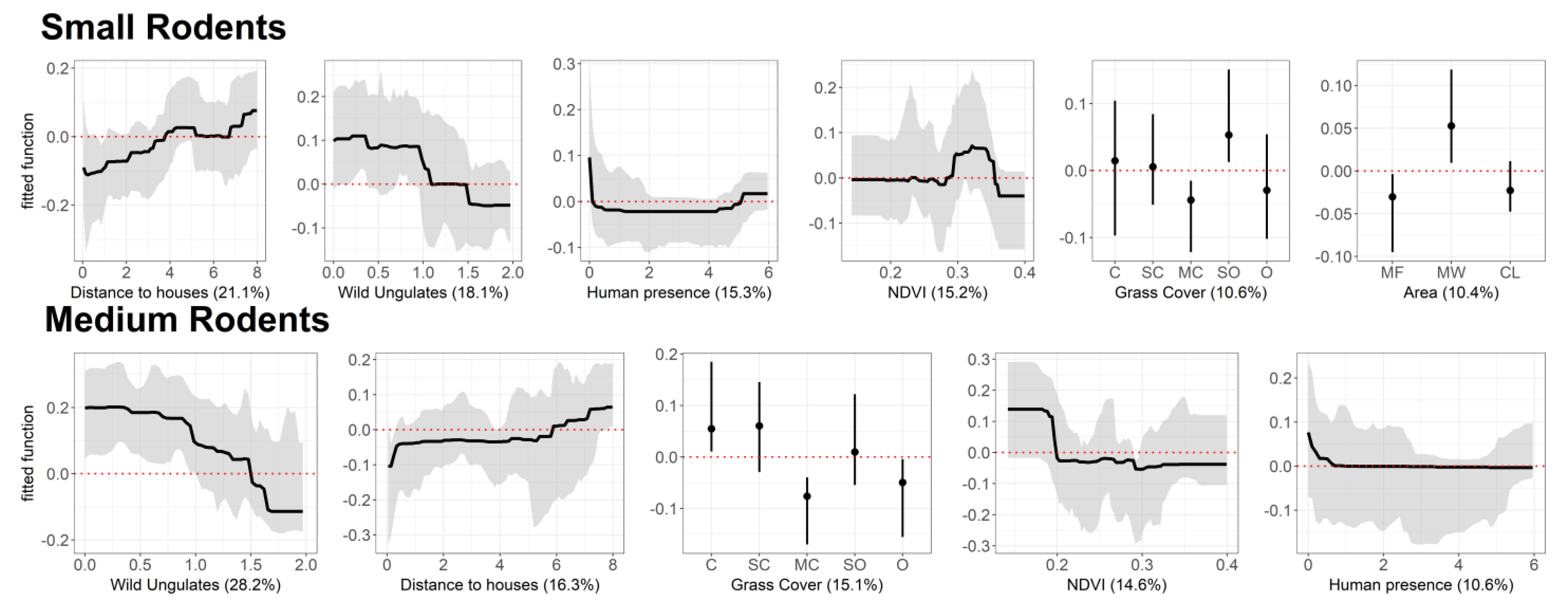
3.2. Drivers of Abundance
3.2.1. Small-Size Rodents
3.2.2. Medium-Size Rodents
4. Discussion
4.1. Context-Specific Responses and Variation Across Management Schemes
4.2. Fine-Scale Environmental Drivers of Rodent Abundance Across the Landscape
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCusker, B. Land use and cover change as an indicator of transformation on recently redistributed farms in Limpopo Province, South Africa. Hum. Ecol. 2004, 32, 49–75. [Google Scholar] [CrossRef]
- Smith, N.; Wilson, S.L. Changing Land Use Trends in the Thicket Biome: Pastoralism to Game Farming; Blackwell Publishing: Oxford, UK, 2002. [Google Scholar]
- Maitima, J.M.; Mugatha, S.M.; Reid, R.S.; Gachimbi, L.N.; Majule, A.; Lyaruu, H.; Pomery, D.; Mathai, S.; Mugisha, S. The linkages between land use change, land degradation and biodiversity across East Africa. Afr. J. Environ. Sci. Technol. 2009, 2, 310–325. [Google Scholar]
- Bender, D.J.; Contreras, T.A.; Fahrig, L. Habitat loss and population decline: A meta-analysis of the patch size effect. Ecology 1998, 79, 517–533. [Google Scholar] [CrossRef]
- Pitman, R.T.; Fattebert, J.; Williams, S.T.; Williams, K.S.; Hill, R.A.; Hunter, L.T.B.; Slotow, R.; Balme, G.A. The conservation costs of game ranching. Conserv. Lett. 2016, 10, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, P.A.; Romanach, S.S.; Davies-Mostert, H.T. The importance of conservancies for enhancing the value of game ranch land for large mammal conservation in southern Africa. J. Zool. 2009, 277, 99–105. [Google Scholar] [CrossRef]
- Child, B.A. The economics and governance of the wildlife economy in drylands in Southern Africa. In Natural Resources, Tourism and Community Livelihoods in Southern Africa; Routledge: Oxfordshire, UK, 2019; pp. 161–175. [Google Scholar]
- Kerley, G.I.; Pressey, R.L.; Cowling, R.M.; Boshoff, A.F.; Sims-Castley, R. Options for the conservation of large and medium-sized mammals in the Cape Floristic Region hotspot, South Africa. Biol. Conserv. 2003, 112, 169–190. [Google Scholar] [CrossRef]
- Rautenbach, A. Patterns and processes of rodent and shrew assemblages in the Savanna Biome of KwaZulu-Natal, South Africa. Ph.D. Thesis, University of KwaZulu-Natal, KwaZulu-Natal, South Africa, 2013. [Google Scholar]
- Di Minin, E.; Macmillan, D.C.; Goodman, P.S.; Escott, B.; Slotow, R.; Moilanen, A. Conservation businesses and conservation planning in a biological diversity hotspot. Conserv. Biol. 2013, 27, 808–820. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.T.; Stolton, S.U.E.; Dudley, N. Private protected areas in East and southern Africa: Contributing to biodiversity conservation and rural development. Parks 2005, 15, 67–76. [Google Scholar]
