Enzymes Present in Neutrophil Extracellular Traps May Stimulate the Fibrogenic PGF2α Pathway in the Mare Endometrium
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Mare’s Uteri and Blood and Experimental Design
2.2. Mare Endometrial Cytological and Histopathological Evaluation and Classification
2.3. Endometrial Explants In Vitro Culture
2.4. Assessment of Endometrial Explants Viability
2.5. Hormone Assays
2.6. Gene Transcription Analysis
2.7. Statistical Analysis
3. Results
3.1. Viability of Endometrial Explants
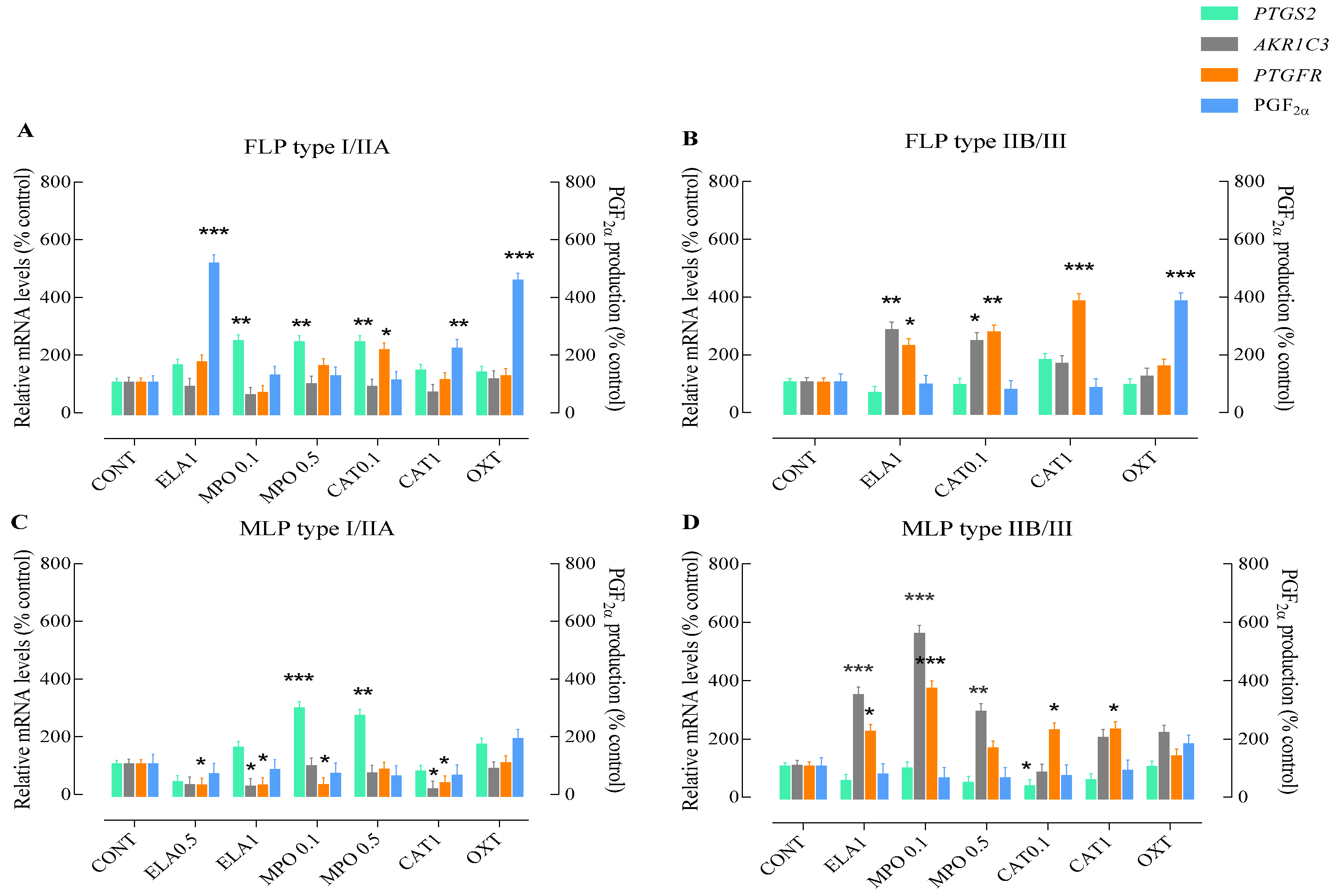
3.2. Effects of NETs Enzymes on the PGF2α Synthesis
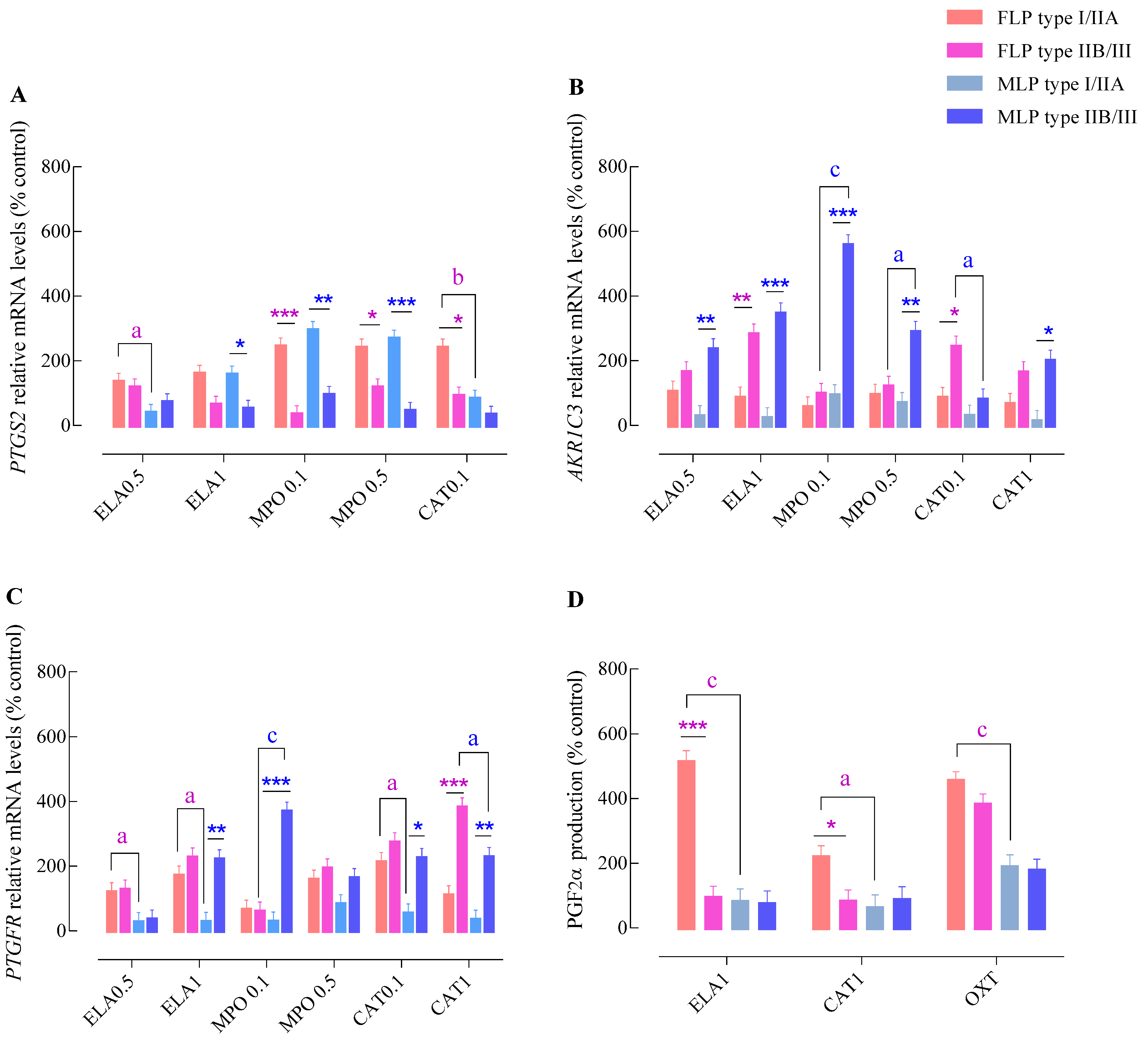
3.3. Influence of the Endometrial Category on the PGF2α Pathway Activation by NETs Enzymes
3.4. Influence of the Estrous Cycle Phase on the PGF2α—Pathway Activation by NETs Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Lögters, T.; Margraf, S.; Altrichter, J.; Cinatl, J.; Mitzner, S.; Windolf, J.; Scholz, M. The clinical value of neutrophil extracellular traps. Med. Microbiol. Immunol. 2009, 198, 211–219. [Google Scholar] [CrossRef]
- Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menegazzi, R.; Decleva, E.; Dri, P. Killing by neutrophil extracellular traps: Fact or folklore? Blood 2012, 119, 1214e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, B.E.; Grinstein, S. Unconventional roles of the NADPH oxidase: Signaling, ion homeostasis, and cell death. Sci. STKE 2007, 379, pe11. [Google Scholar] [CrossRef] [PubMed]
- Segel, G.B.; Halterman, M.W.; Lichtman, M.A. The paradox of the neutrophil’s role in tissue injury. J. Leukoc. Biol. 2011, 89, 359–372. [Google Scholar] [CrossRef]
- Sharony, R.; Yu, P.-J.; Park, J.; Galloway, A.C.; Mignatti, P.; Pintucci, G. Protein targets of inflammatory serine proteases and cardiovascular disease. J. Inflamm. 2010, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Ikegame, Y.; Yamashita, K.; Hayashi, S.; Yoshimura, S.; Nakashima, S.; Iwama, T. Neutrophil elastase inhibitor prevents ischemic brain damage via reduction of vasogenic edema. Hypertens. Res. 2010, 33, 703–707. [Google Scholar] [CrossRef]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015, 14, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Pulli, B.; Ali, M.; Iwamoto, Y.; Zeller, M.W.G.; Schob, S.; Linnoila, J.J.; Chen, J.W. Myeloperoxidase–Hepatocyte–Stellate Cell Cross Talk Promotes Hepatocyte Injury and Fibrosis in Experimental Nonalcoholic Steatohepatitis. Antioxid. Redox Signal. 2015, 23, 1255–1269. [Google Scholar] [CrossRef] [Green Version]
- Chrysanthopoulou, A.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Mikroulis, D.; Konstantinidis, T.; Sivridis, E.; Koffa, M.; Giatromanolaki, A.; Boumpas, D.T.; et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J. Pathol. 2014, 233, 294–307. [Google Scholar] [CrossRef]
