Modified Spirulina maxima Pectin Nanoparticles Improve the Developmental Competence of In Vitro Matured Porcine Oocytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of MSmPNPs
2.3. Oocyte In Vitro Maturation and MSmPNPs Treatment
2.4. Measurement of Intracellular GSH and ROS Levels
2.5. In Vitro Embryo Production
2.6. Analysis of mRNA Transcript Expression by Relative Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Experimental Design
2.8. Statistical Analysis
3. Results
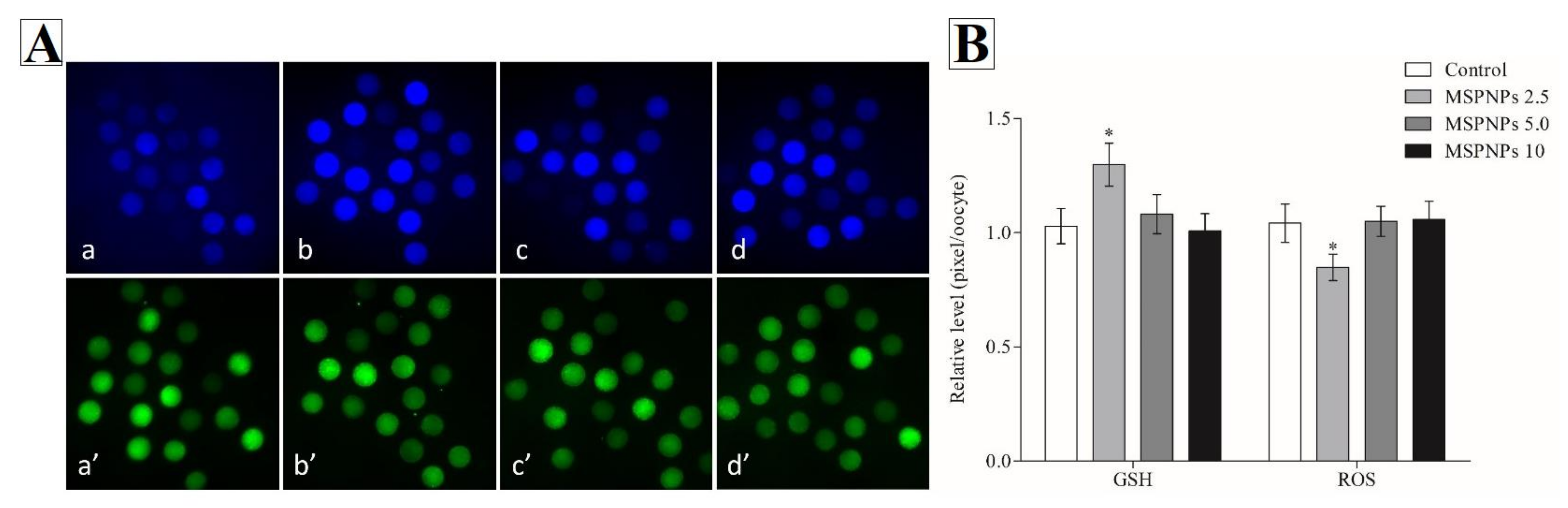
3.1. GSH and ROS Intracellular Levels Treated with/without MSmPNPs
3.2. The Effect of MSmPNPs on the Developmental Competence of PA Embryos
3.3. MSmPNPs Effects on Cell Number of PA Embryos
3.4. MSmPNPs Effects on the Developmental Competence of Cloned Embryos
3.5. MSmPNPs Effects on Cloned Cell Number
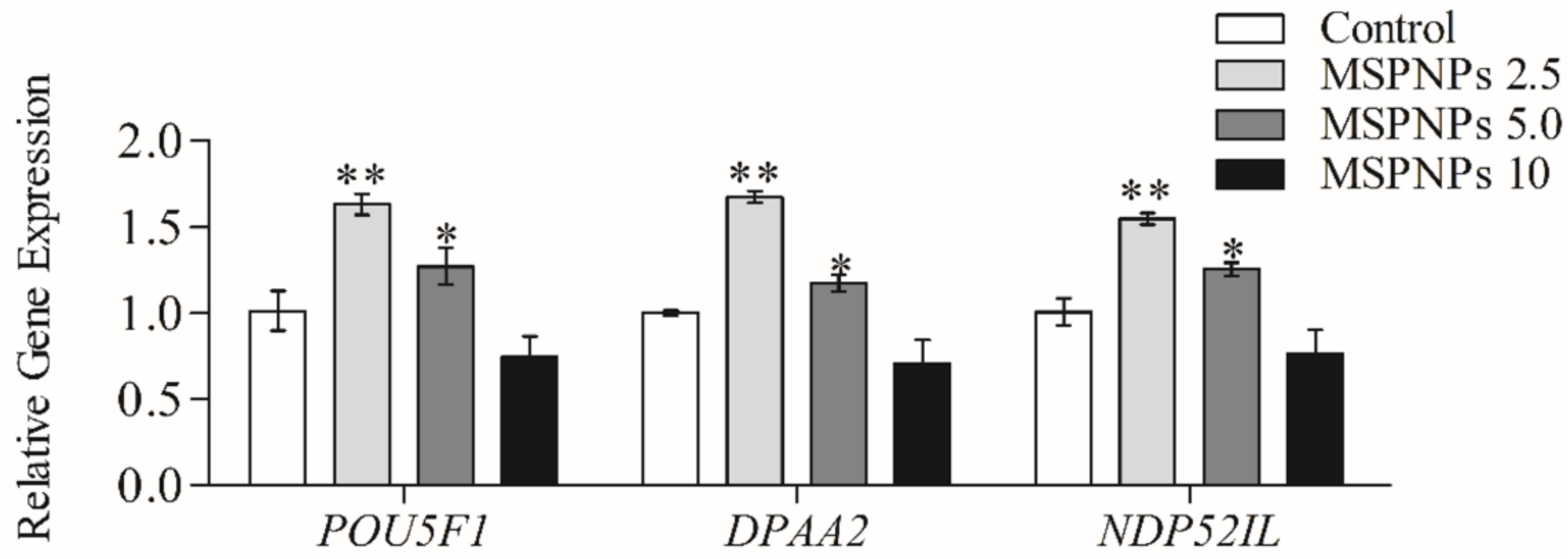
3.6. MSmPNPs Effects on Reprogramming-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Saadeldin, I.M.; Khalil, W.A.; Alharbi, M.G.; Lee, S.H. The Current Trends in Using Nanoparticles, Liposomes, and Exosomes for Semen Cryopreservation. Animals 2020, 10, 2281. [Google Scholar] [CrossRef]
- Lucas, C.G.; Chen, P.R.; Seixas, F.K.; Prather, R.S.; Collares, T. Applications of omics and nanotechnology to improve pig embryo production in vitro. Mol. Reprod. Dev. 2019, 86, 1531–1547. [Google Scholar] [CrossRef]
- Neculai-Valeanu, A.S.; Ariton, A.M.; Mădescu, B.M.; Rîmbu, C.M.; Creangă, Ş. Nanomaterials and Essential Oils as Candidates for Developing Novel Treatment Options for Bovine Mastitis. Animals 2021, 11, 1625. [Google Scholar] [CrossRef] [PubMed]
- Abo-Al-Ela, H.G.; El-Kassas, S.; El-Naggar, K.; Abdo, S.E.; Jahejo, A.R.; Al Wakeel, R.A. Stress and immunity in poultry: Light management and nanotechnology as effective immune enhancers to fight stress. Cell Stress Chaperones 2021, 26, 457–472. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Alagawany, M.; Hashem, N.M.; Farag, M.R.; Alghamdi, E.S.; Hassan, F.U.; Bilal, R.M.; Elnesr, S.S.; Dawood, M.A.O.; Nagadi, S.A.; et al. Nanominerals: Fabrication Methods, Benefits and Hazards, and Their Applications in Ruminants with Special Reference to Selenium and Zinc Nanoparticles. Animals 2021, 11, 1916. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A. Nanotechnology and Reproductive Management of Farm Animals: Challenges and Advances. Animals 2021, 11, 1932. [Google Scholar] [CrossRef] [PubMed]
- Guisbiers, G.; Mejía-Rosales, S.; Leonard Deepak, F. Nanomaterial Properties: Size and Shape Dependencies. J. Nanomater. 2012, 2012, 1–2. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Lucas, C.G.; Remiao, M.H.; Bruinsmann, F.A.; Lopes, I.A.R.; Borges, M.A.; Feijo, A.L.S.; Basso, A.C.; Pohlmann, A.R.; Guterres, S.S.; Campos, V.F.; et al. High doses of lipid-core nanocapsules do not affect bovine embryonic development in vitro. Toxicol. Vitr. 2017, 45, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, B.R. Protective effect of Chitosan nanoparticles against the inhibitory effect of linoleic acid supplementation on maturation and developmental competence of bovine oocytes. Theriogenology 2018, 114, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Qamar, A.Y.; Fang, X.; Kim, G.; Bang, S.; De Zoysa, M.; Shin, S.T.; Cho, J. Chitosan nanoparticles enhance developmental competence of in vitro-matured porcine oocytes. Reprod. Domest. Anim. 2021, 56, 342–350. [Google Scholar] [CrossRef]
- Bakhtari, A.; Nazari, S.; Alaee, S.; Kargar-Abarghouei, E.; Mesbah, F.; Mirzaei, E.; Molaei, M.J. Effects of Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles on Mouse Embryo Development, Antioxidant Enzymes and Apoptosis Genes Expression, and Ultrastructure of Sperm, Oocytes and Granulosa Cells. Int. J. Fertil. Steril. 2020, 14, 161–170. [Google Scholar] [CrossRef]
- Shehata, A.M.; Salem, F.M.S.; El-Saied, E.M.; Abd El-Rahman, S.S.; Mahmoud, M.Y.; Noshy, P.A. Zinc Nanoparticles Ameliorate the Reproductive Toxicity Induced by Silver Nanoparticles in Male Rats. Int. J. Nanomed. 2021, 16, 2555–2568. [Google Scholar] [CrossRef]
- Abbasi, Y.; Hajiaghalou, S.; Baniasadi, F.; Mahabadi, V.P.; Ghalamboran, M.R.; Fathi, R. Fe3O4 magnetic nanoparticles improve the vitrification of mouse immature oocytes and modulate the pluripotent genes expression in derived pronuclear-stage embryos. Cryobiology 2021, 100, 81–89. [Google Scholar] [CrossRef] [PubMed]
- El-Naby, A.-s.A.-H.H.; Ibrahim, S.; Hozyen, H.F.; Sosa, A.S.A.; Mahmoud, K.G.M.; Farghali, A.A. Impact of nano-selenium on nuclear maturation and genes expression profile of buffalo oocytes matured in vitro. Mol. Biol. Rep. 2020, 47, 8593–8603. [Google Scholar] [CrossRef] [PubMed]
- Ariu, F.; Bogliolo, L.; Pinna, A.; Malfatti, L.; Innocenzi, P.; Falchi, L.; Bebbere, D.; Ledda, S. Cerium oxide nanoparticles (CeO2 NPs) improve the developmental competence of in vitro-matured prepubertal ovine oocytes. Reprod. Fertil. Dev. 2017, 29, 1046. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, X.; Dai, J.; Zhang, D.; Liu, B.; Wang, H.; Xu, L. Effect of hydroxyapatite nanoparticles on MII-stage porcine oocytes vitrification and the study of its mechanism. J. Biomed. Eng. = Shengwu Yixue Gongchengxue Zazhi 2013, 30, 789–793. [Google Scholar]
- Huang, C.H.; Yeh, J.M.; Chan, W.H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ. Toxicol. 2018, 33, 1039–1049. [Google Scholar] [CrossRef]
- Santacruz-Márquez, R.; González-De los Santos, M.; Hernández-Ochoa, I. Ovarian toxicity of nanoparticles. Reprod. Toxicol. 2021, 103, 79–95. [Google Scholar] [CrossRef]
- Lin, Y.H.; Zhuang, S.X.; Wang, Y.L.; Lin, S.; Hong, Z.W.; Liu, Y.; Xu, L.; Li, F.P.; Xu, B.H.; Chen, M.H.; et al. The effects of graphene quantum dots on the maturation of mouse oocytes and development of offspring. J. Cell. Physiol. 2019, 234, 13820–13831. [Google Scholar] [CrossRef]