- Curveira-Santos, G.; Sutherland, C.; Santos-Reis, M.; Swanepoel, L.H. Responses of carnivore assemblages to decentralized conservation approaches in a South African landscape. J. Appl. Ecol. 2021, 58, 92–103. [Google Scholar] [CrossRef]
- Makundi, R.H.; Oguge, N.O.; Mwanjabe, P.S. Rodent pest management in East Africa—an ecological approach. In Book Ecologically-Based Rodent Management, 1st ed.; Singleton, G., Hinds, L., Leirs, H., Zhang, Z., Eds.; Australian Center for International Agricultural Research: Camberra, Australia, 1999; pp. 460–476. [Google Scholar]
- Mahlaba, T.A.A.; Monadjem, A.; McCleery, R.; Belmain, S.R. Domestic cats and dogs create a landscape of fear for pest rodents around rural homesteads. PLoS ONE 2017, 12, e0171593. [Google Scholar] [CrossRef] [Green Version]
- Thibault, M.; Brescia, F.; Jourdan, H.; Vidal, E. Invasive rodents, an overlooked threat for skinks in a tropical island hotspot of biodiversity. N. Z. J. Ecol. 2017, 41, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Avenant, N.L.; Cavallini, P. Correlating rodent community structure with ecological integrity, Tussen-die-Riviere Nature Reserve, Free State province, South Africa. Integr. Zool. 2007, 2, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.; Erlinge, S. Influence of predation on rodent populations. Oikos 1977, 29, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, P.; Koskela, E.; Mappes, T. Does risk of predation by mammalian predators affect the spacing behaviour of rodents? Two large-scale experiments. Oecologia 2000, 122, 487–492. [Google Scholar] [CrossRef]
- Cameron, G.N.; Scheel, D. Getting warmer: Effect of global climate change on distribution of rodents in Texas. J. Mammal. 2001, 82, 652–680. [Google Scholar] [CrossRef] [Green Version]
- Panzacchi, M.; Linnell, J.D.; Melis, C.; Odden, M.; Odden, J.; Gorini, L.; Andersen, R. Effect of land-use on small mammal abundance and diversity in a forest–farmland mosaic landscape in south-eastern Norway. For. Ecol. Manag. 2010, 259, 1536–1545. [Google Scholar] [CrossRef]
- Williams, S.E.; Marsh, H.; Winter, J. Spatial scale, species diversity, and habitat structure: Small mammals in Australian tropical rain forest. Ecology 2002, 83, 1317–1329. [Google Scholar] [CrossRef]
- Layme, V.M.G.; Lima, A.P.; Magnusson, W.E. Effects of fire, food availability and vegetation on the distribution of the rodent Bolomys lasiurus in an Amazonian savanna. J. Trop. Ecol. 2004, 20, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Holland, G.J.; Bennett, A.F. Differing responses to landscape change: Implications for small mammal assemblages in forest fragments. Biodivers. Conserv. 2009, 18, 2997–3016. [Google Scholar] [CrossRef]
- Hoffmann, A.; Zeller, U. Influence of variations in land use intensity on species diversity and abundance of small mammals in the Nama Karoo, Namibia. Belg. J. Zool. 2005, 135, 91–96. [Google Scholar]