- Atamas, S. Complex cytokine regulation of tissue fibrosis. Life Sci. 2002, 72, 631–643. [Google Scholar] [CrossRef]
- Oga, T.; Matsuoka, T.; Yao, C.; Nonomura, K.; Kitaoka, S.; Sakata, D.; Kita, Y.; Tanizawa, K.; Taguchi, Y.; Chin, K.; et al. Prostaglandin F2α receptor signaling facilitates bleomycin induced pulmonary fibrosis independently of transforming growth factor-β. Nat. Med. 2009, 15, 1426–1430. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arter. Thromb Vasc Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Fortier, M.A.; Krishnaswamy, K.; Danyod, G.; Boucher-Kovalik, S.; Chapdalaine, P. A postgenomic integrated view of prostaglandins in reproduction: Implications for other body systems. J. Physiol. Pharmacol. 2008, 59, 65–89. [Google Scholar] [PubMed]
- Ueno, N.; Takegoshi, Y.; Kamei, D.; Kudo, I.; Murakami, M. Coupling between cyclooxygenases and terminal prostanoid synthases. Biochem. Biophys. Res. Commun. 2005, 338, 70–76. [Google Scholar] [CrossRef]
- Hata, A.N.; Breyer, R.M. Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004, 103, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Aihara, K.; Handa, T.; Oga, T.; Watanabe, K.; Tanizawa, K.; Ikezoe, K.; Taguchi, Y.; Sato, H.; Chin, K.; Nagai, S.; et al. Clinical relevance of plasma prostaglandin F2α metabolite concentrations in patients with idiopathic pulmonary fibrosis. PLoS ONE 2013, 8, e66017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oga, T.; Handa, T.; Mishima, M.; Chin, K.; Narumyia, S. erRoles of eicosanoids in pulmonary fibrosis. Inflamm. Regen. 2013, 33, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Olman, M.A. Beyond TGF-β: A prostaglandin promotes fibrosis. Nat. Med. 2009, 15, 1360–1361. [Google Scholar] [CrossRef]
- Ding, W.Y.; Ti, Y.; Wang, J.; Wang, Z.H.; Xie, G.L.; Shang, Y.Y.; Tang, M.-X.; Zhang, Y.; Zhang, W.; Zhong, M. The Prostaglandin F2α facilitates collagen synthesis in cardiac fibroblasts via an F-prostanoid receptor/protein kinase C/Rho kinase pathway independent of transforming growth factor β1. Int. J. Biochem. Cell Biol. 2012, 44, 1031–1039. [Google Scholar] [CrossRef]
- Ding, W.; Liu, L.; Wang, Z.; Tang, M.-X.; Ti, Y.; Han, L.; Zhang, L.; Zhang, Y.; Zhong, M.; Zhang, W. FP-receptor gene silencing ameliorates myocardial fibrosis and protects from diabetic cardiomyopathy. J. Mol. Med. 2014, 92, 629. [Google Scholar] [CrossRef]
