Effect of Sustained Administration of Thymol on Its Bioaccessibility and Bioavailability in Rabbits
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Care and Use
2.2. Animals and Housing
2.3. Diet and Chemical Analyses
2.4. Thymol Stability in Feed
2.5. Sampling
2.6. Thymol Analyses in Plasma, Tissues, Large Intestinal Content and Faeces
2.7. Statistical Analysis
3. Results
3.1. Thymol Stability in Feed
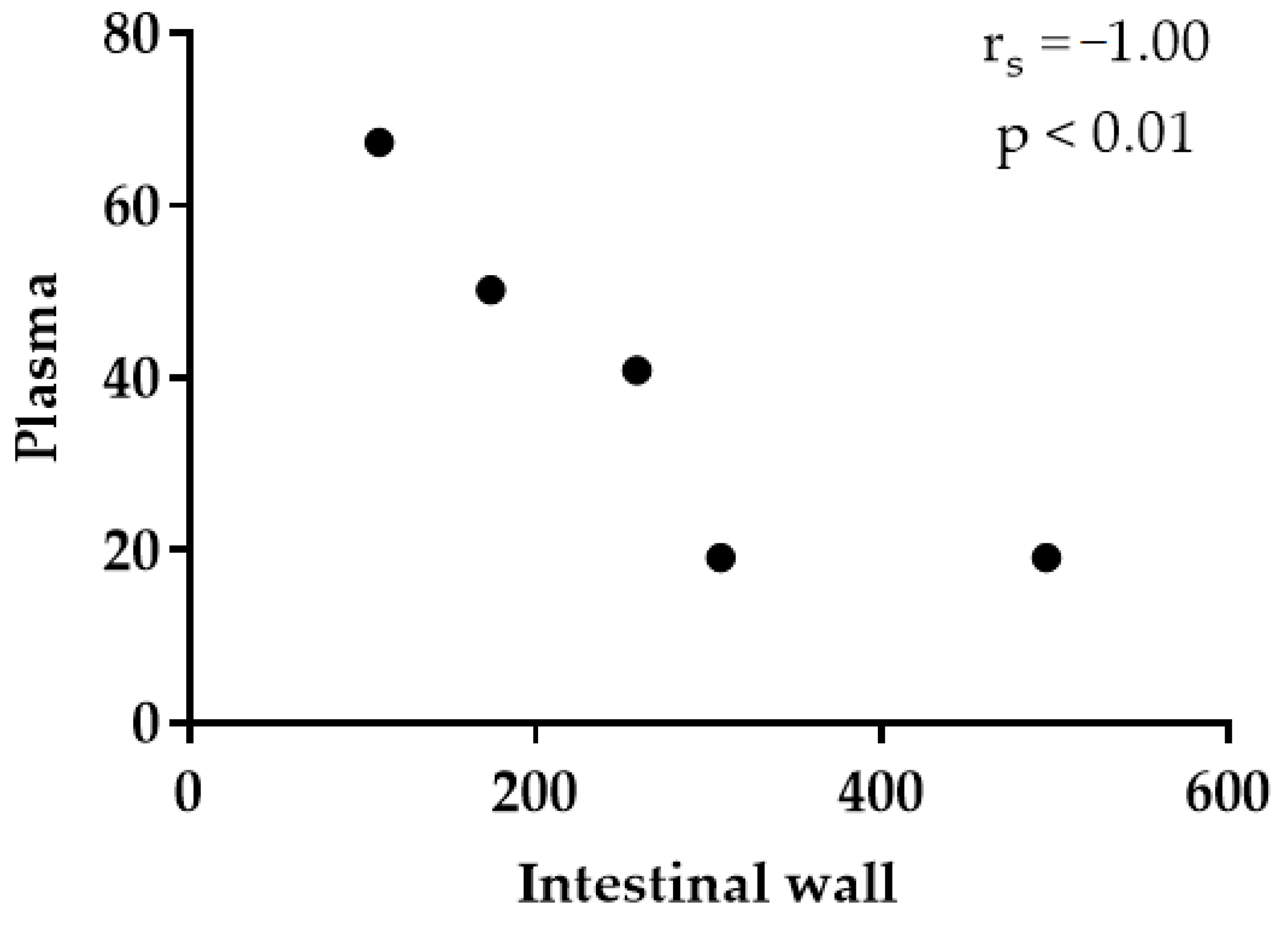
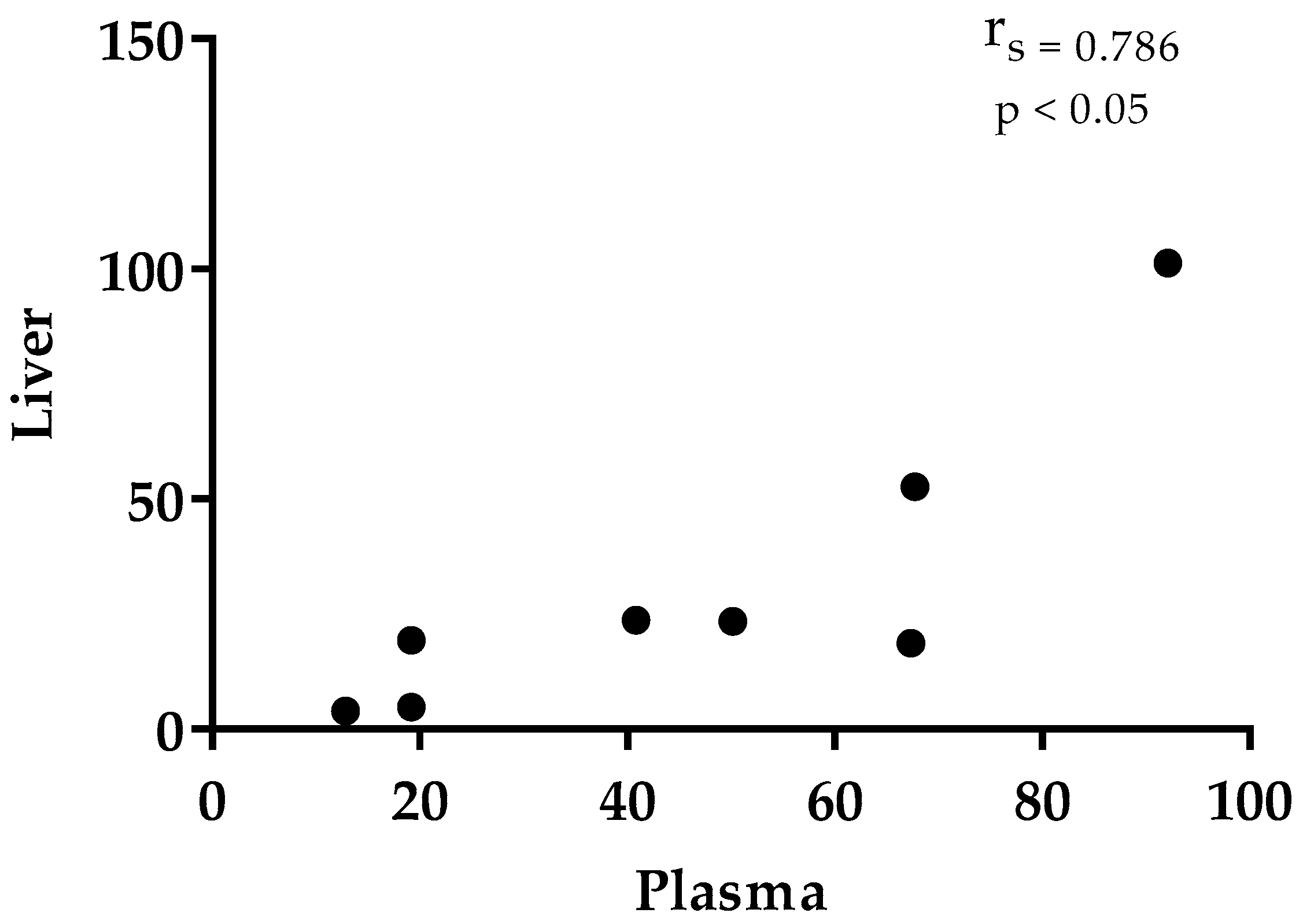
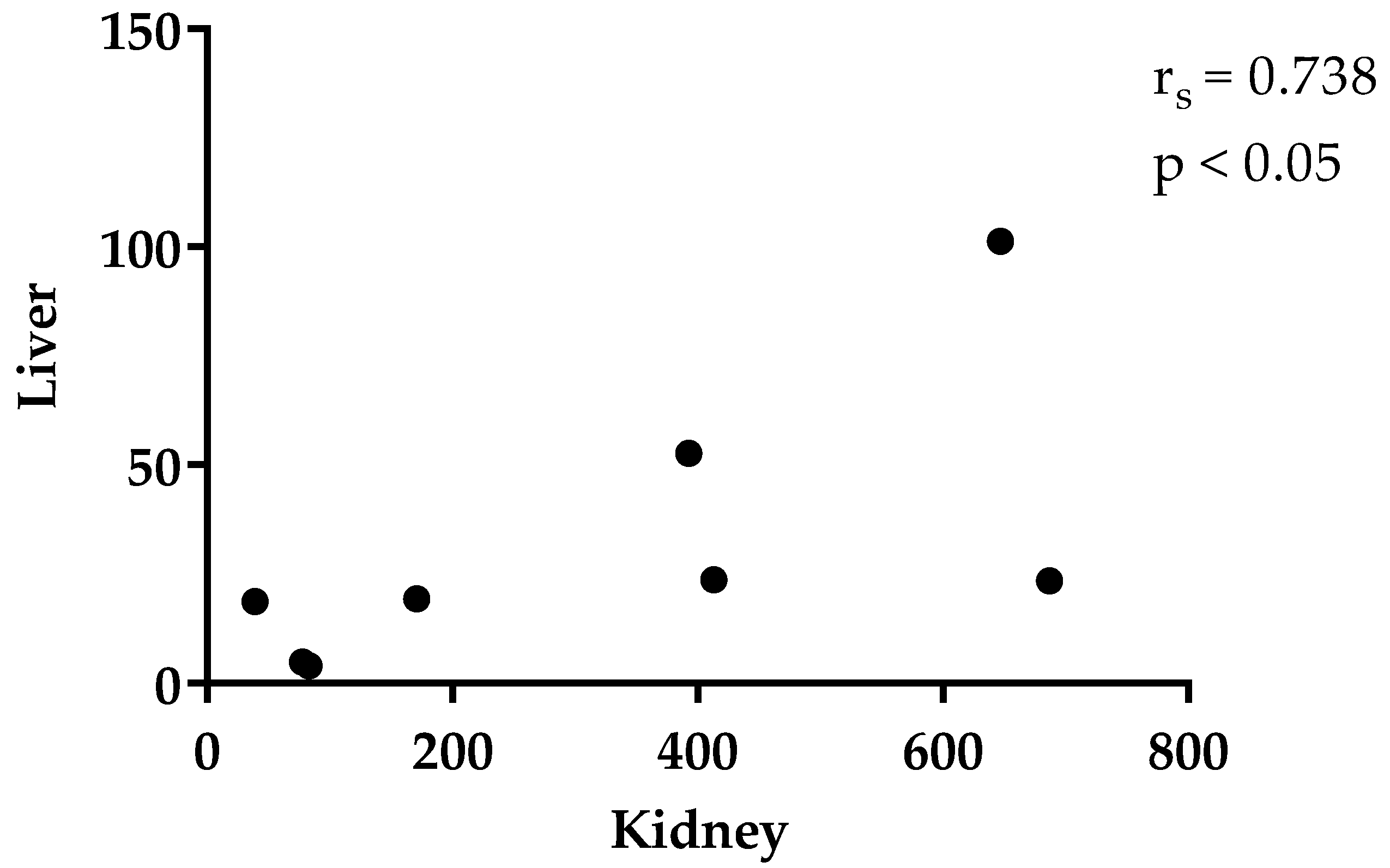
3.2. Thymol in Plasma and Tissues
3.3. Thymol in Caecum, Colon and Feces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kostadinović, L.; Lević, J. Effect of phytoadditives in poultry and pig diseases. J. Agron. Technol. Eng. Manag. 2018, 1, 1–7. [Google Scholar]
- Diaz-Sanchez, S.; D’Souza, D.; Biswas, D.; Hanning, I. Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 2015, 94, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef]
- Rubió, L.; Farràs, M.; de La Torre, R.; Macià, A.; Romero, M.P.; Valls, R.M.; Solà, R.; Farré, M.; Fitó, M.; Motilva, M.J. Metabolite profiling of olive oil and thyme phenols after a sustained intake of two phenol-enriched olive oils by humans: Identification of compliance markers. Food Res. Int. 2014, 65, 59–68. [Google Scholar] [CrossRef]
- Rubió, L.; Macià, A.; Castell-Auví, A.; Pinent, M.; Blay, M.T.; Ardévol, A.; Romero, M.P.; Motilva, M.J. Effect of the co-occurring olive oil and thyme extracts on the phenolic bioaccessibility and bioavailability assessed by in vitro digestion and cell models. Food Chem. 2014, 149, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ocel’ová, V. Plant Additives in Relation to the Animal Gastrointestinal Tract and Metabolism of Their Main Compounds. Ph.D Thesis, Institute of Animal Physiology, Slovak Academy of Sciences, Košice, Slovakia, 2017. [Google Scholar]
