Wider Angle Egg Turning during Incubation Enhances Yolk Utilization and Promotes Goose Embryo Development
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eggs and Experimental Design
2.2. Data and Tissue Collection
2.3. Lipid Extraction and LC–MS Analysis of the Yolk
2.4. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.5. Statistical Analysis
3. Results
3.1. Embryonic Development and Hatching Performance
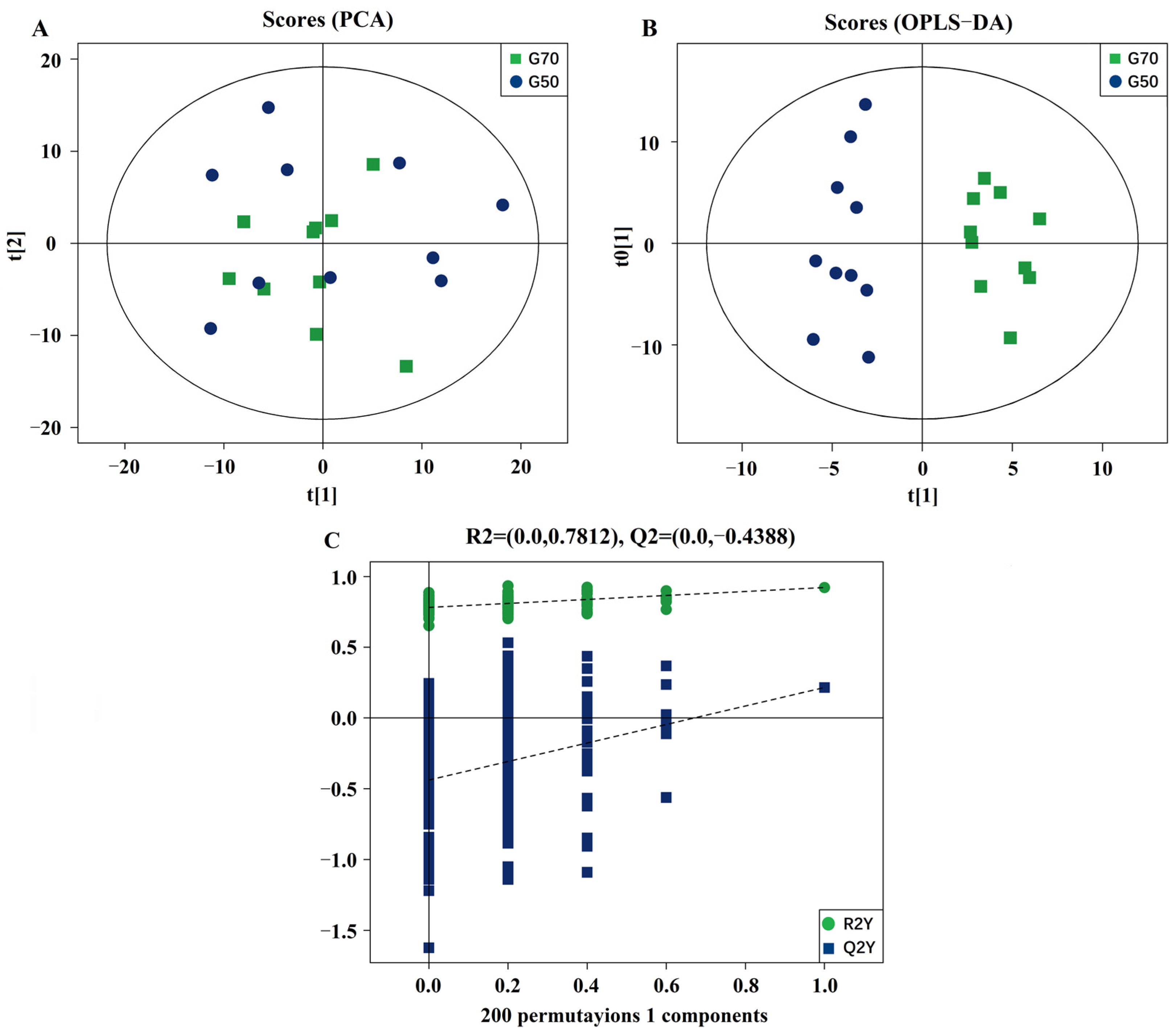
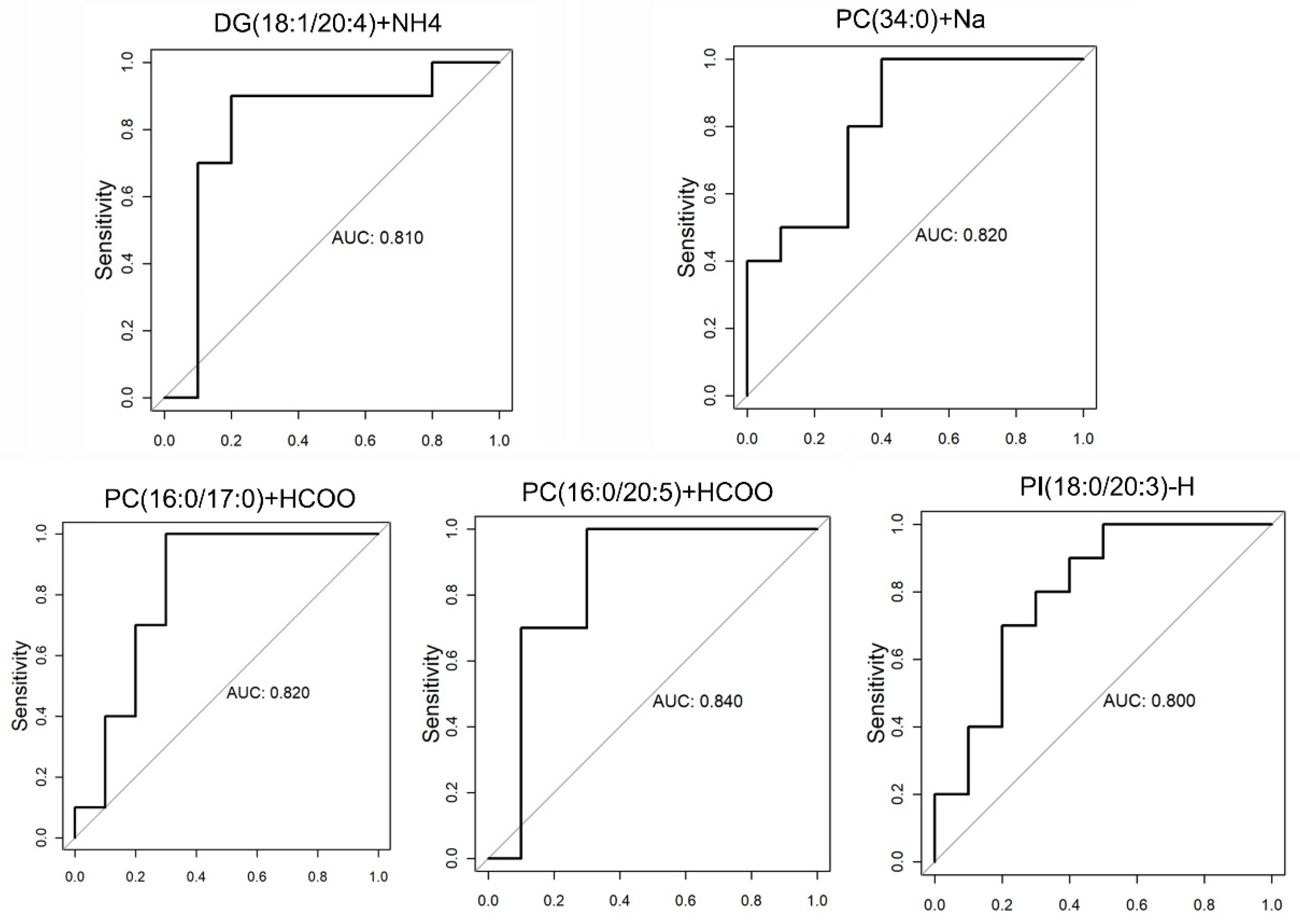
3.2. Validation and Multivariate Analysis of LC-MS Results
3.3. Yolk Lipid Mobilization
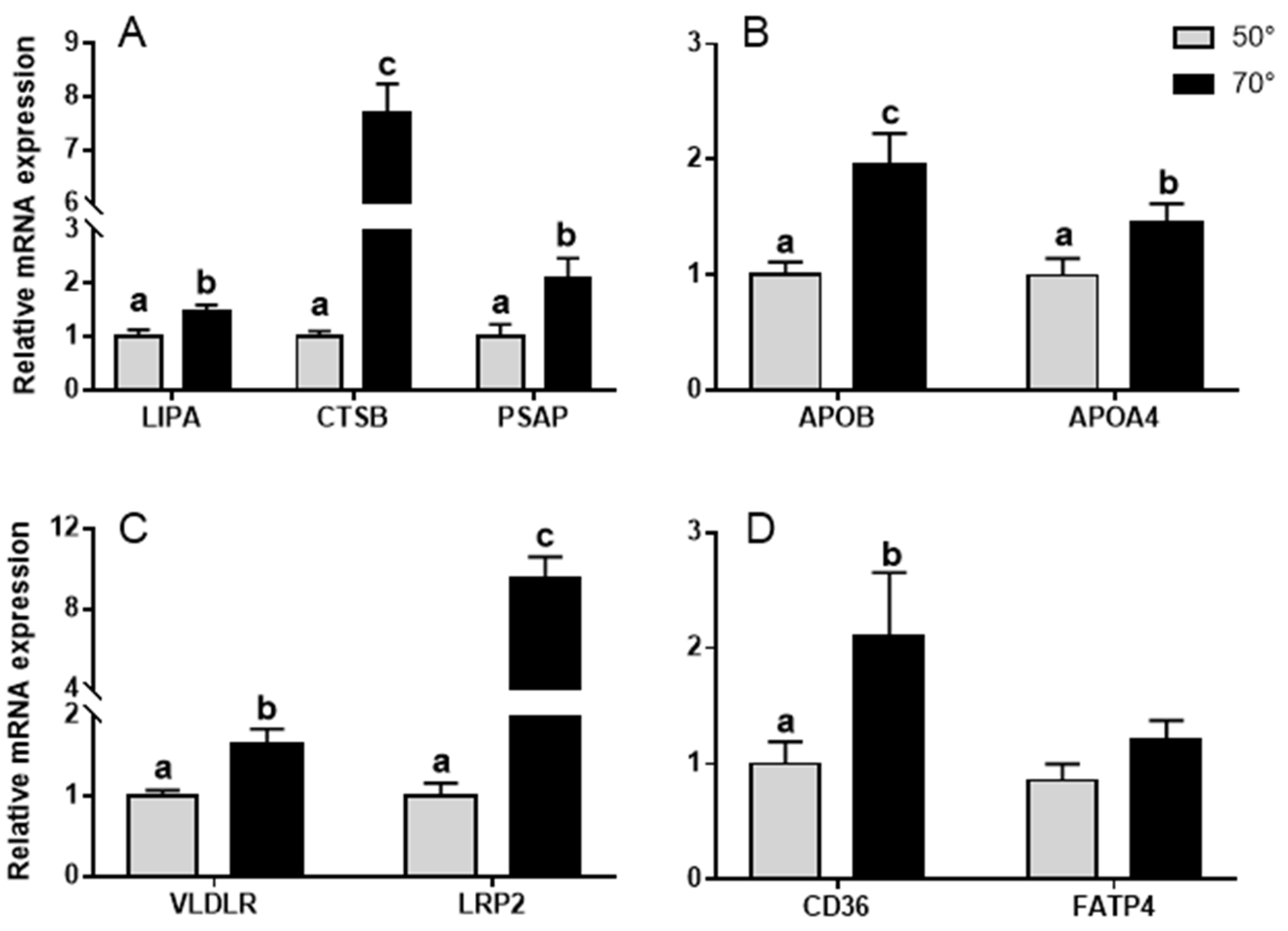
3.4. Lipid Utilization Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yoshizaki, N.; Saito, H. Changes in shell membranes during the development of quail embryos. Poult. Sci. 2002, 81, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Elibol, O.; Peak, S.D.; Brake, J. Effect of flock age, length of egg storage, and frequency of turning during storage on hatchability of broiler hatching eggs. Poult. Sci. 2002, 81, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Deeming, D.C. The role of egg turning during incubation. Avian Biol. Res. 2009, 2, 67–71. [Google Scholar] [CrossRef]
- Tona, K.; Onagbesan, O.; Bruggeman, V.; Mertens, K.; Decuypere, E. Effects of turning duration during incubation on embryo growth, utilization of albumen, and stress regulation. Poult. Sci. 2005, 84, 315–320. [Google Scholar] [CrossRef]
- Wilson, H.R. Physiological Requirements of the Developing Embryo: Temperature and Turning in Avian Incubation; Butterworth-Heinemann: London, UK, 1991; pp. 145–156. [Google Scholar]
- Elibol, O.; Brake, J. Effect of egg turning angle and frequency during incubation on hatchability and incidence of un-hatched broiler embryos with head in the small end of the egg. Poult. Sci. 2006, 85, 1433–1437. [Google Scholar] [CrossRef]
- Elibol, O.; Brake, J. Effect of egg position during three and fourteen days of storage and turning frequency during subsequent incubation on hatchability of broiler hatching eggs. Poult. Sci. 2008, 87, 1237–1241. [Google Scholar] [CrossRef]
