Differential Effects of Green Tea Powders on the Protection of Brown Tsaiya and Kaiya Ducklings against Trichothecene T-2 Toxin Toxicity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Use
2.2. Production and Analysis of T-2 Toxin
2.3. Compositions and Antioxidative Capacity of Green Tea Powder
2.3.1. Analyses of the Chemical Composition and Catechin Concentration
2.3.2. Antioxidative Capacity of Green Tea Powder
2.4. Experimental Designs and Feeding Management of Ducklings
2.5. Growth Performances
2.6. Relative Weights of Organs and Carcass, and Plasma Biochemical Parameters
2.7. Statistical Analysis
3. Results
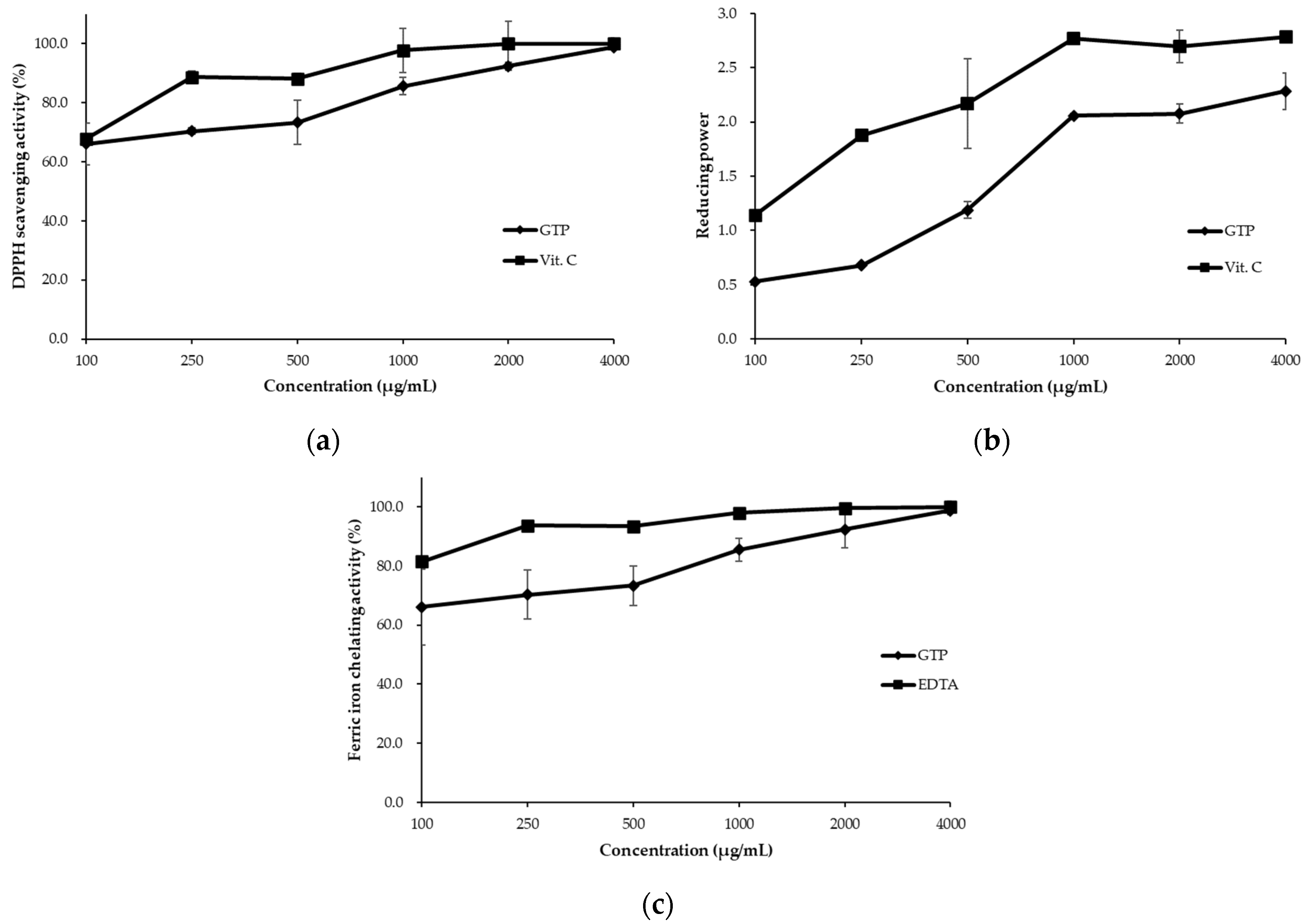
3.1. Antioxidative Capacity of the Green Tea Powder
3.2. Growth Performance
3.3. Plasma Biochemical Parameters
3.4. Relative Weights of Organs and Carcass
4. Discussion
4.1. Antioxidative Capacity of Green Tea Powder
4.2. Effects of T-2 Toxin and Green Tea Powder on the Growth Performance of Ducklings
4.3. Hematological Alterations after T-2 Toxin Challenge Followed by GTP Supplementation
4.4. Effects of T-2 Toxin and Green Tea Powders on the Relative Weights of Organs and Carcass
4.5. Detoxification and Antioxidative Activities of Green Tea Powder
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Loh, Z.H.; Ouwerkerk, D.; Klieve, A.V.; Hungerford, N.L.; Fletcher, M.T. Toxin degradation by rumen microorganisms: A review. Toxins 2020, 12, 664. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Su, R.; Yin, R.; Lai, D.; Wang, M.; Liu, Y.; Zhou, L. Detoxification of mycotoxins through biotransformation. Toxins 2020, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Burstmayr, M.; Burstmayr, H. Trichothecenes in cereal grains—An update. Toxins 2019, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Ferse, I.; Mulac, D.; Wurthwein, E.U.; Humpf, H.U. Structural elucidation of T-2 toxin thermal degradation products and investigations toward their occurrence in retail food. J. Agric. Food Chem. 2009, 57, 1867–1875. [Google Scholar] [CrossRef]
- Bretz, M.; Beyer, M.; Cramer, B.; Knecht, A.; Humpf, H.U. Thermal degradation of the Fusarium mycotoxin deoxynivalenol. J. Agric. Food Chem. 2006, 54, 6445–6451. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Beier, R.C.; Shen, J.; De Smet, D.; De Saeger, S.; Zhang, S. T-2 toxin, a trichothecene mycotoxin: Review of toxicity, metabolism, and analytical methods. J. Agric. Food Chem. 2011, 59, 3441–3453. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Sun, Y.; Ye, W.; Zheng, T.; Wen, J.; Deng, Y. T-2 toxin inhibits the production of mucin via activating the IRE1/XBP1 pathway. Toxicology 2019, 424, 152230. [Google Scholar] [CrossRef] [PubMed]
- Rezar, V.; Frankic, T.; Narat, M.; Levart, A.; Salobir, J. Dose-dependent effects of T-2 toxin on performance, lipid peroxidation, and genotoxicity in broiler chickens. Poult. Sci. 2007, 86, 1155–1160. [Google Scholar] [CrossRef]
- Goossens, J.; Pasmans, F.; Verbrugghe, E.; Vandenbroucke, V.; De Baere, S.; Meyer, E.; Haesebrouck, F.; De Backer, P.; Croubels, S. Porcine intestinal epithelial barrier disruption by the Fusarium mycotoxins deoxynivalenol and T-2 toxin promotes transepithelial passage of doxycycline and paromomycin. BMC Vet. Res. 2012, 8, 245. [Google Scholar] [CrossRef]
- Kutasi, J.; Papp, Z.; Jakab, L.; Brydl, E.; Rafai, P. Deactivation of T-2 toxin in broiler ducks by biotransformation. J. Appl. Poult. Res. 2012, 21, 13–20. [Google Scholar] [CrossRef]