- Kanno, Y.; Kawashita, E.; Kokado, A.; Okada, K.; Ueshima, S.; Matsuo, O.; Matsuno, H. Alpha2-Antiplasmin Regulates the Development of dermal fibrosis in mice by Prostaglandin F2α synthesis through adipose triglyceride Lipase/Calcium-independent phospholipase. Arthritis Rheum. 2013, 65, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Bastiaansen-Jenniskens, Y.M.; Wei, W.; Feijt, C.; Waarsing, J.H.; Verhaar, J.A.; Zuurmond, A.M.; Hanemaaijer, R.; Stoop, R.; van Osch, G.J. Stimulation of fibrotic processes by the infrapatellar fat pad in cultured synoviocytes from patients with osteoarthritis: A possible role for prostaglandin f2α. Arthritis Rheum. 2013, 65, 2070–2080. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, C.; Ellenberger, C.; Mattos, R.C.; Aupperle, H.; Dhein, S.; Stief, B.; Schoon, H.-A. The equine endometrosis: New insights into the pathogenesis. Anim. Reprod. Sci. 2009, 111, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.; Cabezas, J.; Ramírez, G.; Saravia, F.; Lleretny, R.A.; Castro, F.O. Complimentary Diagnostic Tools for Endometrosis in Biopsies of Mares with Clinical Subfertility. Acta Sci. Vet. 2020, 48, 1717. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Carneiro, C.; Alexandre-Pires, G.; Brito, P.; Pereira, C.; Nunes, T.; Galvão, A.; Leitão, A.; Vilela, C.; Ferreira-Dias, G. Neutrophil extracellular traps formation by bacteria causing endometritis in the mare. J. Reprod. Immunol. 2014, 106, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Szóstek, A.Z.; Siemieniuch, M.J.; Lukasik, K.; Galvão, A.M.; Ferreira-Dias, G.M.; Skarzynski, D.J. mRNA transcription of prostaglandin synthases and their products in the equine endometrium in the course of fibrosis. Theriogenology 2012, 78, 768–776. [Google Scholar] [CrossRef]
- Ginther, O.J. Characteristics of the ovulatory season. In Reproductive Biology of the Mare: Basic and Applied Aspects, 2nd ed.; Equiservices: Cross Plains, WI, USA, 1992; pp. 173–232. [Google Scholar]
- Da Costa, R.R.; Serrão, P.M.; Monteiro, S.; Pessa, P.; Silva, J.R.; Ferreira-Dias, G. Caspase-3 mediated apoptosis and cell proliferation in the equine endometrium during the oestrous cycle. Reprod. Fertil. Dev. 2007, 19, 925–932. [Google Scholar] [CrossRef]
- Riddle, W.T.; LeBlanc, M.M.; Stromberg, A.J. Relationships between uterine culture, ccytology and pregnancy rates in a Thoroughbred practice. Theriogenology 2007, 68, 395–402. [Google Scholar] [CrossRef]
- Urban, C.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, A.V.; Gauthier, A.; Brea, D.; Varaigne, F.; Diot, P.; Gauthier, F.; Attucci, S. Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. Am. J. Respir. Cell Mol. Biol. 2012, 47, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Voynow, J.A.; Fischer, B.M.; Zheng, S. Proteases and Cystic Fibrosis. Int. J. Biochem. Cell Biol. 2008, 40, 1238–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glusa, E.; Adam, C. Endothelium-dependent relaxation induced by cathepsin G in porcine pulmonary arteries. Br. J. Pharmacol. 