- Sakaguchi, E. Digestive strategies of small hindgut fermenters. Anim. Sci. J. 2003, 74, 327–337. [Google Scholar] [CrossRef]
- Hirakawa, H. Coprophagy in leporids and other mammalian herbivores. Mammal Rev. 2001, 31, 61–80. [Google Scholar] [CrossRef] [Green Version]
- Irlbeck, N.A. How to feed the rabbit (Oryctolagus cuniculus) gastrointestinal tract. J. Anim. Sci. 2001, 79, E343–E346. [Google Scholar] [CrossRef]
- Ho, P.C. Biological and physiological features of the gastrointestinal tract relevant to oral drug absorption. In Oral Bioavailability: Basic Principles, Advanced Concepts, and Applications; Hu, M., Li, X., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 51–61. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). International Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B. A methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Pisarčíková, J.; Oceľová, V.; Faix, Š.; Plachá, I.; Calderón, A.I. Identification and quantification of thymol metabolites in plasma, liver and duodenal wall of broiler chickens using UHPLC-ESI-QTOF-MS. Biomed. Chromatogr. 2017, 31, e3881. [Google Scholar] [CrossRef] [PubMed]
- Bacova, K.; Zitterl-Eglseer, K.; Chrastinova, L.; Laukova, A.; Madarova, M.; Gancarcikova, S.; Sopkova, D.; Andrejcakova, Z.; Placha, I. Effect of thymol addition and withdrawal on some blood parameters, antioxidative defence system and fatty acid profile in rabbit muscle. Animals 2020, 10, 1248. [Google Scholar] [CrossRef] [PubMed]