- Wyatt, R.D.; Hamilton, P.B.; Burmeister, H.R. The effects of T-2 toxin in broiler chickens. Poult. Sci. 1973, 52, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Maldjian, A.; Cristofori, C.; Noble, R.C.; Speake, B.K. The fatty acid composition of brain phospholipids from chicken and duck embryos. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 115, 153–158. [Google Scholar] [CrossRef]
- Mezes, M.; Barta, M.; Nagy, G. Comparative investigation on the effect of T-2 mycotoxin on lipid peroxidation and antioxidant status in different poultry species. Res. Vet. Sci. 1999, 66, 19–23. [Google Scholar] [CrossRef]
- Fernye, C.; Ancsin, Z.; Bocsai, A.; Balogh, K.; Mezes, M.; Erdelyi, M. Role of glutathione redox system on the T-2 toxin tolerance of Pheasant (Phasianus colchicus). Toxicol. Res. 2018, 34, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; He, Q.; Deng, X.; Hao, F.; Tang, H.; Wang, Y. Systemic metabolic responses of broiler chickens and piglets to acute T-2 toxin intravenous exposure. J. Agric. Food Chem. 2016, 64, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Kuenzel, W.J.; Kuenzel, N.T. Basal metabolic rate in growing chicks Gallus domesticus. Poult. Sci. 1977, 56, 619–627. [Google Scholar] [CrossRef]
- Monbaliu, S.; Van Poucke, C.; Van Peteghem, C.; Van Poucke, K.; Heungens, K.; De Saeger, S. Development of a multi-mycotoxin liquid chromatography/tandem mass spectrometry method for sweet pepper analysis. Rapid Commun. Mass Spectrom. 2009, 23, 3–11. [Google Scholar] [CrossRef]
- Tso, K.H.; Ju, J.C.; Fan, Y.K.; Chiang, H.I. Enzyme Degradation reagents effectively remove mycotoxins deoxynivalenol and zearalenone from pig and poultry artificial digestive juices. Toxins 2019, 11, 599. [Google Scholar] [CrossRef]
- Bocsai, A.; Pelyhe, C.; Zandoki, E.; Ancsin, Z.; Szabo-Fodor, J.; Erdelyi, M.; Mezes, M.; Balogh, K. Short-term effects of T-2 toxin exposure on some lipid peroxide and glutathione redox parameters of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2016, 100, 520–525. [Google Scholar] [CrossRef]
- Shokri, F.; Heidari, M.; Gharagozloo, S.; Ghazi-Khansari, M. In vitro inhibitory effects of antioxidants on cytotoxicity of T-2 toxin. Toxicology 2000, 146, 171–176. [Google Scholar] [CrossRef]
- Weber, M.; Stiller, S.; Balogh, K.; Wagner, L.; Erdelyi, M.; Mezes, M. Effect of feeding T-2 toxin contaminated feed on the utilisation of vitamin E in chickens. Acta Vet. Hung. 2007, 55, 21–27. [Google Scholar] [CrossRef]
- Dvorska, J.E.; Pappas, A.C.; Karadas, F.; Speake, B.K.; Surai, P.F. Protective effect of modified glucomannans and organic selenium against antioxidant depletion in the chicken liver due to T-2 toxin-contaminated feed consumption. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Satoh, E.; Tohyama, N.; Nishimura, M. Comparison of the antioxidant activity of roasted tea with green, oolong, and black teas. Int. J. Food Sci. Nutr. 2005, 56, 551–559. [Google Scholar] [CrossRef]
- Weisburger, J.H. Mechanisms of action of antioxidants as exemplified in vegetables, tomatoes and tea. Food Chem. Toxicol. 1999, 37, 943–948. [Google Scholar] [CrossRef]
- Jeong, C.H.; Ryu, H.; Zhang, T.; Lee, C.H.; Seo, H.G.; Han, S.G. Green tea powder supplementation enhances fermentation and antioxidant activity of set-type yogurt. Food Sci. Biotechnol. 2018, 27, 1419–1427. [Google Scholar] [CrossRef]
