Choniomyzon taiwanensis n. sp. (Crustacea: Copepoda: Nicothoidae) Parasitic on the External Egg Mass of the Longlegged Spiny Lobster Panulirus longipes longipes (Crustacea: Decapoda: Palinuridae) from Taiwanese Waters
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Morphological Studies
3. Results
3.1. Taxonomic Account
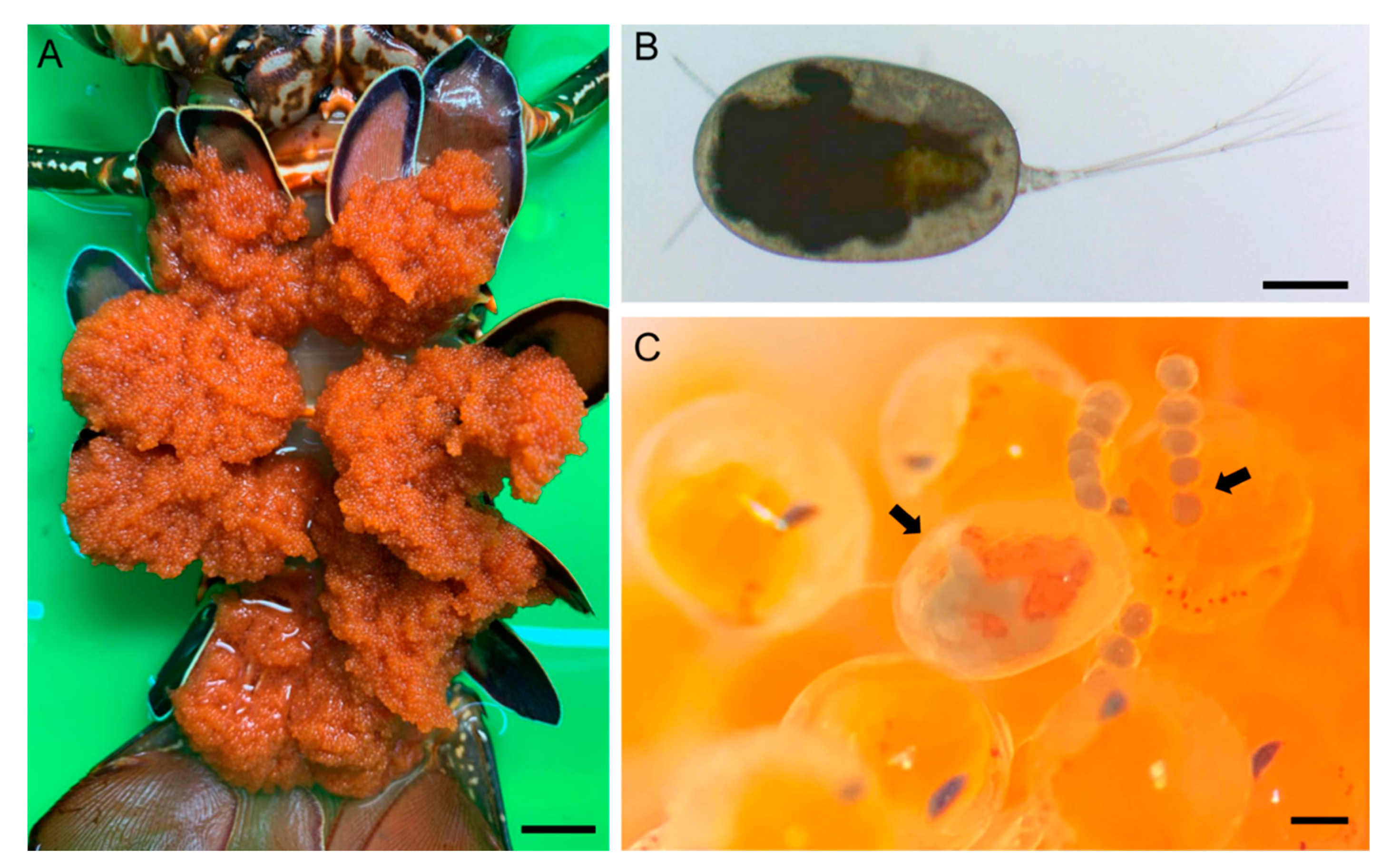
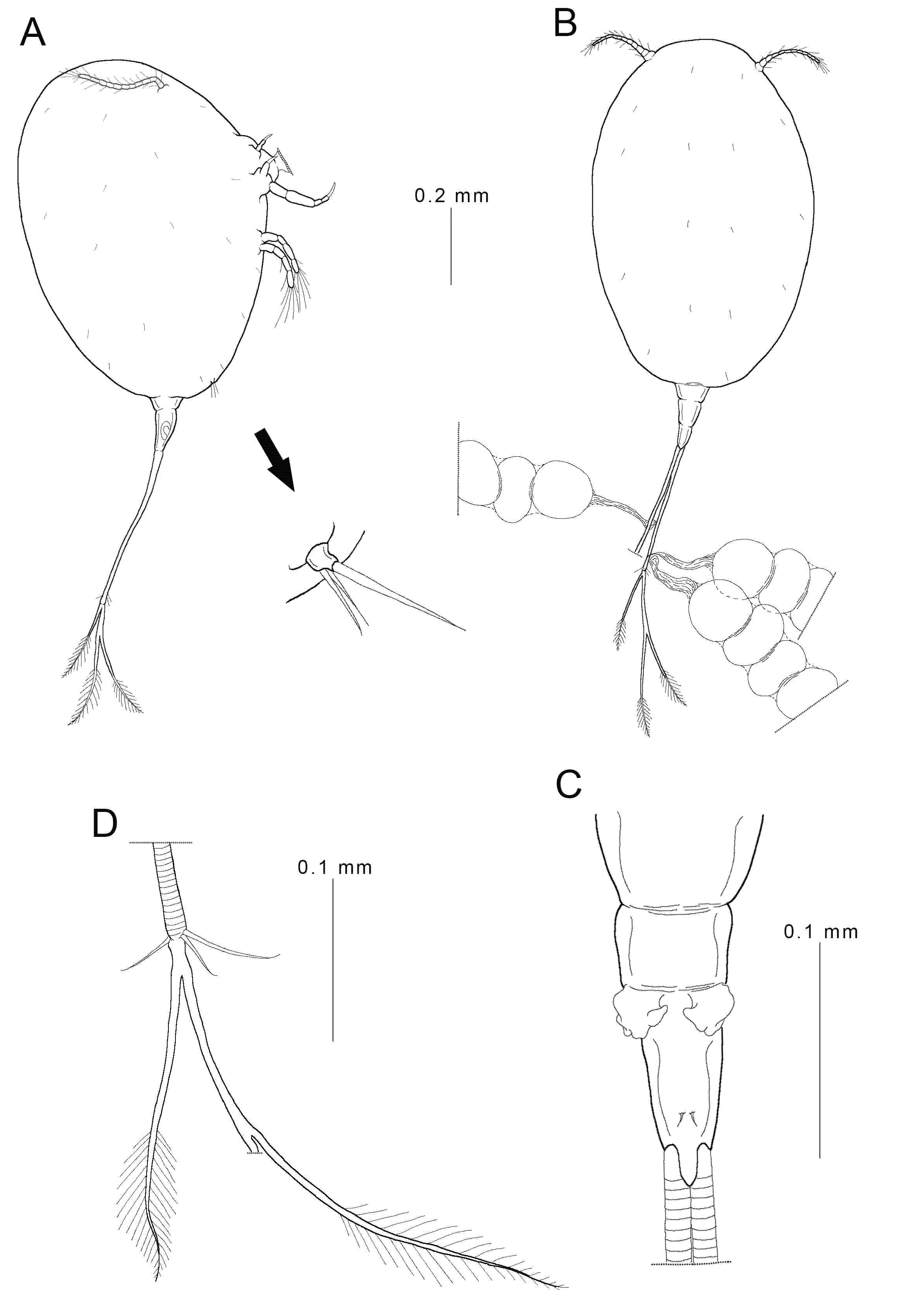
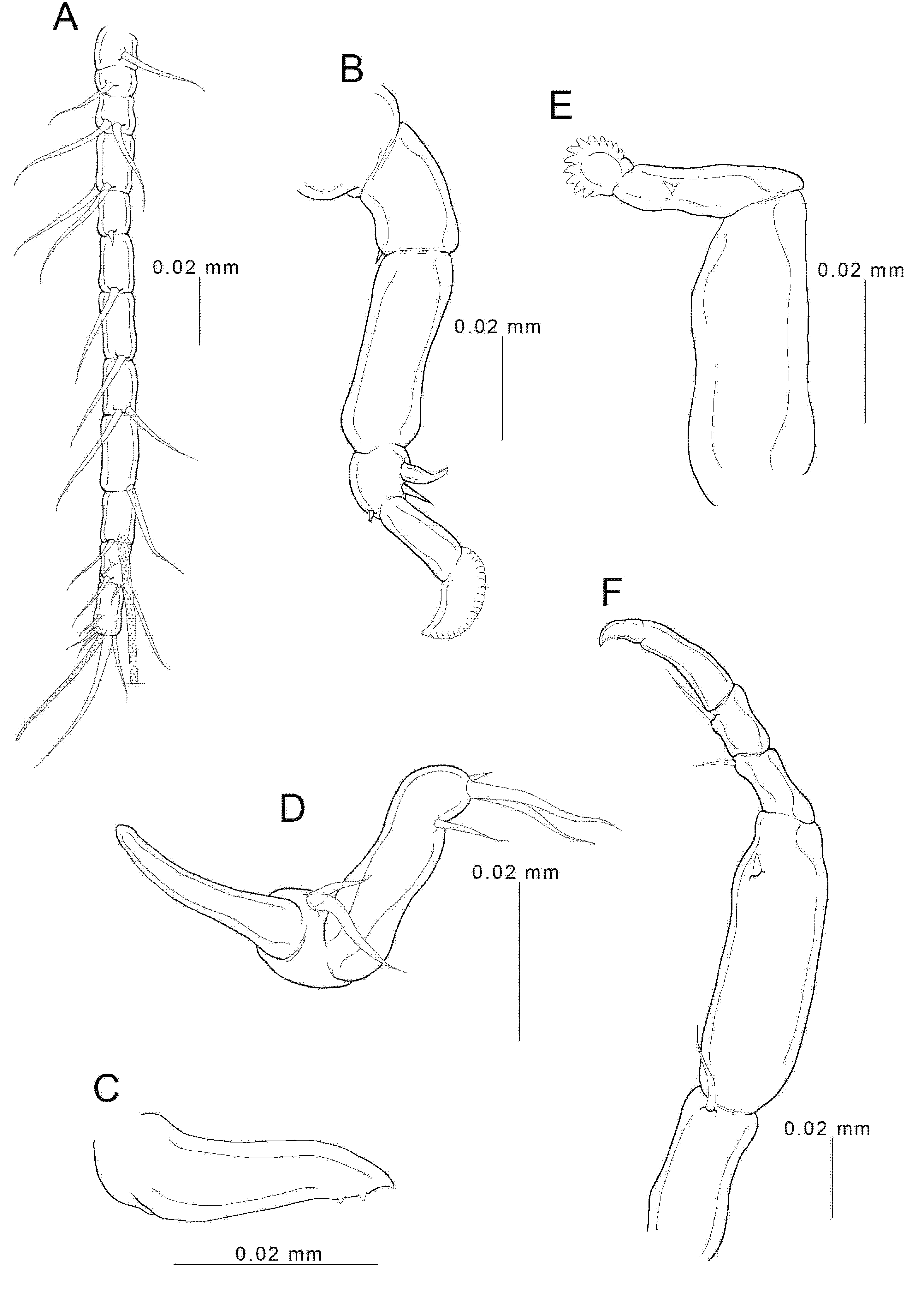
