Hybrid Genotype of Anisakis simplex (s.s.) and A. pegreffii Identified in Third- and Fourth-Stage Larvae from Sympatric and Allopatric Spanish Marine Waters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Samples, Larvae Collection, and Morphological Identification
2.2. In Vitro Culture of Anisakis Larvae
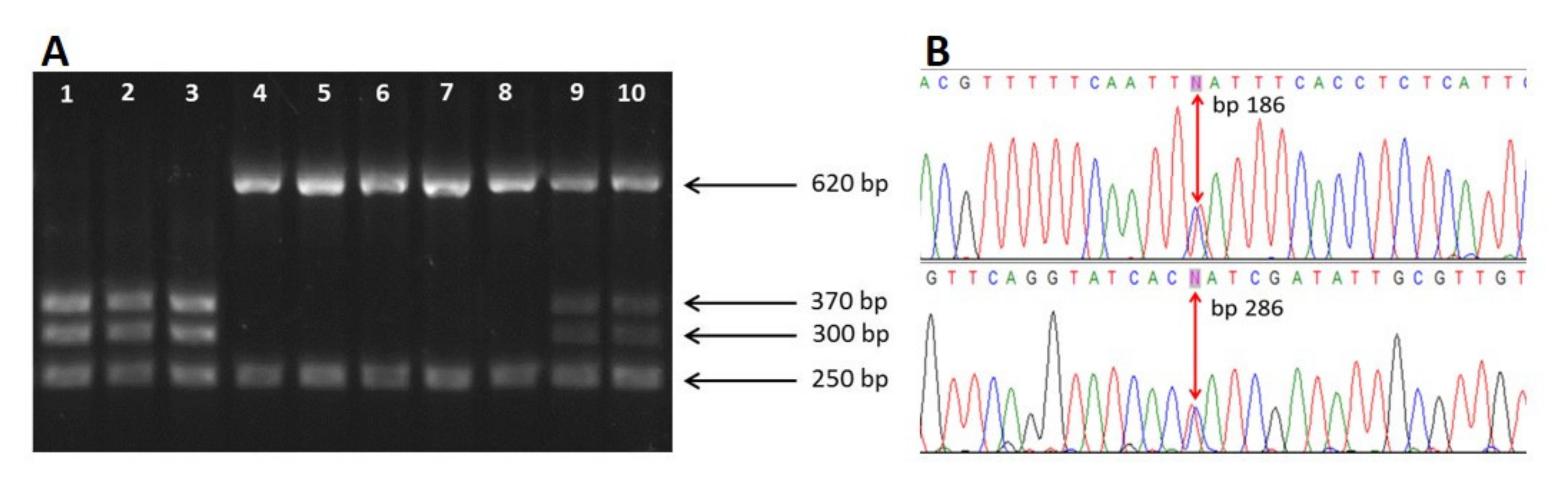
2.3. Molecular Identification
2.4. Statistical Analysis
3. Results
3.1. Morphological Identification and In Vitro Culture of A. simplex (s.l.)
3.2. Molecular Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Levsen, A.; Svanevik, C.S.; Cipriani, P.; Mattiucci, S.; Gay, M.; Hastie, L.C.; Bušelić, I.; Mladineo, I.; Karl, H.; Ostermeyer, U.; et al. A survey of zoonotic nematodes of commercial key fish species from major European fishing grounds—Introducing the FP7 PARASITE exposure assessment study. Fish. Res. 2018, 202, 4–21. [Google Scholar] [CrossRef]
- Mattiucci, S.; Cipriani, P.; Levsen, A.; Paoletti, M.; Nascetti, G. Molecular epidemiology of Anisakis and Anisakiasis: An ecological and evolutionary road map. Adv. Parasitol. 2018, 99, 93–263. [Google Scholar] [PubMed]
- Smaldone, G.; Abollo, E.; Marrone, R.; Bernardi, C.E.M.; Chirollo, C.; Anastasio, A.; Del Hierro, S.P. Risk-based scoring and genetic identification for anisakids in frozen fish products from Atlantic FAO areas. BMC Vet. Res. 2020, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audícana, M.T.; Ansotegui, I.J.; Fernández de Corres, L.; Kennedy, M.W. Anisakis simplex: Dangerous dead and alive? Trends Parasitol. 2002, 18, 20–25. [Google Scholar] [CrossRef]
- Mattiucci, S.; D’Amelio, S. Helminth Infections and Their Impact on Global Public Health; Bruschi, F., Ed.; Springer: Wein, Austria, 2014; pp. 325–365. [Google Scholar]
- Smaldone, G.; Ambrosio, R.L.; Marrone, R.; Ceruso, M.; Anastasio, A. Anisakis spp. Larvae in deboned, in-oil fillets made of anchovies (engraulis encrasicolus) and sardines (sardina pilchardus) sold in eu retailers. Animals 2020, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, G.; Marrone, R.; Palma, G.; Sarnelli, P.; Anastasio, A. Preliminary study on the inactivation of anisakid larvae in baccalà prepared according to traditional methods. Ital. J. Food Saf. 2017, 6, 195–198. [Google Scholar] [CrossRef]
- Mattiucci, S.; Bello, E.; Paoletti, M.; Webb, S.C.; Timi, J.T.; Levsen, A.; Cipriani, P.; Nascetti, G. Novel polymorphic microsatellite loci in Anisakis pegreffii and A. simplex (s.s.) (Nematoda: Anisakidae): Implications for species recognition and population genetic analysis. Parasitology 2019, 146, 1387–1403. [Google Scholar] [CrossRef] [Green Version]
- Roca-Geronès, X.; Segovia, M.; Godínez-González, C.; Fisa, R.; Montoliu, I. Anisakis and Hysterothylacium species in Mediterranean and North-East Atlantic fishes commonly consumed in Spain: Epidemiological, molecular and morphometric discriminant analysis. Int. J. Food Microbiol. 2020, 325, 108642. [Google Scholar] [CrossRef]
- Umehara, A.; Kawakami, Y.; Matsui, T.; Araki, J.; Uchida, A. Molecular identification of Anisakis simplex sensu stricto and Anisakis pegreffii (Nematoda: Anisakidae) from fish and cetacean in Japanese waters. Parasitol. Int. 2006, 55, 267–271. [Google Scholar] [CrossRef]