- Xia, B.; Liu, Y.L.; Sun, D.; Liu, J.; Zhu, Y.J.; Lu, L.Z. Effects of green tea powder supplementation on egg production and egg quality in laying hens. J. Appl. Anim. Res. 2018, 46, 927–931. [Google Scholar] [CrossRef]
- Uuganbayar, D.; Bae, I.H.; Choi, K.S.; Shin, I.S.; Firman, J.D.; Yang, C.J. Effects of green tea powder on laying performance and egg quality in laying hens. Asian Australas. J. Anim. Sci. 2005, 18, 1769–1774. [Google Scholar] [CrossRef]
- Wang, W.; Tan, H.; Liu, H.; Peng, H.; Li, X.; Dang, X.; He, X. Green tea polyphenols protect against preglomerular arteriopathy via the jagged1/notch1 pathway. Am. J. Transl. Res. 2018, 10, 3276–3290. [Google Scholar] [PubMed]
- Sharath Babu, G.R.; Ilaiyaraja, N.; Khanum, F.; Anand, T. Cytoprotective propensity of green tea polyphenols against citrinin-induced skeletal-myotube damage in C2C12 cells. Cytotechnology 2017, 69, 681–697. [Google Scholar] [CrossRef]
- Rahman, S.U.; Huang, Y.; Zhu, L.; Feng, S.; Khan, I.M.; Wu, J.; Li, Y.; Wang, X. Therapeutic role of green tea polyphenols in improving fertility: A review. Nutrients 2018, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural properties of green tea catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Neves, V.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.; Varela, J.; Barreira, L.; Custodio, L. In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers’ infusions and decoctions: A comparison with green tea (Camellia sinensis). Food Chem. 2016, 200, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Gramza-Michalowska, A.; Kobus-Cisowska, J.; Kmiecik, D.; Korczak, J.; Helak, B.; Dziedzic, K.; Gorecka, D. Antioxidative potential, nutritional value and sensory profiles of confectionery fortified with green and yellow tea leaves (Camellia sinensis). Food Chem. 2016, 211, 448–454. [Google Scholar] [CrossRef]
- Suzuki, T.; Kumazoe, M.; Kim, Y.; Yamashita, S.; Nakahara, K.; Tsukamoto, S.; Sasaki, M.; Hagihara, T.; Tsurudome, Y.; Huang, Y.; et al. Green tea extract containing a highly absorbent catechin prevents diet-induced lipid metabolism disorder. Sci. Rep. 2013, 3, 2749. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.S.; Tang, L.; Cai, Q.; Shen, Y.; Su, J.; Wang, J.S. Mitigation of fumonisin biomarkers by green tea polyphenols in a high-risk population of hepatocellular carcinoma. Sci. Rep. 2015, 5, 17545. [Google Scholar] [CrossRef]
- Tulayakul, P.; Dong, K.S.; Li, J.Y.; Manabe, N.; Kumagai, S. The effect of feeding piglets with the diet containing green tea extracts or coumarin on in vitro metabolism of aflatoxin B1 by their tissues. Toxicon 2007, 50, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Cullen, D.; Smalley, E.B.; Caldwell, R.W. New process for T-2 toxin production. Appl. Environ. Microbiol. 1982, 44, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Lattanzio, V.M.; Pascale, M.; Haidukowski, M. Analysis of T-2 and HT-2 toxins in cereal grains by immunoaffinity clean-up and liquid chromatography with fluorescence detection. J. Chromatogr. A 2005, 1075, 151–158. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Fay, J.P.; Guaita, M.S.; Danelon, J.L.; Chifflet, S.; Wawrzckiewicz, M.; Diaz, C.; Ross, D.A.; Fernandez, H.M. Variability among four laboratories of the filter bag technique to determine acid and neutral detergent fiber contents in ruminant feeds from Argentina. J. AOAC Int. 2009, 92, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, K.; Li, H.; Wang, L.; Ruan, L.; Pang, D.; Cheng, H. Identification of key genes involved in catechin metabolism in tea seedlings based on transcriptomic and HPLC analysis. Plant Physiol. Biochem. 2018, 133, 107–115. [Google Scholar] [CrossRef]