- Placha, I.; Ocelova, V.; Chizzola, R.; Battelli, G.; Gai, F.; Bacova, K.; Faix, S. Effect of thymol on the broiler chicken antioxidative defence system after sustained dietary thyme oil application. Br. Poult. Sci. 2019, 60, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Ocel’ová, V.; Chizzola, R.; Pisarčíková, J.; Novak, J.; Ivanišinová, O.; Faix, Š. Effect of thyme essential oil supplementation on thymol content in blood plasma, liver, kidney and muscle in broiler chickens. Nat. Prod. Commun. 2016, 11, 1545–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Hu, M. Drug metabolism in gastroinestinal tract. In Oral Bioavailability: Basic Principles, Advanced Concepts, and Applications; Hu, M., Li, X., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 91–109. [Google Scholar]
- Hu, M.; Li, X. Barriers to oral bioavailability-an overview. In Oral Bioavailability: Basic Principles, Advanced Concepts, and Applications; Hu, M., Li, X., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1–5. [Google Scholar]
- Rubió, L.; Serra, A.; Macià, A.; Borràs, X.; Romero, M.P.; Motilva, M.J. Validation of determination of plasma metabolites derived from thyme bioactive compounds by improved liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B 2012, 905, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ocel’ová, V.; Chizzola, R.; Battelli, G.; Pisarcikova, J.; Faix, S.; Gai, F.; Placha, I. Thymol in the intestinal tract of broiler chickens after sustained administration of thyme essential oil in feed. J. Anim. Physiol. Anim. Nutr. 2018, 103, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell-Ward, M.L. Gastrointestinal physiology and nutrition. In Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery, 3rd ed.; Quesenberry, K.E., Carpenter, J.W., Eds.; Elsevier: Saint-Louis, MO, USA, 2012; pp. 183–192. [Google Scholar]
- Skálová, L.; Boušová, I. Metabolismus Léčivých a Jiných Xenobiotik; Karolinum: Praha, Czech Republic, 2011; p. 162. [Google Scholar]