2001, 133, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Boudjeltia, K.Z.; Moguilevsky, N.; Legssyer, I.; Babar, S.; Guillaume, M.; Delree, P.; Vanhaeverbeek, M.; Brohee, D.; Ducobu, J.; Remacle, C. Oxidation of low density lipoproteins by myeloperoxidase at the surface of endothelial cells: An additional mechanism to subendothelium oxidation. Biochem. Biophys. Res. Commun. 2004, 325, 434–438. [Google Scholar] [CrossRef]
- Parrilla-Hernandez, S.; Ponthier, J.; Franck, T.Y.; Serteyn, D.D.; Deleuze, S.C. High concentrations of myeloperoxidase in the equine uterus as an indicator of endometritis. Theriogenology 2014, 81, 936–940. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, J.M. Endometritis in the mare: A diagnostic study comparing cultures from swab and biopsy. Theriogenology 2005, 64, 510–518. [Google Scholar] [CrossRef]
- Kenney, R.M.; Doig, P.A. Equine endometrial biopsy. In Current Therapy Theriogenology; Morrow, D.A., Ed.; WB Saunders: Philadelphia, PA, USA, 1986; pp. 723–729. [Google Scholar]
- Szóstek, A.Z.; Lukasik, K.; Galvão, A.M.; Ferreira-Dias, G.M.; Skarzynski, D.J. Impairment of the interleukin system in equine endometrium during the course of endometrosis. Biol. Reprod. 2013, 89, 79. [Google Scholar] [CrossRef]
- Nash, D.; Lane, E.; Herath, S.; Sheldon, I.M. Endometrial explant culture for characterizing equine endometritis. Am. J. Reprod. Immunol. 2008, 59, 105–117. [Google Scholar] [CrossRef]
- Szóstek, A.Z.; Galvão, A.M.; Ferreira-Dias, G.M.; Skarzynski, D.J. Ovarian steroids affect prostaglandin production in equine endometrial cells in vitro. J. Endocrinol. 2014, 220, 263–276. [Google Scholar] [CrossRef] [Green Version]
- Carranza-Torres, I.E.; Guzman-Delgado, N.E.; Coronado-Martínez, C.; Viveros-Valdez, E.; Moran-Martínez, J.; Carranza-Rosales, P. Culture of breast tumor explants as a multicellular system for the screening of natural compounds with antineoplastic potential. BioMed Res. Int. 2015, 2015, 618021. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Galvão, A.; Pinto-Bravo, P.; Pinheiro, J.; Gamboa, S.; Silva, E.; Mateus, L.; Ferreira-Dias, G. Expression of prostaglandin synthases, ovarian steroids and oxytocin receptors in equine endometrium during luteal maintenance induced by chronic administration of oxytocin. Theriogenology 2017, 87, 193–204. [Google Scholar] [CrossRef]
- Da Costa, R.R.; Ferreira-Dias, G.; Mateus, L.; Korzekwa, A.; Andronowska, A.; Platek, R.; Skarzynski, D.J. Endometrial nitric oxide production and nitric oxide synthases in the equine endometrium: Relationship with microvascular density during the estrous cycle. Domest. Anim. Endocrinol. 2007, 32, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.; Skarzynski, D.J.; Lukasik, K.; Ramilo, D.; Tramontano, A.; Mollo, A.; Mateus, L.M.; Ferreira-Dias, G.M. Is the Fas/Fas ligand system involved in equine corpus luteum functional regression? Biol. Reprod. 2010, 83, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e1148. [Google Scholar] [CrossRef] [Green Version]