- Villano, D.; Fernandez-Pachon, M.S.; Moya, M.L.; Troncoso, A.M.; Garcia-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid-peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Wyatt, R.D.; Colwell, W.M.; Hamilton, P.B.; Burmeister, H.R. Neural disturbances in chickens caused by dietary T-2 toxin. Appl. Microbiol. 1973, 26, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Lambert, J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, J.-Y.; Lin, Y.; Lin, I.F.; Lu, Y.-R.; Liu, L.-H.; Wang, H.-M.D. Antioxidative and antimelanoma effects of various tea extracts via a green extraction method. J. Food Qual. 2018, 2018, 5156073. [Google Scholar] [CrossRef]
- Liu, W.; Rouzmehr, F.; Wang, X.; Seidavi, A. Green tea dietary supplementation in broiler chickens: Effect on the development of chicken intestine. Food Sci. Nutr. 2021, 9, 1530–1541. [Google Scholar] [CrossRef]
- Hamid, M.A.; Nabi, M.R.; Abid, M.H.; Hossain, M.S.; Rahman, M.M. Effect of green tea powder as an alternative of antibiotic on growth performance, meat quality and blood lipid profile of broiler. Bangladesh J. Vet. Med. 2018, 16, 23–29. [Google Scholar] [CrossRef][Green Version]
- Toyomizu, M.; Kikusato, M.; Kawabata, Y.; Azad, M.A.K.; Inui, E.; Amo, T. Meat-type chickens have a higher efficiency of mitochondrial oxidative phosphorylation than laying-type chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 159, 75–81. [Google Scholar] [CrossRef]
- Lin, M.J.; Chang, S.C.; Tso, K.H.; Lin, W.C.; Chang, C.L.; Lee, T.T. Effect of T-2 toxin and antioxidants on angel wing incidence and severity in White Roman geese. J. Agric. Food Chem. 2017, 46, 340–348. [Google Scholar] [CrossRef]
- Biswas, M.A.H.; Wakita, M. Effect of dietary Japanese green tea powder supplementation on feed utilization and carcass profiles in broilers. J. Poult. Sci. 2001, 38, 8. [Google Scholar] [CrossRef]
- Rehman, H.; Krishnasamy, Y.; Haque, K.; Thurman, R.G.; Lemasters, J.J.; Schnellmann, R.G.; Zhong, Z. Green tea polyphenols stimulate mitochondrial biogenesis and improve renal function after chronic cyclosporin a treatment in rats. PLoS ONE 2014, 8, e65029. [Google Scholar] [CrossRef]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.; Vernekar, S.N. Markers of renal function tests. N. Am. J. Med. Sci 2010, 2, 170–173. [Google Scholar] [PubMed]
- Gowda, S.; Desai, P.B.; Hull, V.V.; Math, A.A.; Vernekar, S.N.; Kulkarni, S.S. A review on laboratory liver function tests. Pan. Afr. Med. J. 2009, 3, 17. [Google Scholar] [PubMed]
- Kubena, L.F.; Harvey, R.B.; Huff, W.E.; Corrier, D.E.; Philips, T.D.; Rottinghaus, G.E. Influence of ochratoxin A and T-2 toxin singly and in combination on broiler chickens. Poult. Sci. 1989, 68, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Huff, W.E.; Harvey, R.B.; Kubena, L.F.; Rottinghaus, G.E. Toxic synergism between aflatoxin and T-2 toxin in broiler chickens. Poult. Sci. 1988, 67, 1418–1423. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Piliia, N.M.; Tutel’ian, V.A.; Spirichev, V.B. Mechanism of impairment of calcium metabolism caused by toxin T-2; the role of the vitamin D-dependent endocrine system. Vopr. Med. Khim. 1990, 36, 45–48. [Google Scholar]