- Ding, X.; Kaminsky, L.S. Human extrahepatic cytochromes P450: Function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Chang, J.H. Absorption of drugs via passive diffusion and carrier-mediated pathways. In Oral Bioavailability: Basic Principles, Advanced Concepts, and Applications; Hu, M., Li, X., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 63–75. [Google Scholar]
- Takada, M.; Agata, I.; Sakamoto, M.; Yagi, N.; Hayashi, N. On the metabolic detoxication of thymol in rabbit and man. J. Toxicol. Sci. 1979, 4, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohlert, C.; Schindler, G.; März, R.W.; Abel, G.; Brinkhaus, B.; Derendorf, H.; Gräfe, E.U.; Veit, M. Systemic availability and pharmacokinetics of thymol in humans. J. Clin. Pharmacol. 2002, 42, 731–737. [Google Scholar] [CrossRef]
- Haselmeyer, A.; Zentek, J.; Chizzola, R. Effects of thyme as a feed additive in broiler chickens on thymol in gut contents, blood plasma, liver and muscle. J. Sci. Food Agric. 2015, 95, 504–508. [Google Scholar] [CrossRef]
| Ingredients (%) | Chemical Composition (g/kg Feed) | ||
|---|---|---|---|
| Dehydrated lucerne meal | 36.0 | Dry matter (g/kg) | 900.9 |
| Dry malting sprouts | 15.0 | Organic compounds | 831.8 |
| Oats | 13.0 | Nitrogen free extract | 444.3 |
| Wheat bran | 9.0 | Neutral detergent fibre (NDF) | 352.9 |
| Barley | 8.0 | Acid detergent fibre (ADF) | 208.1 |
| Extracted sunflower meal | 5.5 | Crude fibre | 177.8 |
| Extracted rapeseed meal | 5.5 | Crude protein | 176.6 |
| Dried distiller grains with solubles | 5.0 | Cellulose | 163.1 |
| Premix 1 | 1.7 | Hemicellulose | 144.8 |
| Limestone | 1.0 | Starch | 133.1 |
| Sodium chloride | 0.3 | Ash | 69.2 |
| Fat | 33.1 | ||
| Metabolic energy, MJ/kg | 9.9 | ||