- Taylor, U.; Tiedemann, D.; Rehbock, C.; Kues, W.A.; Barcikowski, S.; Rath, D. Influence of gold, silver and gold–Silver alloy nanoparticles on germ cell function and embryo development. Beilstein J. Nanotechnol. 2015, 6, 651–664. [Google Scholar] [CrossRef]
- Karimipour, M.; Zirak Javanmard, M.; Ahmadi, A.; Jafari, A. Oral administration of titanium dioxide nanoparticle through ovarian tissue alterations impairs mice embryonic development. Int. J. Reprod. Biomed. 2018, 16, 397–404. [Google Scholar] [CrossRef]
- Lei, R.; Bai, X.; Chang, Y.; Li, J.; Qin, Y.; Chen, K.; Gu, W.; Xia, S.; Zhang, J.; Wang, Z.; et al. Effects of Fullerenol Nanoparticles on Rat Oocyte Meiosis Resumption. Int. J. Mol. Sci. 2018, 19, 699. [Google Scholar] [CrossRef]
- Wang, R.; Song, B.; Wu, J.; Zhang, Y.; Chen, A.; Shao, L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018, 13, 8487–8506. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Y.; Ge, W.; Zhang, P.; Liu, X.; Zhang, W.; Hao, Y.; Yu, S.; Li, L.; Chu, M.; et al. Oocyte exposure to ZnO nanoparticles inhibits early embryonic development through the γ-H2AX and NF-κB signaling pathways. Oncotarget 2017, 8, 42673–42692. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Abdel-Khalek, A.E.; Khalil, W.A.; Yousif, A.I.; Saadeldin, I.M.; Abomughaid, M.M.; El-Harairy, M.A. Effects of mint, thyme, and curcumin extract nanoformulations on the sperm quality, apoptosis, chromatin decondensation, enzyme activity, and oxidative status of cryopreserved goat semen. Cryobiology 2020, 97, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Mbemya, G.T.; Vieira, L.A.; Canafistula, F.G.; Pessoa, O.D.L.; Rodrigues, A.P.R. Reports on in vivo and in vitro contribution of medicinal plants to improve the female reproductive function. Reprod. Clim. 2017, 32, 109–119. [Google Scholar] [CrossRef]
- Yang, L.; Lei, L.; Zhao, Q.; Gao, Z.; Xu, X. Lycium barbarum polysaccharide improves the development of mouse oocytes vitrified at the germinal vesicle stage. Cryobiology 2018, 85, 7–11. [Google Scholar] [CrossRef]
- Willats, W.G.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- de Moura, F.A.; Macagnan, F.T.; Dos Santos, L.R.; Bizzani, M.; de Oliveira Petkowicz, C.L.; da Silva, L.P. Characterization and physicochemical properties of pectins extracted from agroindustrial by-products. J. Food Sci. Technol. 2017, 54, 3111–3117. [Google Scholar] [CrossRef]
- Fracasso, A.F.; Perussello, C.A.; Carpiné, D.; Petkowicz, C.L.O.; Haminiuk, C.W.I. Chemical modification of citrus pectin: Structural, physical and rheologial implications. Int. J. Biol. Macromol. 2018, 109, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M. The potential of pectin to impact pig nutrition and health: Feeding the animal and its microbiome. FEMS Microbiol. Lett. 2019, 366, fnz029. [Google Scholar] [CrossRef] [PubMed]