- Sergeev, I.N.; Arkhapchev Iu, P.; Kravchenko, L.V.; Kodentsova, V.M.; Piliia, N.M. Effect of mycotoxins aflatoxin B1 and T-2 toxin on the vitamin D3 metabolism and binding of its hormonal form 1,25-dihydroxyvitamin D3 in rats. Vopr. Med. Khim. 1988, 34, 51–57. [Google Scholar]
- Moe, S.M. Disorders involving calcium, phosphorus, and magnesium. Prim. Care 2008, 35, 215–237. [Google Scholar] [CrossRef]
- Rodriguez-Ortiz, M.E.; Canalejo, A.; Herencia, C.; Martinez-Moreno, J.M.; Peralta-Ramirez, A.; Perez-Martinez, P.; Navarro-Gonzalez, J.F.; Rodriguez, M.; Peter, M.; Gundlach, K. Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expression at moderately low calcium concentration. Nephrol. Dial. Transplant. 2014, 29, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.C.; Simao, A.M.; Narisawa, S.; Huesa, C.; McKee, M.D.; Farquharson, C.; Millan, J.L. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: A unified model of the mechanisms of initiation of skeletal calcification. J. Bone Miner. Res. 2011, 26, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Madheswaran, R.; Balachandran, C.; Murali Manohar, B. Influence of dietary culture material containing aflatoxin and T-2 toxin on certain serum biochemical constituents in Japanese quail. Mycopathologia 2004, 158, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.H.; Kubena, L.F.; Harvey, R.B.; Buckley, S.A.; Rottinghaus, G.E. Efficacy of various inorganic sorbents to reduce the toxicity of aflatoxin and T-2 toxin in broiler chickens. Poult. Sci. 1998, 77, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Milner, D.J.; Mavroidis, M.; Weisleder, N.; Capetanaki, Y. Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J. Cell Biol. 2000, 150, 1283–1298. [Google Scholar] [CrossRef]
- Edrington, T.S.; Kubena, L.F.; Harvey, R.B.; Rottinghaus, G.E. Influence of a superactivated charcoal on the toxic effects of aflatoxin or T-2 toxin in growing broilers. Poult. Sci. 1997, 76, 1205–1211. [Google Scholar] [CrossRef]
- Xu, Z.; Dai, X.X.; Zhang, Q.Y.; Su, S.L.; Yan, H.; Zhu, Y.; Shang, E.X.; Qian, D.W.; Duan, J.A. Protective effects and mechanisms of Rehmannia glutinosa leaves total glycoside on early kidney injury in db/db mice. Biomed. Pharmacother. 2020, 125, 109926. [Google Scholar] [CrossRef]
- Raju, M.V.; Devegowda, G. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin). Br. Poult. Sci. 2000, 41, 640–650. [Google Scholar] [CrossRef]
- Garcia, A.R.; Avila, E.; Rosiles, R.; Petrone, V.M. Evaluation of two mycotoxin binders to reduce toxicity of broiler diets containing ochratoxin A and T-2 toxin contaminated grain. Avian. Dis. 2003, 47, 691–699. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Saeed, M.; Naveed, M.; Arain, M.A.; Arif, M.; Tiwari, R.; Khandia, R.; Khurana, S.K.; Karthik, K. Nutritional applications and beneficial health applications of green tea and l-theanine in some animal species: A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 245–256. [Google Scholar] [CrossRef]