- Bond, W.; Ferguson, M.; Forsyth, G. Small mammals and habitat structure along altitudinal gradients in the southern Cape mountains. Afr. Zool. 1980, 15, 34–43. [Google Scholar] [CrossRef]
- Keesing, F. Impacts of ungulates on the demography and diversity of small mammals in central Kenya. Oecologia 1998, 116, 381–389. [Google Scholar] [CrossRef]
- Delcros, G.; Taylor, P.J.; Schoeman, M.C. Ecological correlates of small mammal assemblage structure at different spatial scales in the savannah biome of South Africa. Mammalia 2015, 79, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Massawe, A.W.; Leirs, H.; Rwamugira, W.P.; Makundi, R.H. Effect of land preparation methods on spatial distribution of rodents in crop fields. In Book Rats, Mice and People: Rodent Biology and Management, 1st ed.; Australian Centre for International Agricultural Research: Camberra, Australia, 2003. [Google Scholar]
- Mortelliti, A.; Amori, G.; Annesi, F.; Boitani, L. Testing for the relative contribution of patch neighborhood, patch internal structure, and presence of predators and competitor species in determining distribution patterns of rodents in a fragmented landscape. Can. J. Zool. 2009, 87, 662–670. [Google Scholar] [CrossRef]
- Sutherland, G.D.; Harestad, A.S.; Price, K.; Lertzman, K.P. Scaling of natal dispersal distances in terrestrial birds and mammals. Conserv. Ecol. 2000, 4, 16. [Google Scholar] [CrossRef]
- Pacifici, M.; Rondinini, C.; Rhodes, J.R.; Burbidge, A.A.; Cristiano, A.; Watson, J.E.M.; Woinarski, J.C.Z.; Di Marco, M. Global correlates of range contractions and expansions in terrestrial mammals. Nat. Commun. 2020, 11, 2840. [Google Scholar] [CrossRef] [PubMed]
- Roques, K.G.; O’connor, T.G.; Watkinson, A.R. Dynamics of shrub encroachment in an African savanna: Relative influences of fire, herbivory, rainfall and density dependence. J. Appl. Ecol. 2001, 38, 268–280. [Google Scholar] [CrossRef]
- Blaum, N.; Rossmanith, E.; Jeltsch, F. Land use affects rodent communities in Kalahari savannah rangelands. Afr. J. Ecol. 2007, 45, 189–195. [Google Scholar] [CrossRef]
- Hurst, Z.M.; McCleery, R.A.; Collier, B.A.; Silvy, N.J.; Taylor, P.J.; Monadjem, A. Linking changes in small mammal communities to ecosystem functions in an agricultural landscape. Mamm. Biol. 2014, 79, 17–23. [Google Scholar] [CrossRef]
- Mulungu, L.S.; Mahlaba, T.A.; Massawe, A.W.; Kennis, J.; Crauwels, D.; Eiseb, S.; Monadjem, A.; Makundi, R.H.; Katakweba, A.A.S.; Leirs, H.; et al. Dietary differences of the multimammate mouse, Mastomys natalensis (Smith, 1834), across different habitats and seasons in Tanzania and Swaziland. Wildl. Res. 2011, 38, 640–646. [Google Scholar] [CrossRef] [Green Version]
- Dalmagro, A.D.; Vieira, E.M. Patterns of habitat utilization of small rodents in an area of Araucaria forest in Southern Brazil. Austral Ecol. 2005, 30, 353–362. [Google Scholar] [CrossRef]