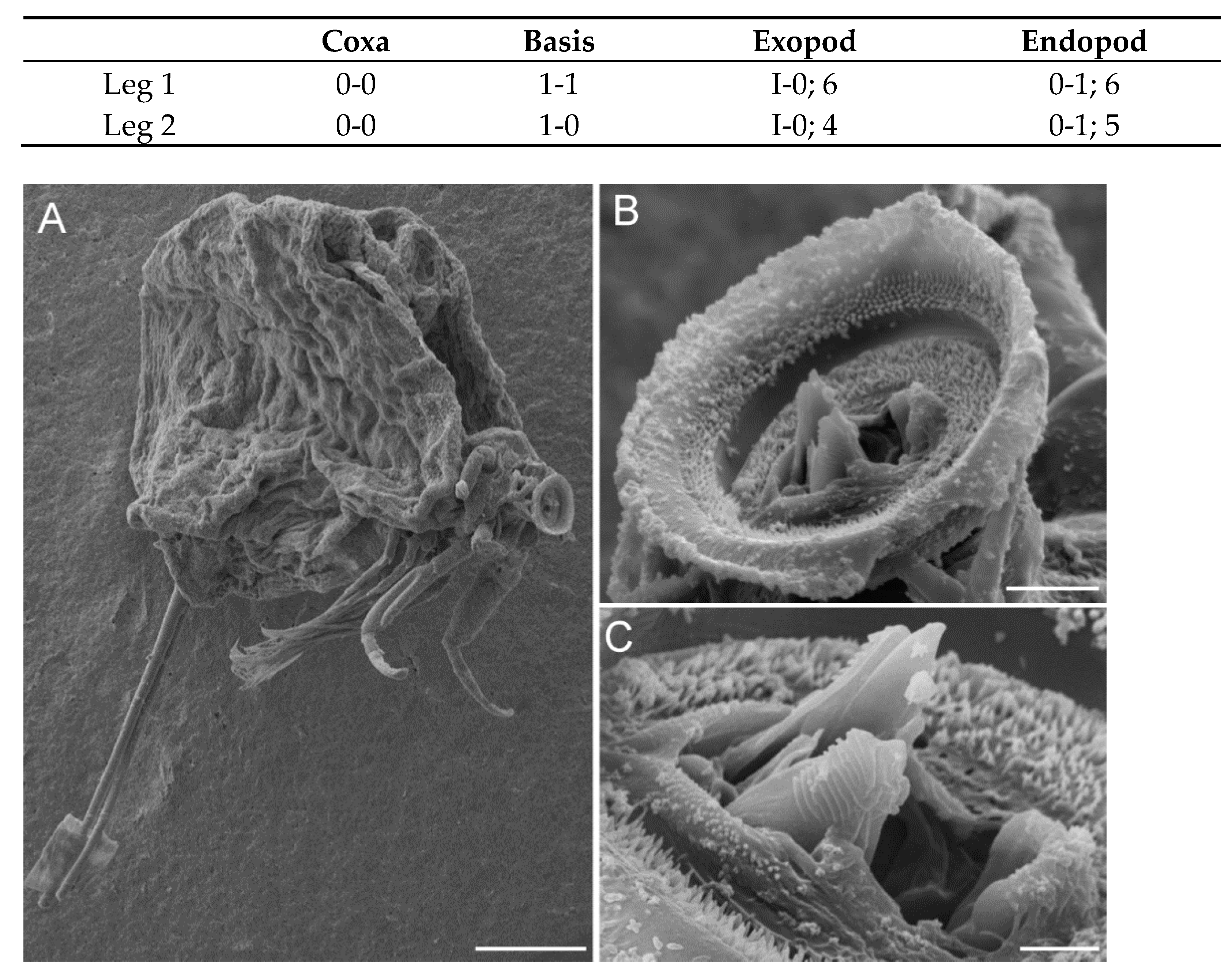
3.2. Description
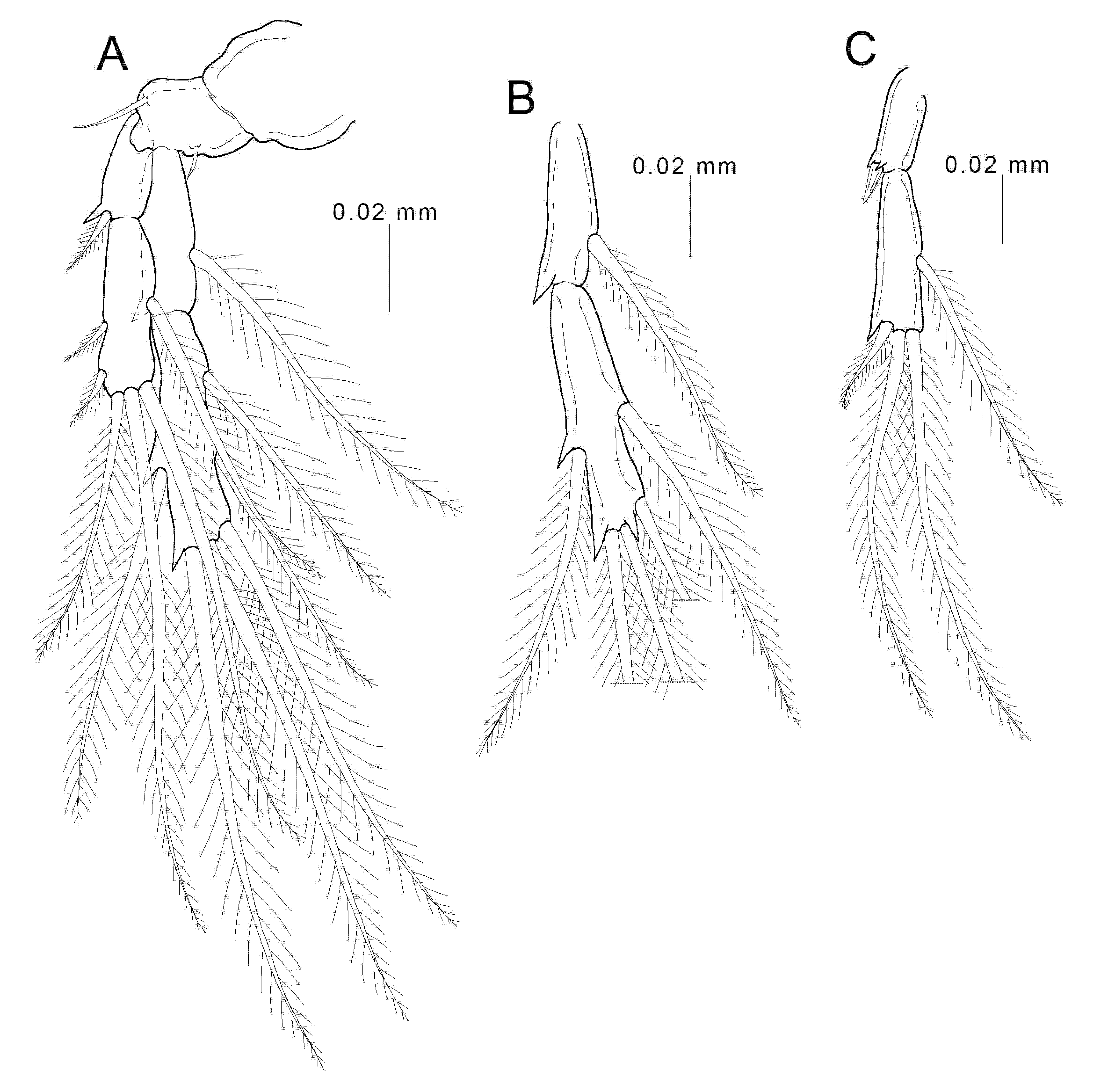
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, H.J. The Choniostomatidae, A Family of Copepoda, Parasites on Crustacea Malacostraca; Andreas Frederik Host & Son: Copenhagen, Denmark, 1897; p. 206. [Google Scholar]
- Shields, J.D. Diseases of spiny lobsters: A review. J. Invertebr. Pathol. 2011, 106, 79–91. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Halsey, S.H. An Introduction to Copepod Diversity; The Ray Society: London, UK, 2004; p. 966. [Google Scholar]
- World of Copepods Database by Walter T.C. and Boxshall G. Available online: http://www.marinespecies.org/copepoda (accessed on 13 May 2021).