- Wang, H.; Li, D.; Hu, Z.; Zhao, S.; Zheng, Z.; Li, W. Protective Effects of green tea polyphenol against renal injury through ROS-mediated JNK-MAPK pathway in lead exposed rats. Mol. Cells 2016, 39, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Avwioro, G.; Iyiola, S.; Aghoghovwia, B. Histological and biochemical markers of the liver of Wistar rats on subchronic oral administration of green tea. N. Am. J. Med. Sci. 2010, 2, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Nootash, S.; Sheikhzadeh, N.; Baradaran, B.; Oushani, A.K.; Maleki Moghadam, M.R.; Nofouzi, K.; Monfaredan, A.; Aghebati, L.; Zare, F.; Shabanzadeh, S. Green tea (Camellia sinensis) administration induces expression of immune relevant genes and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2013, 35, 1916–1923. [Google Scholar] [CrossRef]
- Mathew, J.; Sankar, P.; Varacallo, M. Physiology, Blood Plasma; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Meloche, J.L.; Smith, T.K. Altered tissue amino acid metabolism in acute T-2 toxicosis. Proc. Soc. Exp. Biol. Med. 1995, 210, 260–265. [Google Scholar] [CrossRef]
- Thompson, W.L.; Wannemacher, R.W., Jr. In vivo effects of T-2 mycotoxin on synthesis of proteins and DNA in rat tissues. Toxicol. Appl. Pharmacol. 1990, 105, 483–491. [Google Scholar] [CrossRef]
- Shen, C.L.; Cao, J.J.; Dagda, R.Y.; Tenner, T.E., Jr.; Chyu, M.C.; Yeh, J.K. Supplementation with green tea polyphenols improves bone microstructure and quality in aged, orchidectomized rats. Calcif. Tissue Int. 2011, 88, 455–463. [Google Scholar] [CrossRef]
- Shen, C.L.; Han, J.; Wang, S.; Chung, E.; Chyu, M.C.; Cao, J.J. Green tea supplementation benefits body composition and improves bone properties in obese female rats fed with high-fat diet and caloric restricted diet. Nutr. Res. 2015, 35, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Osselaere, A.; Li, S.J.; De Bock, L.; Devreese, M.; Goossens, J.; Vandenbroucke, V.; Van Bocxlaer, J.; Boussery, K.; Pasmans, F.; Martel, A.; et al. Toxic effects of dietary exposure to T-2 toxin on intestinal and hepatic biotransformation enzymes and drug transporter systems in broiler chickens. Food Chem. Toxicol. 2013, 55, 150–155. [Google Scholar] [CrossRef]
- Liu, W.; Rouzmehr, F.; Seidavi, A. Effect of amount and duration of waste green tea powder on the growth performance, carcass characteristics, blood parameters, and lipid metabolites of growing broilers. Environ. Sci. Pollut. Res. Int. 2018, 25, 375–387. [Google Scholar] [CrossRef]
- Kalaiselvi, P.; Rajashree, K.; Bharathi Priya, L.; Padma, V.V. Cytoprotective effect of epigallocatechin-3-gallate against deoxynivalenol-induced toxicity through anti-oxidative and anti-inflammatory mechanisms in HT-29 cells. Food Chem. Toxicol. 2013, 56, 110–118. [Google Scholar] [CrossRef]
- Sugiyama, K.; Kinoshita, M.; Kamata, Y.; Minai, Y.; Sugita-Konishi, Y. (-)-Epigallocatechin gallate suppresses the cytotoxicity induced by trichothecene mycotoxins in mouse cultural macrophages. Mycotoxin Res. 2011, 27, 281–285. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Contents |
|---|---|
| Proximal analysis, g/kg | |
| Dry matter | 989.1 |
| Crude fiber | 200.5 |
| Acid detergent fiber | 198.3 |
| Neutral detergent fiber | 282.6 |
| Green tea polyphenol compositions, mg/100 mL | |
| Catechin | 0.78 |
| Catechin gallate | 2.31 |
| Epicatechin | 7.16 |
| Epicatechin gallate | 2.04 |
| Epigallo catechin | 41.93 |
| Epilgallocatechin gallate | 20.54 |
| Gallocatechin | 3.22 |
| Gallocatechin gallate | 0.20 |
| Ingredient | g/kg |
|---|---|
| Corn | 590 |
| Soybean meal | 200 |
| Soybean oil | 14.3 |
| Fish meal | 50.0 |
| Wheat | 60.0 |
| Wheat middling | 50.0 |
| Sodium chloride | 5.00 |