- Caro, T.M. Species richness and abundance of small mammals inside and outside an African national park. Biol. Conserv. 2001, 98, 251–257. [Google Scholar] [CrossRef]
- Monadjem, A. Habitat preferences and biomasses of small mammals in Swaziland. Afr. J. Ecol. 1997, 35, 64–72. [Google Scholar] [CrossRef]
- Dunstan, C.; Fox, B. The effects of fragmentation and disturbance of rainforest on ground-dwelling small mammals on the Robertson Plateau, New South Wales, Australia. J. Biogeogr. 1996, 23, 187–201. [Google Scholar] [CrossRef]
- Balme, G.A.; Slotow, R.O.B.; Hunter, L.T. Edge effects and the impact of non-protected areas in carnivore conservation: Leopards in the Phinda–Mkhuze Complex, South Africa. Anim. Conserv. 2010, 13, 315–323. [Google Scholar] [CrossRef]
- Krug, W. Private supply of protected land in southern Africa: A review of markets, approaches, barriers and issues. In Workshop Paper, World Bank/OECD International Workshop on Market Creation for Biodiversity Products and Services, Paris; OECD: Paris, France, 2001; Volume 25. [Google Scholar]
- King, C.M.; Edgar, R.L. Techniques for trapping and tracking stoats (Mustela erminea); a review, and a new system. N. Z. J. Zool. 1977, 4, 193–212. [Google Scholar] [CrossRef] [Green Version]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations—The CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef] [Green Version]
- ASTER GDEM Data Version 2. Available online: https://search.earthdata.nasa.gov/search/ (accessed on 27 April 2019).
- Department of Environmental Affairs (DEA). 2013-14 SA National Land-Cover—Broad Parent Classes. Available online: http://www.sasdi.net/metaview.aspx?uuid=20c6336b75031c73041ec8b88372c3b0 (accessed on 27 April 2019).
- Glennon, M.J.; Porter, W.F.; Demers, C.L. An alternative field technique for estimating diversity of small-mammal populations. J. Mammal. 2002, 83, 734–742. [Google Scholar] [CrossRef] [Green Version]
- Hughes, J.J.; Ward, D.; Perrin, M.R. Predation risk and competition affect habitat selection and activity of Namib Desert gerbils. Ecology 1994, 75, 1397–1405. [Google Scholar] [CrossRef]
- Wilkinson, E.B.; Branch, L.C.; Miller, D.L.; Gore, J.A. Use of track tubes to detect changes in abundance of beach mice. J. Mammal. 2012, 93, 791–798. [Google Scholar] [CrossRef]
- Palma, A.R.T.; Gurgel-Gonçalves, R. Morphometric identification of small mammal footprints from ink tracking tunnels in the Brazilian Cerrado. Rev. Bras. Zool. 2007, 24, 333–343. [Google Scholar] [CrossRef]
- Greene, D.U.; Oddy, D.M.; Gore, J.A.; Gillikin, M.N.; Evans, E.; Gann, S.L.; Leone, E.H. Differentiating footprints of sympatric rodents in coastal dune communities: Implications for imperiled beach mice. J. Fish Wildl. Manag. 2018, 9, 593–601. [Google Scholar] [CrossRef]
- Delany, M.J. Ecology of small rodents in Africa. Mammal. Rev. 1986, 16, 1–41. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 12 March 2019).