- Bednarczyk, M.; Rosinski, A. Comparison of egg hatchability and in vitro survival of goose embryos of various origins. Poult. Sci. 1999, 78, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.L.; Li, L.; Wang, C.L.; Chen, F.; Sun, A.D.; Shi, Z.D. Raising on water stocking density reduces geese reproductive performances via water bacteria and lipopolysaccharide contaminations in “geese-fish” production system. Agric. Sci. China 2011, 10, 1459–1466. [Google Scholar] [CrossRef]
- Yang, X.W.; Liu, L.; Jiang, D.L.; Wang, C.L.; Sun, A.D.; Shi, Z.D. Improving geese production performance in “goose-fish” production system by competitive reduction of pathogenic bacteria in pond water. J. Integr. Agric. 2012, 11, 993–1001. [Google Scholar] [CrossRef]
- Shao, X.B.; Lei, M.M.; Chen, X.P.; Shi, Z.D. Analysis of related factors affecting hatching performance of out-of-season breeding geese. China Poult. 2014, 36, 52–54. [Google Scholar]
- Dai, Z.C.; Yao, J.J.; Ren, Y.H.; Shao, X.B.; Huang, Y.M.; Shi, Z.D. Development of large angle egg turning incubator and its application in goose egg hatching. China Poult. 2017, 39, 63–66. [Google Scholar]
- Guo, B.B.; Dai, Z.C.; Ren, Y.H.; Zhu, H.X.; Shao, X.B.; Sun, A.D.; Shi, Z.D. Improvement of goose embryonic and muscular developments by wider angle egg turning during incubation and the regulatory mechanisms. Poult. Sci. 2000, 1, 114–119, under review. [Google Scholar]
- Romanoff, A.L. The Extraembryonic Membranes in the Avian Embryo, Structural and Functional Development; MacMillan Company: New York, NY, USA, 1960; pp. 1041–1140. [Google Scholar]
- Tona, K.; Onagbesan, O.; De, K.B.; Decuypere, E.; Bruggeman, V. Effects of turning duration during incubation on corticosterone and thyroid hormone levels, gas pressures in air cell, chick quality, and juvenile growth. Poult. Sci. 2003, 82, 1974–1979. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Xie, Y.; Xu, S.; Lv, X.; Yin, H.; Xiang, J.; Chen, H.; Wei, F. Comprehensive lipidomics analysis reveals the effects of different omega-3 polyunsaturated fatty acid-rich diets on egg yolk lipids. J. Agric. Food Chem. 2020, 68, 15048–15060. [Google Scholar] [CrossRef]