| Calcium carbonate | 9.50 |
| Dicalcium phosphate | 14.0 |
| dL-methionine | 1.60 |
| L-lysine | 8.00 |
| Choline | 1.50 |
| Vitamin premix a | 2.00 |
| Mineral premix b | 1.30 |
| Total | 1000 |
| Calculated nutrient composition | |
| Metabolizable energy, kcal/kg | 2913 |
| Crude protein, g/kg | 192 |
| Crude fiber, g/kg | 36.3 |
| Calcium, g/kg | 9.80 |
| Available phosphorus, g/kg | 4.60 |
| Treatment | Feed Intake, g/bird/day | BWG, g/bird/day | FCR, g/g | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Week | 2nd Week | 3rd Week | 1st Week | 2nd Week | 3rd Week | 1st Week | 2nd Week | 3rd Week | |
| T-2 toxin (T), mg/kg | |||||||||
| 0 | 25.9 | 60.3 a | 79.5 a | 12.7 | 29.2 a | 38.6 a | 2.27 | 2.35 | 2.21 |
| 0.5 | 26.4 | 59.2 ab | 77.8 ab | 12.7 | 27.2 b | 36.0 a | 2.44 | 2.62 | 2.34 |
| 5 | 25.4 | 57.3 b | 75.2 b | 11.7 | 26.8 b | 33.2 b | 2.54 | 2.51 | 2.45 |
| Green tea powder (G), % | |||||||||
| 0 | 25.8 | 59.0 | 77.4 | 12.6 | 27.9 | 33.7 b | 2.44 | 2.46 | 2.50 a |
| 0.5 | 26.0 | 58.6 | 77.7 | 12.2 | 27.5 | 38.2 a | 2.39 | 2.52 | 2.17 b |
| Breed (B) | |||||||||
| BTD 1 | 24.3 b | 57.1 b | 74.5 b | 7.91 b | 17.0 b | 26.6 b | 3.19 a | 3.40 a | 2.88 a |
| KD | 27.4 a | 60.5 a | 80.5 a | 16.9 a | 38.4 a | 45.4 a | 1.64 b | 1.58 b | 1.80 b |
| SEM | 0.851 | 1.293 | 1.926 | 0.732 | 1.156 | 2.202 | 0.212 | 0.162 | 0.206 |
| Source of variation | p-Values | ||||||||
| T | 0.2698 | 0.0141 | 0.0066 | 0.0787 | 0.0101 | 0.0021 | 0.2179 | 0.0774 | 0.1525 |
| G | 0.7052 | 0.6343 | 0.7600 | 0.3423 | 0.5655 | 0.0004 | 0.7104 | 0.5521 | 0.0024 |
| B | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| T × G | 0.7839 | 0.1898 | 0.2785 | 0.6109 | 0.8407 | 0.7586 | 0.8118 | 0.9784 | 0.5457 |
| T × B | 0.0196 | 0.1997 | 0.9445 | 0.2354 | 0.4165 | 0.3424 | 0.5272 | 0.3158 | 0.8393 |
| G × B | 0.7814 | 0.0016 | 0.1823 | 0.0124 | 0.6165 | 0.8757 | 0.0980 | 0.2232 | 0.1688 |
| T × G × B | 0.6305 | 0.3593 | 0.9224 | 0.1981 | 0.6651 | 0.4780 | 0.3967 | 0.7332 | 0.7325 |
| Treatment | Mineral, mg/dL | Renal Function, mg/dL | Liver Function, U/L | Protein, g/dL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Mg | BUN | CREA | UA | CPK | CHE | GOT | GPT | ALK | ALB 1 | GLO | TP | |
| T-2 toxin (T), mg/kg | |||||||||||||
| 0 | 10.89 a | 9.39 a | 3.34 b | 0.171 | 3.57 | 2787 a | 2911 | 56.0 | 44.2 | 458 a | 1.50 | 1.60 | 3.08 |
| 0.5 | 8.26 b | 8.21 a | 3.41 ab | 0.159 | 2.86 | 2315 b | 2878 | 65.6 | 45.9 | 373 b | 1.49 | 1.55 | 3.06 |
| 5 | 6.73 c | 6.76 b | 3.75 a | 0.166 | 3.62 | 2188 b | 2929 | 68.9 | 41.8 | 356 b | 1.41 | 1.54 | 2.94 |
| Green tea powder (G), % | |||||||||||||
| 0 | 8.04 b | 8.25 | 3.60 a | 0.165 | 3.78 a | 2439 | 3042 a | 70.1 a | 44.7 | 410 | 1.42 | 1.51 b | 2.92 b |
| 0.5 | 9.21 a | 7.99 | 3.40 b | 0.166 | 2.93 b | 2421 | 2769 b | 56.9 b | 43.3 | 382 | 1.51 | 1.62 a | 3.13 a |
| Breed (B) | |||||||||||||
| BTD | 8.55 | 8.06 | 3.15 b | 0.175 a | 3.81 a | 2421 | 2913 | 71.7 a | 48.1 a | 399 | 1.65 a | 1.74 a | 3.39 a |
| KD | 8.70 | 8.18 | 3.85 a | 0.155 b | 2.89 b | 2439 | 2899 | 55.3 b | 39.8 b | 393 | 1.28 b | 1.38 b | 2.66 b |
| SEM | 0.655 | 0.744 | 0.214 | 0.011 | 0.705 | 154 | 74.7 | 9.99 | 3.44 | 31.1 | 0.081 | 0.073 | 0.149 |
| Source of variation | p-Values | ||||||||||||
| T | <0.0001 | <0.0001 | 0.0188 | 0.3407 | 0.2375 | <0.0001 | 0.6113 | 0.1718 | 0.2552 | <0.0001 | 0.2061 | 0.4324 | 0.3877 |