- Edwards, E. A broad-scale structural classification of vegetation for practical purposes. Bothalia 1983, 14, 705–712. [Google Scholar] [CrossRef]
- FAO Country Analysis (GIEWS). Available online: http://www.fao.org/giews/countrybrief/country.jsp?code=ZAF&lang=ru&fbclid=IwAR3fdjZN3f-NmB1RDGa5Z7Q01OdiKy4ndt3xuFmGrWsHHiYisK_pWEdY30Y (accessed on 24 August 2021).
- Earth Explorer (Landsat 8 Images). Available online: https://earthexplorer.usgs.gov/ (accessed on 18 April 2019).
- Freitas, S.R.; Cerqueira, R.; Vieira, M.V. A device and standard variables to describe microhabitat structure of small mammals based on plant cover. Braz. J. Biol. 2002, 62, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.H.G.; Dickinson, N.M. Small mammal abundance in relation to microhabitat in a dry sub-humid grassland in Kenya. Afr. J. Ecol. 1985, 23, 223–234. [Google Scholar] [CrossRef]
- Dueser, R.D.; Shugart, H.H., Jr. Microhabitats in a forest-floor small mammal fauna. Ecology 1978, 59, 89–98. [Google Scholar] [CrossRef]
- Monadjem, A. Geographic distribution patterns of small mammals in Swaziland in relation to abiotic factors and human land-use activity. Biodivers. Conserv. 1999, 8, 223–237. [Google Scholar] [CrossRef]
- Christie, J.E.; Wilson, P.R.; Taylor, R.H.; Elliott, G. How elevation affects ship rat (Rattus rattus) capture patterns, Mt Misery, New Zealand. N. Z. J. Ecol. 2017, 41, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Miranda, C.S.; Gamarra, R.M.; Mioto, C.L.; Silva, N.M.; Conceição Filho, A.P.; Pott, A. Analysis of the landscape complexity and heterogeneity of the Pantanal wetland. Braz. J. Biol. 2018, 78, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, M. Mean crowding’. J. Anim. Ecol. 1967, 36, 1–30. [Google Scholar] [CrossRef]
- Revelle, M.W. Package ‘psych’: The Comprehensive R Archive Network. Version 1.9.12. 2015. Available online: https://cran.r-project.org/web/packages/psych/psych.pdf (accessed on 16 April 2019).
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Science & Business Media: New York, NY, USA, 2009. [Google Scholar]
- Filipe, A.F.; Cowx, I.G.; Collares-Pereira, M.J. Spatial modelling of freshwater fish in semi-arid river systems: A tool for conservation. River Res. Appl. 2002, 18, 123–136. [Google Scholar] [CrossRef]
- Package ‘gbm’. Available online: https://cran.r-project.org/web/packages/gbm/index.html (accessed on 16 April 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 12 March 2019).
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA; Available online: http://www.rstudio.com (accessed on 12 March 2019).