- Mizera, A.; Kuczaj, M.; Szul, A. Impact of the Spirulina maxima extract addition to semen extender on bovine sperm quality. Ital. J. Anim. Sci. 2019, 18, 601–607. [Google Scholar] [CrossRef]
- Barkallah, M.; Slima, A.B.; Elleuch, F.; Fendri, I.; Pichon, C.; Abdelkafi, S.; Baril, P. Protective Role of Spirulina platensis Against Bifenthrin-Induced Reprotoxicity in Adult Male Mice by Reversing Expression of Altered Histological, Biochemical, and Molecular Markers Including MicroRNAs. Biomolecules 2020, 10, 753. [Google Scholar] [CrossRef]
- Lee, A.V.; You, L.; Oh, S.Y.; Li, Z.; Fisher-Heffernan, R.E.; Regnault, T.R.H.; de Lange, C.F.M.; Huber, L.; Karrow, N.A. Microalgae supplementation to late gestation sows and its effects on the health status of weaned piglets fed diets containing high- or low-quality protein sources. Vet. Immunol. Immunopathol. 2019, 218, 109937. [Google Scholar] [CrossRef]
- Senosy, W.; Kassab, A.Y.; Mohammed, A.A. Effects of feeding green microalgae on ovarian activity, reproductive hormones and metabolic parameters of Boer goats in arid subtropics. Theriogenology 2017, 96, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, S.L.; Dananjaya, S.H.S.; Nikapitiya, C.; Liyanage, T.D.; Lee, K.A.; Oh, C.; Kang, D.H.; De Zoysa, M. Novel pectin isolated from Spirulina maxima enhances the disease resistance and immune responses in zebrafish against Edwardsiella piscicida and Aeromonas hydrophila. Fish Shellfish Immunol. 2019, 94, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, S.L.; Rajapaksha, D.C.; Nikapitiya, C.; Oh, C.; Lee, K.-A.; Kang, D.-H.; De Zoysa, M. Spirulina maxima derived marine pectin promotes the in vitro and in vivo regeneration and wound healing in zebrafish. Fish Shellfish Immunol. 2020, 107, 414–425. [Google Scholar] [CrossRef]
- Chandrarathna, H.; Liyanage, T.D.; Edirisinghe, S.L.; Dananjaya, S.H.S.; Thulshan, E.H.T.; Nikapitiya, C.; Oh, C.; Kang, D.H.; De Zoysa, M. Marine Microalgae, Spirulina maxima-Derived Modified Pectin and Modified Pectin Nanoparticles Modulate the Gut Microbiota and Trigger Immune Responses in Mice. Mar. Drugs 2020, 18, 175. [Google Scholar] [CrossRef]
- Park, D.S.; Kim, S.; Koo, D.-B.; Kang, M.-J. Current Status of Production of Transgenic Livestock by Genome Editing Technology. J. Anim. Reprod. Biotechnol. 2019, 34, 148–156. [Google Scholar] [CrossRef]
- Roy, P.K.; Qamar, A.Y.; Fang, X.; Hassan, B.M.S.; Cho, J. Effects of cobalamin on meiotic resumption and developmental competence of growing porcine oocytes. Theriogenology 2020, 154, 24–30. [Google Scholar] [CrossRef]
- Lin, T.; Lee, J.E.; Kang, J.W.; Oqani, R.K.; Cho, E.S.; Kim, S.B.; Il Jin, D. Melatonin supplementation during prolonged in vitro maturation improves the quality and development of poor-quality porcine oocytes via anti-oxidative and anti-apoptotic effects. Mol. Reprod. Dev. 2018, 85, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Park, H.-J.; Yang, S.-G.; Koo, D.-B. Anti-oxidative effects of exogenous ganglioside GD1a and GT1b on embryonic developmental competence in pigs. J. Anim. Reprod. Biotechnol. 2020, 35, 347–356. [Google Scholar] [CrossRef]