- Abattouy, N.; Valero, A.; Lozano, J.; Barón, S.D.; Romero, C.; Martín-Sánchez, J. Population genetic analysis of Anisakis simplex s.l. and Anisakis pegreffii (Nematoda, Anisakidae) from parapatric areas and their contact zone. Parasite Epidemiol. Control 2016, 1, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Mattiucci, S.; Acerra, V.; Paoletti, M.; Cipriani, P.; Levsen, A.; Webb, S.C.; Canestrelli, D.; Nascetti, G. No more time to stay ‘single’ in the detection of Anisakis pegreffii, A. simplex (s.s.) and hybridization events between them: A multi-marker nuclear genotyping approach. Parasitology 2016, 143, 998–1011. [Google Scholar] [CrossRef] [Green Version]
- Mladineo, I.; Bušelić, I.; Hrabar, J.; Vrbatović, A.; Radonić, I. Population parameters and mito-nuclear mosaicism of Anisakis spp. in the Adriatic Sea. Mol. Biochem. Parasitol. 2017, 212, 46–54. [Google Scholar] [CrossRef]
- Berland, B. Nematodes from some norwegian marine fishes. Sarsia 1961, 2, 1–50. [Google Scholar] [CrossRef]
- Iglesias, L.; Valero, A.; Adroher, F.J. Some factors which influence the in vitro maintenance of Anisakis simplex (Nematoda). Folia Parasitol. 1997, 44, 297–301. [Google Scholar]
- Iglesias, L.; Valero, A.; Benítez, R.; Adroher, F.J. In vitro cultivation of anisakis simplex: Pepsin increases survival and moulting from fourth larval to adult stage. Parasitology 2001, 123, 285–291. [Google Scholar] [CrossRef] [Green Version]
- D’Amelio, S.; Mathiopoulos, K.; Santos, C.; Pugachev, O.; Webb, S.; Picanço, M.; Paggi, L. Genetic markers in ribosomal DNA for the identification of members of the genus Anisakis (Nematoda: Ascaridoidea) defined by polymerase-chain-reaction-based restriction fragment length polymorphism. Int. J. Parasitol. 2000, 30, 223–226. [Google Scholar] [CrossRef]
- Nadler, S.A.; D’Amelio, S.; Dailey, M.D.; Paggi, L.; Siu, S.; Sakanari, J.A. Molecular phylogenetics and diagnosis of Anisakis, Pseudoterranova, and Contracaecum from northern Pacific marine mammals. J. Parasitol. 2005, 91, 1413–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamsi, S.; Gasser, R.; Beveridge, I. Description and genetic characterisation of Hysterothylacium (Nematoda: Raphidascarididae) larvae parasitic in Australian marine fishes. Parasitol. Int. 2013, 62, 320–328. [Google Scholar] [CrossRef]
- Anderson, T.J.C. The dangers of using single locus markers in parasite epidemiology: Ascaris as a case study. Trends Parasitol. 2001, 17, 183–188. [Google Scholar] [CrossRef]
- Gómez-Mateos, M.; Merino-Espinosa, G.; Corpas-López, V.; Valero-López, A.; Martín-Sánchez, J. A multi-restriction fragment length polymorphism genotyping approach including the beta-tubulin gene as a new differential nuclear marker for the recognition of the cryptic species Anisakis simplex s.s. and Anisakis pegreffii and their hybridization event. Vet. Parasitol. 2020, 283, 109162. [Google Scholar] [CrossRef]
- Palomba, M.; Paoletti, M.; Webb, S.C.; Nascetti, G.; Mattiucci, S. A novel nuclear marker and development of an ARMS-PCR assay targeting the metallopeptidase 10 (nas 10) locus to identify the species of the Anisakis simplex (s. l.) complex (Nematoda, Anisakidae). Parasite 2020, 27, 39. [Google Scholar] [CrossRef] [PubMed]