- Bowman, T.E.; Kornicker, L.S. Two new crustaceans: The parasitic copepod Sphaeronellopsis monothrix (Choniostomatidae) and its myodocopid ostracod host Parasterope pollex (Cylindroleberidae) from the southern New England coast. Proc. U. S. Natl. Mus. 1967, 123, 1–28. [Google Scholar] [CrossRef][Green Version]
- Wakabayashi, K.; Otake, S.; Tanaka, Y.; Nagasawa, K. Choniomyzon inflatus n. sp. (Crustacea: Copepoda: Nicothoidae) associated with Ibacus novemdentatus (Crustacea: Decapoda: Scyllaridae) from Japanese waters. Syst. Parasitol. 2013, 84, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Pillai, N.K. Choniomyzon gen. nov. (Copepoda: Choniostomatidae) associated with Panulirus. J. Mar. Biol. Ass. India 1962, 4, 95–99. [Google Scholar]
- Bradford, J.M. New parasitic Choniostomatidae (Copepoda) mainly from Antarctic and Subantarctic Ostracoda. N. Z. Oceanog. Inst. Mem. 1975, 67, 1–36. [Google Scholar]
- Shields, J.D.; Stephens, F.J.; Jones, J.B. Pathogens, parasites and other symbionts. In Lobsters: Biology, Management, Aquaculture and Fisheries; Phillip, B.F., Ed.; Blackwell: Oxford, UK, 2006; pp. 146–204. [Google Scholar]
- Santos, C.; Björnberg, T. Choniomyzon libiniae, sp. n. (Crustacea, Copepoda, Nicothoidae) from São Sebastião, SP, Brazil. Zootaxa 2004, 603, 1–12. [Google Scholar] [CrossRef]
- Humes, A.G.; Gooding, R.U. A method for studying the external anatomy of copepods. Crustaceana 1964, 6, 238–240. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 5, 294–299. [Google Scholar]
- Kakui, K. Descriptions of two new species of Rhizorhina Hansen, 1892 (Copepoda: Siphonostomatoida: Nicothoidae) parasitic on tanaidacean crustaceans, with a note on their phylogenetic position. Syst. Parasitol. 2016, 93, 57–68. [Google Scholar] [CrossRef] [PubMed]
| Copepod Species | Host | Distribution | References |
|---|---|---|---|
| C. panuliri | P. homarus | India | [7] |
| P. versicolor | British Solomon Islands | [8] | |
| Panulirus spp. | Australia | [2,9] | |
| C. libiniae | L. spinosa | Brazil | [10] |
| C. inflatus | I. novemdentatus | Japan | [6] |
| C. taiwanensis n. sp. | P. longipes longipes | Taiwan | This study |
| C. panuliri | C. panuliri | C. taiwanensis n. sp. | C. inflatus | C. libiniae | |
|---|---|---|---|---|---|
| Length of prosome (mm) | 1.30 | 1.07 | 0.84 | 1.16 | 0.62–0.83 |
| Antennule | 1, 2, 2, 2, 2, 2, 1, 1, 1, 1, 1+1 (aesthetasc), 2+1 (aesthetasc) | 1, 1, 2, 2, 1, 1, 1, 2, 1, 1+1 (aesthetasc), 4, 7 | 1, 1, 2, 2, 1, 1, 1, 2, 1, 1+1 (aesthetasc), 4, 6+1 (aesthetasc) | 1, 1, 2, 2, 0, 1, 1, 2, 1, 1+1 (aesthetasc), 3, 4+1 (aesthetasc) | 1, 1, 1, 2, 0, 1, 1, 1, 1, 3+1 (aesthetasc), 2, 4+1 (aesthetasc) |