- Cho, J.; Kim, G.; Qamar, A.Y.; Fang, X.; Roy, P.K.; Tanga, B.M.; Bang, S.; Kim, J.K.; Galli, C.; Perota, A.; et al. Improved efficiencies in the generation of multigene-modified pigs by recloning and using sows as the recipient. Zygote 2021, 1–8. [Google Scholar] [CrossRef]
- Roy, P.K.; Qamar, A.Y.; Tanga, B.M.; Fang, X.; Kim, G.; Bang, S.; Cho, J. Enhancing Oocyte Competence With Milrinone as a Phosphodiesterase 3A Inhibitor to Improve the Development of Porcine Cloned Embryos. Front. Cell Dev. Biol. 2021, 9, 647616. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Kim, G.; Fang, X.; Hassan, B.; Soysa, M.D.; Shin, S.T.; Cho, J.K. Optimization of post-activation systems to improve the embryonic development in porcine parthenogenesis and somatic cell nuclear transfer. J. Embryo Transf. 2017, 32, 95–104. [Google Scholar] [CrossRef]
- Kim, G.; Roy, P.K.; Fang, X.; Hassan, B.M.; Cho, J. Improved preimplantation development of porcine somatic cell nuclear transfer embryos by caffeine treatment. J. Vet. Sci. 2019, 20, e31. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z. Nanoparticles induced embryo–fetal toxicity. Toxicol. Ind. Health 2020, 36, 181–213. [Google Scholar] [CrossRef]
- Wen, X.; Han, Z.; Liu, S.-J.; Hao, X.; Zhang, X.-J.; Wang, X.-Y.; Zhou, C.-J.; Ma, Y.-Z.; Liang, C.-G. Phycocyanin Improves Reproductive Ability in Obese Female Mice by Restoring Ovary and Oocyte Quality. Front. Cell Dev. Biol. 2020, 8, 1208. [Google Scholar] [CrossRef]
- Liang, S.; Guo, J.; Jin, Y.X.; Yuan, B.; Zhang, J.B.; Kim, N.H. C-Phycocyanin supplementation during in vitro maturation enhances pre-implantation developmental competence of parthenogenetic and cloned embryos in pigs. Theriogenology 2018, 106, 69–78. [Google Scholar] [CrossRef]
- Wathoni, N.; Yuan Shan, C.; Yi Shan, W.; Rostinawati, T.; Indradi, R.B.; Pratiwi, R.; Muchtaridi, M. Characterization and antioxidant activity of pectin from Indonesian mangosteen (Garcinia mangostana L.) rind. Heliyon 2019, 5, e02299. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, V.V.; Golovchenko, V.V.; Vityazev, F.V.; Patova, O.A.; Selivanov, N.Y.; Selivanova, O.G.; Popov, S.V. The Antioxidant Properties of Pectin Fractions Isolated from Vegetables Using a Simulated Gastric Fluid. J. Chem. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Hosseini Abari, A.; Amini Rourani, H.; Ghasemi, S.M.; Kim, H.; Kim, Y.-G. Investigation of antioxidant and anticancer activities of unsaturated oligo-galacturonic acids produced by pectinase of Streptomyces hydrogenans YAM1. Sci. Rep. 2021, 11, 8491. [Google Scholar] [CrossRef]