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- De’Ath, G. Boosted trees for ecological modeling and prediction. Ecology 2007, 88, 243–251. [Google Scholar] [CrossRef]
- Carslaw, D.C.; Taylor, P.J. Analysis of air pollution data at a mixed source location using boosted regression trees. Atmos. Environ. 2009, 43, 3563–3570. [Google Scholar] [CrossRef]
- Williams, G.J.; Aeby, G.S.; Cowie, R.O.; Davy, S.K. Predictive modeling of coral disease distribution within a reef system. PLoS ONE 2010, 5, e9264. [Google Scholar] [CrossRef] [Green Version]
- Colin, B.; Clifford, S.; Wu, P.P.; Rathmanner, S.; Mengersen, K. Using boosted regression trees and remotely sensed data to drive decision-making. Open J. Stat. 2017, 7, 859–875. [Google Scholar] [CrossRef] [Green Version]
- Abeare, S. Comparisons of Boosted Regression Tree, GLM and GAM Performance in the Standardization of Yellowfin Tuna Catch-Rate Data from the Gulf of Mexico. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2009. [Google Scholar]
- Averbeck, C.; Apio, A.; Plath, M.; Wronski, T. Environmental parameters and anthropogenic effects predicting the spatial distribution of wild ungulates in the Akagera savannah ecosystem. Afr. J. Ecol. 2009, 47, 756–766. [Google Scholar] [CrossRef]
- Swihart, R.K.; Atwood, T.C.; Goheen, J.R.; Scheiman, D.M.; Munroe, K.E.; Gehring, T.M. Patch occupancy of North American mammals: Is patchiness in the eye of the beholder? J. Biogeogr. 2003, 30, 1259–1279. [Google Scholar] [CrossRef]
- Lagesse, J.V.; Thondhlana, G. The effect of land-use on small mammal diversity inside and outside the Great Fish River Nature Reserve, Eastern Cape, South Africa. J. Arid Environ. 2016, 130, 76–83. [Google Scholar] [CrossRef]
- Keesing, F. Cryptic consumers and the ecology of an African savanna. BioScience 2000, 50, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Iwuala, M.O.; Braide, E.I.; Maduka, N. Observations on the food habits of some African rodents. Rev. Biol. Trop. 1979, 28, 227–236. [Google Scholar]
- Torre, I.; Díaz, M.; Martínez-Padilla, J.; Bonal, R.; Vinuela, J.; Fargallo, J.A. Cattle grazing, raptor abundance and small mammal communities in Mediterranean grasslands. Basic Appl. Ecol. 2007, 8, 565–575. [Google Scholar] [CrossRef]
- Tew, T.E.; Macdonald, D.W. The effects of harvest on arable wood mice Apodemus sylvaticus. Biol. Conserv. 1993, 65, 279–283. [Google Scholar] [CrossRef]
- Pounds, C.J. Niche overlap in sympatric populations of stoats (Mustela erminea) and weasels (M. nivalis) in Northeast Scotland. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 1981. [Google Scholar]
- Carrilho, M.; Teixeira, D.; Santos-Reis, M.; Rosalino, L.M. Small mammal abundance in Mediterranean Eucalyptus plantations: How shrub cover can really make a difference. For. Ecol. Manag. 2017, 391, 256–263. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Ali, Z.; Bhaskar, S.B. Basic statistical tools in research and data analysis. Indian J. Anaesth. 2016, 60, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Ostertagová, E.; Ostertag, O.; Kováč, J. Methodology and Application of the Kruskal-Wallis Test. Appl. Mech. Mater. 2014, 611, 115–120. [Google Scholar] [CrossRef]
- Kingdon, J.; Happold, D.; Butynski, T.; Hoffmann, M.; Happold, M.; Kalina, J. Mammals of Africa; A&C Black: London, UK, 2013; Volume 1. [Google Scholar]