- Rebordão, M.R.; Amaral, A.; Łukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvao, A.M.; Skarzynski, D.J.; Ferreira-Dias, G. Constituents of neutrophil extracellular traps induce in vitro collagen formation in mare endometrium. Theriogenology 2018, 113, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fernald, R.D. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Hogg, J.C.; Senior, R.M. Chronic obstructive pulmonary disease-part 2: Pathology and biochemistry of emphysema. Thorax 2002, 57, 830–834. [Google Scholar] [CrossRef] [Green Version]
- Szóstek-Mioduchowska, A.Z.; Baclawska, A.; Rebordão, M.R.; Ferreira-Dias, G.; Skarzynski, D.J. Prostaglandins effect on matrix metallopeptidases and collagen in mare endometrial fibroblasts. Theriogenology 2020, 153, 74–84. [Google Scholar] [CrossRef]
- Amaral, A.; Fernandes, C.; Lukasik, K.; Szóstek-Mioduchowska, A.; Baclawska, A.; Rebordão, M.R.; Aguiar-Silva, J.; Pinto-Bravo, P.; Skarzynski, D.J.; Dias, G.M.L.F. Elastase inhibition affects collagen transcription and prostaglandin secretion in mare endometrium during the estrous cycle. Reprod. Domest. Anim. 2018, 53 (Suppl. 2), 66–69. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Amaral, A.; Lukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Impairment of the antifibrotic prostaglandin E2 pathway may influence neutrophil extracellular traps–induced fibrosis in the mare endometrium. Domest. Anim. Endocrinol. 2019, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.; Thaler, T.; Meibom, D.; Meininghaus, M.; Jörißen, H.; Dietz, L.; Terjung, C.; Bairlein, M.; von Bühler, C.J.; Anlauf, S.; et al. Potent and Selective Human Prostaglandin F (FP) Receptor Antagonist (BAY-6672) for the Treatment of Idiopathic Pulmonary Fibrosis (IPF). J. Med. Chem. 2020, 63, 11639–11662. [Google Scholar] [CrossRef] [PubMed]
- Keerthisingam, C.B.; Jenkins, R.G.; Harrison, N.K.; Hernandez-Rodriguez, N.A.; Booth, H.; Laurent, G.J.; Hart, S.L.; Foster, M.L.; McAnulty, R.J. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am. J. Pathol. 2001, 158, 1411–1422. [Google Scholar] [CrossRef]
- Szóstek, A.Z.; Galvão, A.M.; Hojo, T.; Okuda, K.; Skarzynski, D.J. Interleukins Affect Equine Endometrial Cell Function: Modulatory Action of Ovarian Steroids. Mediat. Inflamm. 2014, 2014, 208103. [Google Scholar] [CrossRef] [Green Version]
- Vernon, M.W.; Zavy, M.T.; Asquith, R.L.; Sharp, D.C. Prostaglandin F2alpha in the equine endometrium: Steroid modulation and production capacities during the estrous cycle and early pregnancy. Biol. Reprod. 1981, 25, 581–589. [Google Scholar] [CrossRef]