- Ogutu, F.O.; Mu, T.-H. Ultrasonic degradation of sweet potato pectin and its antioxidant activity. Ultrason. Sonochem. 2017, 38, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jin, C.; Tong, Z.; Lu, J.; Tan, L.; Tian, L.; Chang, Q. Optimization extraction, characterization and antioxidant activities of pectic polysaccharide from tangerine peels. Carbohydr. Polym. 2016, 136, 187–197. [Google Scholar] [CrossRef]
- Kang, H.J.; Jo, C.; Kwon, J.H.; Son, J.H.; An, B.J.; Byun, M.W. Antioxidant and Cancer Cell Proliferation Inhibition Effect of Citrus Pectin-Oligosaccharide Prepared by Irradiation. J. Med. Food 2006, 9, 313–320. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Dong, Y.; Zhu, R.; Liu, Y. Antioxidant activity of penta-oligogalacturonide, isolated from haw pectin, suppresses triglyceride synthesis in mice fed with a high-fat diet. Food Chem. 2014, 145, 335–341. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Nie, X.W.; Li, Z.Y.; Wang, Y.M.; Liang, S.; Li, S. Rosmarinic acid treatment during porcine oocyte maturation attenuates oxidative stress and improves subsequent embryo development in vitro. PeerJ 2019, 7, e6930. [Google Scholar] [CrossRef]
- Gao, W.; Jin, Y.; Hao, J.; Huang, S.; Wang, D.; Quan, F.; Ren, W.; Zhang, J.; Zhang, M.; Yu, X. Procyanidin B1 promotes in vitro maturation of pig oocytes by reducing oxidative stress. Mol. Reprod. Dev. 2021, 88, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Kang, J.-T.; Park, S.-J.; Kim, S.-J.; Moon, J.-H.; Saadeldin, I.M.; Jang, G.; Lee, B.-C. Effect of 7,8-Dihydroxyflavone as an Antioxidant on In Vitro Maturation of Oocytes and Development of Parthenogenetic Embryos in Pigs. J. Reprod. Dev. 2013, 59, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-T.; Moon, J.H.; Choi, J.-Y.; Park, S.J.; Kim, S.J.; Saadeldin, I.M.; Lee, B.C. Effect of Antioxidant Flavonoids (Quercetin and Taxifolin) on In Vitro Maturation of Porcine Oocytes. Asian-Australas. J. Anim. Sci. 2016, 29, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Guerin, P. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Somfai, T.; Kaneda, M.; Akagi, S.; Watanabe, S.; Haraguchi, S.; Mizutani, E.; Dang-Nguyen, T.Q.; Geshi, M.; Kikuchi, K.; Nagai, T. Enhancement of lipid metabolism with L-carnitine during in vitro maturation improves nuclear maturation and cleavage ability of follicular porcine oocytes. Reprod. Fertil. Dev. 2011, 23, 912–920. [Google Scholar] [CrossRef]
- Rajapaksha, D.C.; Edirisinghe, S.L.; Nikapitiya, C.; Dananjaya, S.; Kwun, H.J.; Kim, C.H.; Oh, C.; Kang, D.H.; De Zoysa, M. Spirulina maxima Derived Pectin Nanoparticles Enhance the Immunomodulation, Stress Tolerance, and Wound Healing in Zebrafish. Mar. Drugs 2020, 18, 556. [Google Scholar] [CrossRef] [PubMed]
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of Reactive Oxygen Species and Antioxidants in Ovarian Toxicity1. Biol. Reprod. 2012, 86, 1–10. [Google Scholar] [CrossRef]
- Circu, M.L.; Yee Aw, T. Glutathione and apoptosis. Free Radic. Res. 2009, 42, 689–706. [Google Scholar] [CrossRef]