- Havlicek, L.L.; Peterson, N.L. Robustness of the Pearson Correlation against Violations of Assumptions. Percept. Mot. Skills 1976, 43, 1319–1334. [Google Scholar] [CrossRef]

| Variable Acronym | Description | Mean/Range | Resolution | Source | Supporting References |
|---|---|---|---|---|---|
| AREA TYPE (H1) | |||||
| Area | Managment context | Mixed farms Mun-ya-wana Communal lands | Collected at point | - | [37] |
| VEGETATION STUCTURE (H2) | |||||
| Tree_Cover | % Tree Cover | 30.80/6–72% | 30 × 30 m | Global Forest Watch https://www.globalforestwatch.org/ (16 April 2019) | [27,56] |
| Shrub_Cover | % of Shrub cover | Continuous (C)—76–100% Semi-continuous (SC)—51–74% Moderated closed (MC)—26–50% Semi-open (SO)—11–25% Open (O)—0–10% | 30 m buffer | Visually estimated | [22,27,39,57,58,59,60] |
| Grass_Cover | % of Grass cover | Continuous (C)—76–100% Semi-continuous (SC)—51–74% Moderated closed (MC)—26–50% Semi-open (SO)—11–25% Open (O)—0–10% | 30 m buffer | Visually estimated | [22,25,38,57] |
| Land_use | Land use categories | Thicket Grassland Sand Forest Urban Villages | 30 m buffer | 2013–2014 National Land Cover South Africa-SASDI http://www.sasdi.net/ (16 April 2019) | [21,22,23] |
| NDVI | Normalized difference vegetation index calculated from Landsat images | 0.48/0.28–0.67 | 30 × 30 m | Landsat 8 https://earthexplorer.usgs.gov/ (18 April 2019) | [60,61] |
| UNGULATE PRESSURE (H3) | |||||
| Goats | Capture rate of goats (number of records per 100 days of trapping) | 0.16/0–1.88 | Collected at point | Camera-trapping survey | [9,24,26] |
| Livestock | Capture rate of cows (number of records per 100 days of trapping) | 0.20/0–3.17 | Collected at point | Camera-trapping survey | |
| Wild Ungulates | Capture rate of ungulates (number of records per 100 days of trapping) | 0.750/0–3.48 | Collected at point | Camera-trapping survey | |
| DISTURBANCE VARIABLES (H4) | |||||
| HUMANS | Capture rate of humans | 0.84/0–10 | Collected at point | Camera-trapping survey | [39] |
| DIST | Distance to houses | 2.738/0.031–9.867 km | Collected at point | Camera-trapping survey | |
| Area | Lloyd’s Index of Patchiness (γ) | |
|---|---|---|
| Small | Medium | |
| Mun-ya-wana game reserve | 1.128 | 1.529 |
| Mixed farms | 1.372 | 1.296 |
| Communal lands | 1.528 | 1.306 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, B.C.; Swanepoel, L.H.; Rosa, B.P.; Marques, T.A.; Rosalino, L.M.; Santos-Reis, M.; Curveira-Santos, G. Patterns and Drivers of Rodent Abundance across a South African Multi-Use Landscape. Animals 2021, 11, 2618. https://doi.org/10.3390/ani11092618
Afonso BC, Swanepoel LH, Rosa BP, Marques TA, Rosalino LM, Santos-Reis M, Curveira-Santos G. Patterns and Drivers of Rodent Abundance across a South African Multi-Use Landscape. Animals. 2021; 11(9):2618. https://doi.org/10.3390/ani11092618
Chicago/Turabian StyleAfonso, Beatriz C., Lourens H. Swanepoel, Beatriz P. Rosa, Tiago A. Marques, Luís M. Rosalino, Margarida Santos-Reis, and Gonçalo Curveira-Santos. 2021. "Patterns and Drivers of Rodent Abundance across a South African Multi-Use Landscape" Animals 11, no. 9: 2618. https://doi.org/10.3390/ani11092618
APA StyleAfonso, B. C., Swanepoel, L. H., Rosa, B. P., Marques, T. A., Rosalino, L. M., Santos-Reis, M., & Curveira-Santos, G. (2021). Patterns and Drivers of Rodent Abundance across a South African Multi-Use Landscape. Animals, 11(9), 2618. https://doi.org/10.3390/ani11092618