| 56 d of Age (with Thymol) | 63 d of Age (without Thymol) | |||
|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |
| Plasma | 46.2 b | 10.0 | 2.73 b | 0.458 |
| Intestinal wall | 268 a | 65.9 | 20.4 a | 2.70 |
| Liver | 39.9 b | 13.4 | 5.93 ab | 0.285 |
| Kidney | 314 a | 91.7 | 16.1 ab | 6.53 |
| Spleen | 181 ab | 40.1 | ND | - |
| 56 d of Age (with Thymol) | 63 d of Age (without Thymol) | |||
|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |
| Caecum | 882 ab | 231 | 45.8 ab | 12.44 |
| Colon | 672b | 330 | 16.4 b | 9.44 |
| Faeces | 2444 a | 451 | 150 a | 40.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacova, K.; Eglseer, K.Z.; Räuber, G.K.; Chrastinova, L.; Laukova, A.; Takacsova, M.; Simonova, M.P.; Placha, I. Effect of Sustained Administration of Thymol on Its Bioaccessibility and Bioavailability in Rabbits. Animals 2021, 11, 2595. https://doi.org/10.3390/ani11092595
Bacova K, Eglseer KZ, Räuber GK, Chrastinova L, Laukova A, Takacsova M, Simonova MP, Placha I. Effect of Sustained Administration of Thymol on Its Bioaccessibility and Bioavailability in Rabbits. Animals. 2021; 11(9):2595. https://doi.org/10.3390/ani11092595
Chicago/Turabian StyleBacova, Kristina, Karin Zitterl Eglseer, Gesine Karas Räuber, Lubica Chrastinova, Andrea Laukova, Margareta Takacsova, Monika Pogany Simonova, and Iveta Placha. 2021. "Effect of Sustained Administration of Thymol on Its Bioaccessibility and Bioavailability in Rabbits" Animals 11, no. 9: 2595. https://doi.org/10.3390/ani11092595
APA StyleBacova, K., Eglseer, K. Z., Räuber, G. K., Chrastinova, L., Laukova, A., Takacsova, M., Simonova, M. P., & Placha, I. (2021). Effect of Sustained Administration of Thymol on Its Bioaccessibility and Bioavailability in Rabbits. Animals, 11(9), 2595. https://doi.org/10.3390/ani11092595