- Kim, S.J.; Koo, O.J.; Park, H.J.; Moon, J.H.; da Torre, B.R.; Javaregowda, P.K.; Kang, J.T.; Park, S.J.; Saadeldin, I.M.; Choi, J.Y.; et al. Oct4 overexpression facilitates proliferation of porcine fibroblasts and development of cloned embryos. Zygote 2014, 23, 704–711. [Google Scholar] [CrossRef][Green Version]
- Sato, Y.; Kobayashi, H.; Higuchi, T.; Shimada, Y.; Era, T.; Kimura, S.; Eto, Y.; Ida, H.; Ohashi, T. Disease modeling and lentiviral gene transfer in patient-specific induced pluripotent stem cells from late-onset Pompe disease patient. Mol. Ther. Methods Clin. Dev. 2015, 2, 15023. [Google Scholar] [CrossRef]
- Eckersley-Maslin, M.A. Keeping your options open: Insights from Dppa2/4 into how epigenetic priming factors promote cell plasticity. Biochem. Soc. Trans. 2020, 48, 2891–2902. [Google Scholar] [CrossRef]
- Lim, P.S.L.; Meshorer, E. Dppa2 and Dppa4 safeguard bivalent chromatin in order to establish a pluripotent epigenome. Nat. Struct. Mol. Biol. 2020, 27, 685–686. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, C.E.; Shinde, V.; Srinivasan, S.P.; Henry, M.; Rotshteyn, T.; Baumstark-Khan, C.; Schmitz, C.; Feles, S.; Spitta, L.F.; Hemmersbach, R.; et al. Radiation Response of Murine Embryonic Stem Cells. Cells 2020, 9, 650. [Google Scholar] [CrossRef] [PubMed]
- Bortvin, A.; Eggan, K.; Skaletsky, H.; Akutsu, H.; Berry, D.L.; Yanagimachi, R.; Page, D.C.; Jaenisch, R. Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development 2003, 130, 1673–1680. [Google Scholar] [CrossRef]
- You, J.; Lee, J.; Kim, J.; Park, J.; Lee, E. Post-fusion treatment with MG132 increases transcription factor expression in somatic cell nuclear transfer embryos in pigs. Mol. Reprod. Dev. 2010, 77, 149–157. [Google Scholar] [CrossRef] [PubMed]
| Genes | Gene Full Name | Sequences (5′-3′) | Product Size (bp) | NCBI Accession No. |
|---|---|---|---|---|
| ß-actin | Beta actin | F: CCC TGG AGA GCT ACG AG | 172 | XM_003124280.5 |
| R: TCC TTC CTG ATG TCC ACG TC | ||||
| POU5F1 | POU class 5 homeobox 1 | F: AGT GAG AGG CAA CCT GGA GA | 166 | XM_021097869.1 |
| R: TCG TTG CGA ATA GTC ACT GC | ||||
| NDP52 | Nuclear domain 10 protein | F: TGC TGA GTT ACA TGG GTC TGG | 182 | XM_003131552.4 |
| R: ACC AAG GTC TGA TTT GCA GGT | ||||
| DPPA2 | Developmental pluripotency associated 2 | F: TGA GAG AGG GGA AAA GAC CAA | 151 | XM_003358822.4 |
| R: TGG CAG AAA GGT CTC AAC AGA |
| Conc. of MSmPNPs (µg/mL) | No. of COCs | No. of Matured Oocytes (%) | No. of Embryos (Mean ± SEM) | ||
|---|---|---|---|---|---|
| Cultured | Cleaved (%) | Blastocyst (%) | |||
| 0 (Control) | 200 | 167 (83.5 ± 1.8) b | 161 | 137 (85.1 ± 1.3) b | 47 (29.2 ± 1.0) b |
| 2.5 | 200 | 182 (91.0 ± 1.0) a | 168 | 152 (90.5 ± 0.8) a | 58 (34.5 ± 1.4) a |
| 5.0 | 200 | 173 (86.5 ± 1.3) b | 162 | 140 (86.4 ± 1.3) a,b | 48 (29.6 ± 1.7) a,b |
| 10 | 200 | 160 (80.0 ± 0.8) b | 157 | 126 (80.3 ± 1.1) c | 39 (24.9 ± 1.1) b |