- Llorens, C.; Arcos, S.C.; Robertson, L.; Ramos, R.; Futami, R.; Soriano, B.; Ciordia, S.; Careche, M.; González-Muñoz, M.; Jiménez-Ruiz, Y.; et al. Functional insights into the infective larval stage of Anisakis simplex s.s., Anisakis pegreffii and their hybrids based on gene expression patterns. BMC Genom. 2018, 19, 1–21. [Google Scholar] [CrossRef] [Green Version]
- D’amelio, S.; Lombardo, F.; Pizzarelli, A.; Bellini, I.; Cavallero, S. Advances in omic studies drive discoveries in the biology of anisakid nematodes. Genes 2020, 11, 801. [Google Scholar] [CrossRef]
- Romero, M.C.; Valero, A.; Navarro-Moll, M.C.; Martín-Sánchez, J. Experimental comparison of pathogenic potential of two sibling species Anisakis simplex s.s. and Anisakis pegreffii in Wistar rat. Trop. Med. Int. Health 2013, 18, 979–984. [Google Scholar] [CrossRef]
- Arcos, S.C.; Ciordia, S.; Roberston, L.; Zapico, I.; Jiménez-Ruiz, Y.; Gonzalez-Muñoz, M.; Moneo, I.; Carballeda-Sangiao, N.; Rodriguez-Mahillo, A.; Albar, J.P.; et al. Proteomic profiling and characterization of differential allergens in the nematodes Anisakis simplex sensu stricto and A. pegreffii. Proteomics 2014, 14, 1547–1568. [Google Scholar] [CrossRef]
- Detwiler, J.T.; Criscione, C.D. An infectious topic in reticulate evolution: Introgression and hybridization in animal parasites. Genes 2010, 1, 102–123. [Google Scholar] [CrossRef] [Green Version]
- Kong, Q.; Fan, L.; Zhang, J.; Akao, N.; Dong, K.; Lou, D.; Ding, J.; Tong, Q.; Zheng, B.; Chen, R.; et al. Molecular identification of Anisakis and Hysterothylacium larvae in marine fishes from the East China Sea and the Pacific coast of central Japan. Int. J. Food Microbiol. 2015, 199, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Farjallah, S.; Busi, M.; Mahjoub, M.O.; Slimane, B.B.; Paggi, L.; Said, K.; D’Amelio, S. Molecular characterization of larval anisakid nematodes from marine fishes off the Moroccan and Mauritanian coasts. Parasitol. Int. 2008, 57, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Molina-Fernández, D.; Malagón, D.; Gómez-Mateos, M.; Benítez, R.; Martín-Sánchez, J.; Adroher, F.J. Fishing area and fish size as risk factors of Anisakis infection in sardines (Sardina pilchardus) from Iberian waters, southwestern Europe. Int. J. Food Microbiol. 2015, 203, 27–34. [Google Scholar] [CrossRef]
- MacKenzie, K.; Campbell, N.; Mattiucci, S.; Ramos, P.; Pinto, A.L.; Abaunza, P. Parasites as biological tags for stock identification of Atlantic horse mackerel Trachurus trachurus L. Fish. Res. 2008, 89, 136–145. [Google Scholar] [CrossRef]
- Mattiucci, S.; Cimmaruta, R.; Cipriani, P.; Abaunza, P.; Bellisario, B.; Nascetti, G. Integrating Anisakis spp. parasites data and host genetic structure in the frame of a holistic approach for stock identification of selected Mediterranean Sea fish species. Parasitology 2015, 142, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, G.Z.; Onuk, E.E.; Bolukbas, C.S.; Yardimci, B.; Gurler, A.T.; Acici, M.; Umur, S. Molecular identification of Anisakis species (Nematoda: Anisakidae) from marine fishes collected in Turkish waters. Vet. Parasitol. 2014, 201, 82–94. [Google Scholar] [CrossRef]