- Noble, R.C.; Ogunyemi, D. Lipid changes in the residual yolk and liver of the chick immediately after hatching. Biol. Neonate 1989, 56, 228–236. [Google Scholar] [CrossRef]
- Yadgary, L.; Cahaner, A.; Kedar, O.; Uni, Z. Yolk sac nutrient composition and fat uptake in late-term embryos in eggs from young and old broiler breeder hens. Poult. Sci. 2010, 89, 2441–2452. [Google Scholar] [CrossRef]
- Wagt, I.V.D.; Jong, I.C.D.; Mitchell, M.A.; Molenaar, R.; Brand, H.V.D. A review on yolk sac utilization in poultry. Poult. Sci. 2020, 99, 2162–2175. [Google Scholar] [CrossRef]
- Yadgary, L.; Yair, R.; Uni, Z. The chick embryo yolk sac membrane expresses nutrient transporter and digestive enzyme genes. Poult. Sci. 2011, 90, 410–416. [Google Scholar] [CrossRef]
- Yadgary, L.; Kedar, O.; Adepeju, O.; Uni, Z. Changes in yolk sac membrane absorptive area and fat digestion during chick embryonic development. Poult. Sci. 2013, 92, 1634–1640. [Google Scholar] [CrossRef]
- Taguchi, R.; Ishikawa, M. Precise and global identification of phospholipid molecular species by an Orbitrap mass spectrometer and automated search engine Lipid Search. J. Chromatogr. A 2010, 1217, 4229–4239. [Google Scholar] [CrossRef]
- Michopoulos, F.; Whalley, N.; Theodoridis, G.; Wilson, I.D.; Dunkley, T.P.J.; Critchlow, S.E. Targeted profiling of polar intracellular metabolites using ion-pair-high performance liquid chromatography and ultra-high performance liquid chromatography coupled to tandem mass spectrometry: Applications to serum, urine and tissue extracts. J. Chromatogr. A 2014, 1349, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; Santos, V.M.D.; Rodrigues, J.C.; Nascimento, S.T. Effects of different egg turning frequencies on incubation efficiency parameters. Poult. Sci. 2020, 99, 4417–4420. [Google Scholar] [CrossRef]