| No. of segments on the antenna | 5 | 5 | 5 | 4 | 3 |
| Size of terminal spine on the fourth segment of antenna | very long | small | small | incomparable | incomparable |
| Second segment of antenna | without seta | without seta | with 1 inner seta | without seta | without seta |
| Distal part of maxillule | with 2 long setae | with 1 spine+3 setae | with 4 setae | with 3 setae | with 4 setae |
| No. of segments on the maxilla | 3 | 3 | 2 | 3 | 3 |
| Final segment of maxilla | with 1 spine | with 1 spine | with 1 spine | with 1 spine | with 4 spiny protuberances |
| Maxilliped | 1, 3, 2, 1, 1 rake-like structure | 1, 1, 1, 1, 1 rake-like structure | 1, 1, 1, 1, 1 rake-like structure | 1, 1, 1, 1, 1 rake-like structure | 1, 1, 1, 1, 1 comb-like structure |
| A serrate lobe on the distal part of the basis of legs 1 and 2 | present | present | absent | absent | absent |
| Wing-like folds on prosome | absent | absent | absent | absent | present |
| Urosome overlapped by the prosome | no | no | no | no | yes |
| Host | P. homarus | P. versicolor | P. longipes longipes | I. novemdentatus | L. spinosa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-R.; Wakabayashi, K.; Pan, Y.-J. Choniomyzon taiwanensis n. sp. (Crustacea: Copepoda: Nicothoidae) Parasitic on the External Egg Mass of the Longlegged Spiny Lobster Panulirus longipes longipes (Crustacea: Decapoda: Palinuridae) from Taiwanese Waters. Animals 2021, 11, 2475. https://doi.org/10.3390/ani11082475
Cheng Y-R, Wakabayashi K, Pan Y-J. Choniomyzon taiwanensis n. sp. (Crustacea: Copepoda: Nicothoidae) Parasitic on the External Egg Mass of the Longlegged Spiny Lobster Panulirus longipes longipes (Crustacea: Decapoda: Palinuridae) from Taiwanese Waters. Animals. 2021; 11(8):2475. https://doi.org/10.3390/ani11082475
Chicago/Turabian StyleCheng, Yu-Rong, Kaori Wakabayashi, and Yen-Ju Pan. 2021. "Choniomyzon taiwanensis n. sp. (Crustacea: Copepoda: Nicothoidae) Parasitic on the External Egg Mass of the Longlegged Spiny Lobster Panulirus longipes longipes (Crustacea: Decapoda: Palinuridae) from Taiwanese Waters" Animals 11, no. 8: 2475. https://doi.org/10.3390/ani11082475
APA StyleCheng, Y.-R., Wakabayashi, K., & Pan, Y.-J. (2021). Choniomyzon taiwanensis n. sp. (Crustacea: Copepoda: Nicothoidae) Parasitic on the External Egg Mass of the Longlegged Spiny Lobster Panulirus longipes longipes (Crustacea: Decapoda: Palinuridae) from Taiwanese Waters. Animals, 11(8), 2475. https://doi.org/10.3390/ani11082475