- Costa, A.; Cammilleri, G.; Graci, S.; Buscemi, M.D.; Vazzana, M.; Principato, D.; Giangrosso, G.; Ferrantelli, V. Survey on the presence of A. simplex s.s. and A. pegreffii hybrid forms in Central-Western Mediterranean Sea. Parasitol. Int. 2016, 65, 696–701. [Google Scholar] [CrossRef]
- Cavallero, S.; Costa, A.; Caracappa, S.; Gambetta, B.; D’Amelio, S. Putative hybrids between two Anisakis cryptic species: Molecular genotyping using High Resolution Melting. Exp. Parasitol. 2014, 146, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bello, E.; Palomba, M.; Webb, S.C.; Paoletti, M.; Cipriani, P.; Nascetti, G.; Mattiucci, S. Investigating the genetic structure of the parasites Anisakis pegreffii and A. berlandi (Nematoda: Anisakidae) in a sympatric area of the southern Pacific Ocean waters using a multilocus genotyping approach: First evidence of their interspecific hybridiza. Infect. Genet. Evol. 2021, 92, 104887. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.O.; Jeon, C.H.; Nam, U.H.; Subramaniyam, S.; Yoo, S.I.; Park, J.H. Comparative transcriptome analyses of the third and fourth stage larvae of Anisakis simplex (Nematoda: Anisakidae). Mol. Biochem. Parasitol. 2018, 226, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Nam, U.H.; Kim, J.O.; Kim, J.H. De novo transcriptome sequencing and analysis of Anisakis pegreffii (Nematoda: Anisakidae) third-stage and fourth stage larvae. J. Nematol. 2020, 52, 1–16. [Google Scholar] [CrossRef] [Green Version]
| Developmental Stage | Area | Host Species | A.s. (s.s.) 1 | A.p.1 | H g 1 | H g 2 |
|---|---|---|---|---|---|---|
| L3 | West Mediterranean | T. trachurus | - | 28 | 3 | 1 |
| M. poutassou | - | 13 | - | - | ||
| North-East Atlantic | T. trachurus | 10 | 11 | 1 | 1 | |
| M. poutassou | 74 | 20 | 5 | 2 | ||
| L4 | North-East Atlantic | M. poutassou | 56 | 24 | 9 | 7 |
| Total | 140 | 96 | 18 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roca-Geronès, X.; Alcover, M.M.; Godínez-González, C.; Montoliu, I.; Fisa, R. Hybrid Genotype of Anisakis simplex (s.s.) and A. pegreffii Identified in Third- and Fourth-Stage Larvae from Sympatric and Allopatric Spanish Marine Waters. Animals 2021, 11, 2458. https://doi.org/10.3390/ani11082458
Roca-Geronès X, Alcover MM, Godínez-González C, Montoliu I, Fisa R. Hybrid Genotype of Anisakis simplex (s.s.) and A. pegreffii Identified in Third- and Fourth-Stage Larvae from Sympatric and Allopatric Spanish Marine Waters. Animals. 2021; 11(8):2458. https://doi.org/10.3390/ani11082458
Chicago/Turabian StyleRoca-Geronès, Xavier, M. Magdalena Alcover, Carla Godínez-González, Isabel Montoliu, and Roser Fisa. 2021. "Hybrid Genotype of Anisakis simplex (s.s.) and A. pegreffii Identified in Third- and Fourth-Stage Larvae from Sympatric and Allopatric Spanish Marine Waters" Animals 11, no. 8: 2458. https://doi.org/10.3390/ani11082458
APA StyleRoca-Geronès, X., Alcover, M. M., Godínez-González, C., Montoliu, I., & Fisa, R. (2021). Hybrid Genotype of Anisakis simplex (s.s.) and A. pegreffii Identified in Third- and Fourth-Stage Larvae from Sympatric and Allopatric Spanish Marine Waters. Animals, 11(8), 2458. https://doi.org/10.3390/ani11082458