- Woodward, E.M.; Troedsson, M.H. Equine breeding-induced endometritis: A review. J. Equine Vet. Sci. 2013, 33, 673–682. [Google Scholar] [CrossRef]
- Katila, T. Onset and duration of uterine inflammatory response of mares after insemination with fresh semen. Biol. Reprod. Monogr. 1995, 1, 515–517. [Google Scholar] [CrossRef]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Betancourt, A.; Horohov, D.; Scoggin, K.E.; Squires, E.L.; Troedsson, M.H.T. Endometrial inflammatory markers of the early immune response in mares susceptible or resistant to persistent breeding-induced endometritis. Reproduction 2013, 145, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Cadario, M.E.; Thatcher, W.; Klapstein, E.; Merrit, A.; Archbald, L.; Thatcher, M.; LeBlanc, M. Dynamics of prostaglandin secretion, intrauterine fluid and uterine clearance in reproductively normal mares and mares with delayed uterine clearance. Theriogenology 1999, 52, 1181–1192. [Google Scholar] [CrossRef]
- Christoffersen, M.; Woodward, E.; Bojesen, A.M.; Jacobsen, S.; Petersen, M.R.; Troedsson, M.H.; Lehn-Jensen, H. Inflammatory responses to induced infectious endometritis in mares resistant or susceptible to persistent endometritis. BMC Vet. Res. 2012, 29, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nash, D.M.; Sheldon, I.M.; Herath, S.; Lane, E.A. Markers of the uterine innate immune response of the mare. Anim. Reprod. Sci. 2010, 119, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, H.K.; Beg, M.A.; Burnette, R.R.; Ginther, O.J. Plasma clearance and half-life of prostaglandin F2alpha: A comparison between mares and heifers. Biol. Reprod. 2012, 87, 18. [Google Scholar] [CrossRef]
- Kozai, K.; Tokuyama, S.; Szóstek, A.Z.; Toishi, Y.; Tsunoda, N.; Taya, K.; Sakatani, M.; Takahashi, M.; Nambo, Y.; Skarzynski, D.J.; et al. Evidence for a PGF2α auto-amplification system in the endometrium in mares. Reproduction 2016, 151, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Blesson, C.S.; Sahlin, L. Prostaglandin E and F receptors in the uterus. Recept. Clin. Investig. 2014, 1, e115. [Google Scholar]
- Ruijter-Villani, M.; van Tol, H.T.; Stout, T.A. Effect of pregnancy on endometrial expression of luteolytic pathway components in the mare. Reprod. Fertil. Dev. 2015, 27, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Rebordão, M.R.; Amaral, A.; Galvão, A.; Szótek, A.Z.; Pinto-Bravo, P.; Skarzynski, D.S.; Dias, G.M.L.F. Dual effect of hormones on mare reproductive physiology and dysfunction. Pferdeheilkunde Equine Med. 2016, 32, 36–38. [Google Scholar] [CrossRef]