- Dayan, J.; Reicher, N.; Melkman-Zehavi, T.; Uni, Z. Incubation temperature affects yolk utilization through changes in expression of yolk sac tissue functional genes. Poult. Sci. 2020, 99, 6128–6138. [Google Scholar] [CrossRef]
- Tazawa, H.; Wakayama, H.; Turner, J.; Paganelli, C. Metabolic compensation for gradual cooling in developing chick embryos. Comp. Biochem. Physiol. 1988, 89, 125–129. [Google Scholar] [CrossRef]
- Lourens, A.; Brand, V.D.H.; Heetkamp, M.J.W.; Meijerhof, R.; Kemp, B. Metabolic responses of chick embryos to short-term temperature fluctuations. Poult. Sci. 2006, 85, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Funk, E.M.; Forward, J. The Effect of Angle of Turning Eggs during Incubation on Hatchability; University of Missouri: Columbia, MO, USA, 1953. [Google Scholar]
- Funk, E.M.; Forward, J. The relation of angle of turning and position of the egg to hatchability of chicken eggs. Poult. Sci. 1960, 39, 784–785. [Google Scholar] [CrossRef]
- Schalkwyk, V.S.J.; Cloete, S.W.; Brown, C.R.; Brand, Z. Hatching success of ostrich eggs in relation to setting, turning and angle of rotation. Br. Poult. Sci. 2000, 41, 46–52. [Google Scholar] [CrossRef]
- Wood, P.L.; Muir, W.; Christmann, U.; Gibbons, P.; Hancock, C.L.; Poole, C.M.; Emery, A.L.; Poovey, J.R.; Hagg, C.; Scarborough, J.H.; et al. Lipidomics of the chicken egg yolk: High-resolution mass spectrometric characterization of nutritional lipid families. Poult. Sci. 2021, 100, 887–899. [Google Scholar] [CrossRef]
- Speake, B.K.; Murray, A.M.; Noble, R.C. Transport and transformations of yolk lipids during develo pment of the avian embryo. Prog. Lipid Res. 1998, 37, 1–32. [Google Scholar] [CrossRef]
- Sato, M.; Tachibana, T.; Furuse, M. Heat production and lipid metabolism in broiler and layer chickens during embryonic development. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 143, 382–388. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Kosińska, J.; Chwojnicka, M.; Tuchendler, D.; Tabin, M.; Tuchendler, R.; Bobak, L.; Trziszka, T.; Szuba, A. Positive effects of egg-derived phospholipids in patients with metabolic syndrome. Adv. Med. Sci. 2016, 61, 169–174. [Google Scholar] [CrossRef]