| Conc. of MSmPNPs (µg/mL) | No. of Cells (Mean ± SEM) | Ratio (%) of ICM: TE | ||
|---|---|---|---|---|
| Total Cells | TE (%) | ICM (%) | ||
| 0 (Control) | 42.8 ± 1.2 b,c | 33.8 (78.7 ± 1.0) a | 9.0 (21.3 ± 1.0) b | 27.9 ± 1.7 b |
| 2.5 | 48.7 ± 1.4 a | 36.6 (74.8 ± 0.8) b | 12.0 (25.2 ± 0.8) a | 34.5 ± 1.4 a |
| 5.0 | 44.0 ± 1.1 b | 33.4 (75.6 ± 0.7) b,c | 10.7 (24.4 ± 0.7) a,b | 32.8 ± 1.2 a,b |
| 10 | 40.2 ± 1.0 c | 32.0 (79.6 ± 0.9) a | 8.2 (20.4 ± 0.9) b | 26.1 ± 1.5 b |
| Conc. of MSmPNPs (µg/mL) | No. of COCs | No. of Matured Oocytes (%) | No. of Embryos (Mean ± SEM) | ||
|---|---|---|---|---|---|
| Cultured | Cleaved (%) | Blastocyst (%) | |||
| 0 (Control) | 200 | 166 (83.0 ± 1.5) b | 137 | 116 (84.7 ± 1.0) | 34 (24.8 ± 0.9) b |
| 2.5 | 200 | 179 (89.5 ± 1.8) a | 148 | 131 (88.5 ± 1.6) | 46 (31.1 ± 1.1) a |
| 5.0 | 200 | 173 (86.5 ± 1.3) b | 143 | 125 (87.4 ± 2.1) | 37 (25.9 ± 0.9) b |
| 10 | 200 | 161 (80.5 ± 1.4) b | 132 | 109 (82.6 ± 1.3) | 30 (22.7 ± 1.4) b |
| Conc. of MSmPNPs (µg/mL) | No. of Cells (Mean ± SEM) | Ratio (%) of ICM: TE | ||
|---|---|---|---|---|
| Total Cells | TE (%) | ICM (%) | ||
| 0 (Control) | 41.9 ± 1.9 b,c | 33.2 (78.7 ± 1.2) a | 8.8 (21.3 ± 1.2) b | 27.9 ± 2.2 b |
| 2.5 | 48.9 ± 1.5 a | 36.4 (74.0 ± 0.9) b | 12.5 (26.0 ± 0.9) a | 35.7 ± 1.8 a |
| 5.0 | 44.4 ± 1.0 b | 34.6 (77.7 ± 1.0) a | 9.7 (22.3 ± 1.0) b | 29.0 ± 1.7 b |
| 10 | 39.2 ± 2.5 c | 31.0 (78.5 ± 1.1) a | 8.2 (21.5 ± 1.1) b | 27.6 ± 1.7 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, P.-K.; Qamar, A.-Y.; Tanga, B.-M.; Bang, S.; Seong, G.; Fang, X.; Kim, G.; Edirisinghe, S.-L.; De Zoysa, M.; Kang, D.-H.; et al. Modified Spirulina maxima Pectin Nanoparticles Improve the Developmental Competence of In Vitro Matured Porcine Oocytes. Animals 2021, 11, 2483. https://doi.org/10.3390/ani11092483
Roy P-K, Qamar A-Y, Tanga B-M, Bang S, Seong G, Fang X, Kim G, Edirisinghe S-L, De Zoysa M, Kang D-H, et al. Modified Spirulina maxima Pectin Nanoparticles Improve the Developmental Competence of In Vitro Matured Porcine Oocytes. Animals. 2021; 11(9):2483. https://doi.org/10.3390/ani11092483
Chicago/Turabian StyleRoy, Pantu-Kumar, Ahmad-Yar Qamar, Bereket-Molla Tanga, Seonggyu Bang, Gyeonghwan Seong, Xun Fang, Ghangyong Kim, Shan-Lakmal Edirisinghe, Mahanama De Zoysa, Do-Hyung Kang, and et al. 2021. "Modified Spirulina maxima Pectin Nanoparticles Improve the Developmental Competence of In Vitro Matured Porcine Oocytes" Animals 11, no. 9: 2483. https://doi.org/10.3390/ani11092483
APA StyleRoy, P.-K., Qamar, A.-Y., Tanga, B.-M., Bang, S., Seong, G., Fang, X., Kim, G., Edirisinghe, S.-L., De Zoysa, M., Kang, D.-H., Saadeldin, I. M., & Cho, J. (2021). Modified Spirulina maxima Pectin Nanoparticles Improve the Developmental Competence of In Vitro Matured Porcine Oocytes. Animals, 11(9), 2483. https://doi.org/10.3390/ani11092483