- Coffman, E.A.; Pinto, C.R. A Review on the Use of Prostaglandin F2α for Controlling the Estrous Cycle in Mares. J. Equine Veter- Sci. 2016, 40, 34–40. [Google Scholar] [CrossRef]
- Fabian, E.; Gomes, C.; Birk, B.; Williford, T.; Hernandez, T.R.; Haase, C.; Zbranek, R.; Van Ravenzwaay, B.; Landsiedel, R. In vitro-to-in vivo extrapolation (IVIVE) by PBTK modeling for animal-free risk assessment approaches of potential endocrine-disrupting compounds. Arch. Toxicol. 2019, 93, 401–416. [Google Scholar] [CrossRef] [Green Version]
| Gene (Acession Number) | Sequence 5′-3 | Amplicon (Base Pairs) |
|---|---|---|
| AKR1C3 (XM_001500921.1) | Forward: TGGGTCACTTTCCTTCAACCA | 200 |
| Reverse: CTTCTCCATTGCCTCCCATGT | ||
| PTGFR (NM_001081806.3) | Forward: GTGCAATGCCATCACAGGAA | 225 |
| Reverse: GCCATTCGGAGAGCAAACAG | ||
| PTGS2 (NM_001081775.2) | Forward: TGCTGTTCCAACCCGTGTC | 204 |
| Reverse: GACAATGTTCCAGACTCCCTTGA | ||
| RPL32 (XM_001492042.6) | Forward: AGCCATCTACTCGGCGTCA | 144 |
| Reverse: GTCAATGCCTCTGGGTTTCC |
| Interactions | PTGS2 | AKR1C3 | PTGFR | PGF2α |
|---|---|---|---|---|
| estrous cycle X endometrial categories | 0.6387 | <0.0001 | 0.0008 | <0.0001 |
| estrous cycle X treatments | 0.0004 | <0.0001 | <0.0001 | <0.0001 |
| endometrial categories X treatments | <0.0001 | <0.0001 | <0.0001 | 0.003 |
| estrous cycle X endometrial categories X treatments | 0.2969 | <0.0001 | 0.00005 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebordão, M.R.; Amaral, A.; Fernandes, C.; Silva, E.; Lukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Enzymes Present in Neutrophil Extracellular Traps May Stimulate the Fibrogenic PGF2α Pathway in the Mare Endometrium. Animals 2021, 11, 2615. https://doi.org/10.3390/ani11092615
Rebordão MR, Amaral A, Fernandes C, Silva E, Lukasik K, Szóstek-Mioduchowska A, Pinto-Bravo P, Galvão A, Skarzynski DJ, Ferreira-Dias G. Enzymes Present in Neutrophil Extracellular Traps May Stimulate the Fibrogenic PGF2α Pathway in the Mare Endometrium. Animals. 2021; 11(9):2615. https://doi.org/10.3390/ani11092615
Chicago/Turabian StyleRebordão, Maria Rosa, Ana Amaral, Carina Fernandes, Elisabete Silva, Karolina Lukasik, Anna Szóstek-Mioduchowska, Pedro Pinto-Bravo, António Galvão, Dariusz J. Skarzynski, and Graça Ferreira-Dias. 2021. "Enzymes Present in Neutrophil Extracellular Traps May Stimulate the Fibrogenic PGF2α Pathway in the Mare Endometrium" Animals 11, no. 9: 2615. https://doi.org/10.3390/ani11092615
APA StyleRebordão, M. R., Amaral, A., Fernandes, C., Silva, E., Lukasik, K., Szóstek-Mioduchowska, A., Pinto-Bravo, P., Galvão, A., Skarzynski, D. J., & Ferreira-Dias, G. (2021). Enzymes Present in Neutrophil Extracellular Traps May Stimulate the Fibrogenic PGF2α Pathway in the Mare Endometrium. Animals, 11(9), 2615. https://doi.org/10.3390/ani11092615