- Nangsuay, A.; Meijerhof, R.; Ruangpanit, Y.; Kemp, B.; Brand, V.D.H. Energy utilization and heat production of embryos from eggs originating from young and old broiler breeder flocks. Poult. Sci. 2013, 92, 474–482. [Google Scholar] [CrossRef]
- Xiao, N.; Zhao, Y.; Yao, Y.; Wu, N.; Xu, M.; Du, H.; Tu, Y. Biological activities of egg yolk lipids: A review. J. Agric. Food Chem. 2020, 68, 1948–1957. [Google Scholar] [CrossRef]
- Kanai, M.; Soji, T.; Sugawara, E.; Watari, N.; Oguchi, H.; Matsubara, M.; Herbert, D.C. Participation of endodermal epithelial cells on the synthesis of plasma LDL and HDL in the chick yolk sac. Microsc. Res. Tech. 1996, 35, 340–348. [Google Scholar] [CrossRef]
- Hermann, M.; Mahon, M.G.; Lindstedt, K.A.; Nimpf, J.; Schneider, W.J. Lipoprotein receptors in extraembryonic tissues of the chicken. J. Biol. Chem. 2000, 275, 16837–16844. [Google Scholar] [CrossRef] [Green Version]
- Latour, M.A.; Peebles, E.D.; Boyle, C.R.; Brake, J.D.; Kellogg, T.F. Changes in serum lipid, lipoprotein and corticosterone concentrations during neonatal chick development. Biol. Neonate 1995, 67, 381–386. [Google Scholar] [CrossRef]
- Sklan, D. Fat and carbohydrate use in posthatch chicks. Poult. Sci. 2003, 82, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.C.; Cocchi, M. Lipid metabolism and the neonatal chicken. Prog. Lipid Res. 1990, 29, 107–140. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res. 2018, 59, 1084–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene name | Accession Number | Primer Sequence (5′ to 3′) | PCR Product (bp) |
|---|---|---|---|
| β-actin | M26111 | TGACGCAGATCATGTTTGAGA GCAGAGCGTAGCCCTCATAG | 159 |
| LIPA | XM_013171545.1 | CTGAGCTGCTTCTCAAGGACA CGCAGGGCAATGTGTTGAAT | 200 |
| CTSB | XM_013184419.1 | TGATGTACAAGTCTGGGGTGT CACGATTTCGGACTCGATGC | 200 |
| PSAP | XM_027460545.2 | CAGGAAGCCGTCAGGACAAA ACAGCCTGATCCTTCATGTGC | 167 |