| G | 0.0028 | 0.5491 | 0.0456 | 0.8218 | 0.0396 | 0.8436 | <0.0001 | 0.0248 | 0.5036 | 0.1293 | 0.0601 | 0.0108 | 0.0211 |
| B | 0.6869 | 0.7708 | <0.0001 | 0.0025 | 0.0275 | 0.8451 | 0.7337 | 0.0058 | <0.0001 | 0.7522 | <0.0001 | <0.0001 | <0.0001 |
| T × G | 0.5745 | 0.8717 | 0.1829 | 0.3174 | 0.0039 | <0.0001 | 0.8452 | 0.3352 | 0.5517 | 0.3592 | 0.9189 | 0.5207 | 0.9378 |
| T × B | 0.2801 | 0.6945 | 0.8197 | 0.8335 | 0.5316 | 0.9816 | 0.0587 | 0.0123 | 0.4972 | 0.9911 | 0.8835 | 0.3255 | 0.6349 |
| G × B | 0.9308 | 0.8524 | 0.1810 | 0.3519 | 0.9959 | 0.4574 | 0.7270 | 0.1390 | 0.8342 | 0.0080 | 0.8945 | 0.8829 | 0.9807 |
| T × G × B | 0.1637 | 0.9475 | 0.3536 | 0.8707 | 0.6821 | 0.7940 | 0.4463 | 0.0186 | 0.8984 | 0.7763 | 0.8835 | 0.6429 | 0.8202 |
| Treatment | g/100 g BW | |||||||
|---|---|---|---|---|---|---|---|---|
| Organs | Carcass | |||||||
| Heart | Gizzard | Liver | Kidney | Breast | Leg | Left Tibia | Right Tibia | |
| T-2 toxin (T), mg/kg | ||||||||
| 0 | 0.921 | 5.75 b | 3.56 | 1.13 b | 0.917 a | 7.16 a | 1.56 | 1.49 |
| 0.5 | 0.961 | 8.18 a | 3.73 | 1.27 a | 0.855 ab | 6.76 ab | 1.51 | 1.54 |
| 5 | 0.983 | 8.54 a | 3.86 | 1.29 a | 0.763 b | 6.34 b | 1.44 | 1.53 |
| Green tea powder (G), % | ||||||||
| 0 | 0.993 a | 7.47 | 3.92 a | 1.29 a | 0.823 | 6.53 | 1.42 a | 1.48 |
| 0.5 | 0.917 b | 7.51 | 3.51 b | 1.18 b | 0.866 | 6.98 | 1.58 b | 1.55 |
| Breed (B) | ||||||||
| BTD 1 | 1.141 a | 9.26 a | 4.21 a | 1.58 a | 0.709 b | 7.85 a | 1.85 a | 1.89 a |
| KD | 0.769 b | 5.72 b | 3.22 b | 0.89 b | 0.981 a | 5.66 b | 1.16 b | 1.15 b |
| SEM | 0.051 | 0.405 | 0.215 | 0.084 | 0.081 | 0.431 | 0.102 | 0.114 |
| Source of variation | p-Values | |||||||
| T | 0.2142 | <0.0001 | 0.1458 | 0.0127 | 0.0286 | 0.0294 | 0.2615 | 0.1062 |
| G | 0.0098 | 0.8687 | 0.0012 | 0.0254 | 0.3609 | 0.0773 | 0.0088 | 0.3016 |
| B | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| T × G | 0.3407 | 0.5519 | 0.7071 | 0.2755 | 0.8118 | 0.5560 | 0.6880 | 0.7386 |
| T × B | 0.2282 | <0.0001 | 0.0663 | 0.8104 | 0.3007 | 0.0032 | 0.0597 | 0.5375 |
| G × B | 0.0720 | 0.4704 | 0.0842 | 0.1710 | 0.0810 | 0.1093 | 0.0056 | 0.0424 |
| T × G × B | 0.6305 | 0.3593 | 0.9224 | 0.1981 | 0.6651 | 0.9003 | 0.3967 | 0.7332 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tso, K.-H.; Lumsangkul, C.; Cheng, M.-C.; Ju, J.-C.; Fan, Y.-K.; Chiang, H.-I. Differential Effects of Green Tea Powders on the Protection of Brown Tsaiya and Kaiya Ducklings against Trichothecene T-2 Toxin Toxicity. Animals 2021, 11, 2541. https://doi.org/10.3390/ani11092541
Tso K-H, Lumsangkul C, Cheng M-C, Ju J-C, Fan Y-K, Chiang H-I. Differential Effects of Green Tea Powders on the Protection of Brown Tsaiya and Kaiya Ducklings against Trichothecene T-2 Toxin Toxicity. Animals. 2021; 11(9):2541. https://doi.org/10.3390/ani11092541
Chicago/Turabian StyleTso, Ko-Hua, Chompunut Lumsangkul, Min-Chien Cheng, Jyh-Cherng Ju, Yang-Kwang Fan, and Hsin-I Chiang. 2021. "Differential Effects of Green Tea Powders on the Protection of Brown Tsaiya and Kaiya Ducklings against Trichothecene T-2 Toxin Toxicity" Animals 11, no. 9: 2541. https://doi.org/10.3390/ani11092541
APA StyleTso, K.-H., Lumsangkul, C., Cheng, M.-C., Ju, J.-C., Fan, Y.-K., & Chiang, H.-I. (2021). Differential Effects of Green Tea Powders on the Protection of Brown Tsaiya and Kaiya Ducklings against Trichothecene T-2 Toxin Toxicity. Animals, 11(9), 2541. https://doi.org/10.3390/ani11092541