| APOB | XM_013194833.1 | AAGCAACAAGGAAGCTCCTGA ATGGCCTGTGCAATCATTTCG | 156 |
| APOA4 | XM_021270123.3 | CAAGCAGATCAACACCCTGC CCTTGCGGATCTGCTCCTT | 149 |
| VLDLR | XM_013198844.1 | GGCAGTGCAATGGTGTGAGAGAC GGGGCTCATCACTCCAGTCCTTG | 171 |
| LRP2 | XM_013171457.1 | GACGACTGCAAAATGTGGGG TGACGCAATACCAGCCGAAG | 138 |
| CD36 | XM_013180470 | GGGGAAGTCTGGCAACAAAC TCTTCCTGAGTGAAGCTGCTTTG | 100 |
| FATP4 | XM_013198644.1 | TGCACTTTCTGGTGCAAAGC ACATGCGGAAGTACCTGCAAT | 170 |
| Incubation or Hatch Days | Items | Group | |
|---|---|---|---|
| 50° | 70° | ||
| E7 | Fertilization rate (%) | 93.42 ± 0.27 | 91.91 ± 1.46 |
| Early mortality (%) | 3.89 ± 0.25 | 3.46 ± 0.63 | |
| E18 | Middle mortality (%) | 1.19 ± 0.18 | 1.44 ± 0.16 |
| E22 | Relative embryo weight (%) | 27.54 ± 0.54 a | 29.94 ± 0.59 c |
| Relative yolk weight (%) | 27.30 ± 0.65 | 25.38 ± 0.74 | |
| H0 | Late mortality (%) | 5.66 ± 0.55 a | 3.39 ± 0.29 b |
| Relative yolk weight (%) | 12.77 ± 1.64 a | 8.95 ± 0.87 b | |
| Hatched gosling weight (g) | 100.99 ± 1.43 a | 106.21 ± 1.66 b | |
| Hatchability of fertile eggs (%) | 89.59 ± 0.52 a | 91.92 ± 0.77 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Yan, L.; Lei, M.; Dai, Z.; Shi, Z. Wider Angle Egg Turning during Incubation Enhances Yolk Utilization and Promotes Goose Embryo Development. Animals 2021, 11, 2485. https://doi.org/10.3390/ani11092485
Guo B, Yan L, Lei M, Dai Z, Shi Z. Wider Angle Egg Turning during Incubation Enhances Yolk Utilization and Promotes Goose Embryo Development. Animals. 2021; 11(9):2485. https://doi.org/10.3390/ani11092485
Chicago/Turabian StyleGuo, Binbin, Leyan Yan, Mingming Lei, Zichun Dai, and Zhendan Shi. 2021. "Wider Angle Egg Turning during Incubation Enhances Yolk Utilization and Promotes Goose Embryo Development" Animals 11, no. 9: 2485. https://doi.org/10.3390/ani11092485
APA StyleGuo, B., Yan, L., Lei, M., Dai, Z., & Shi, Z. (2021). Wider Angle Egg Turning during Incubation Enhances Yolk Utilization and Promotes Goose Embryo Development. Animals, 11(9), 2485. https://doi.org/10.3390/ani11092485






