Bacterial Skin Infections in Livestock and Plant-Based Alternatives to Their Antibiotic Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Staphylococcus aureus in Livestock
3. Antibiotic Treatment of Bacterial Skin Infections in Livestock
Resistance of Staphylococcal Species to Antibiotics
4. Alternatives to Conventional Antibiotics Used to Treat Bacterial Skin Infections in Animals
Phytochemicals
Phytochemicals with Antimicrobial Effects
Alkaloids
Polyphenols
Tannins
Essential Oils
Terpenoids
Saponins
Organosulfur Compounds
5. Plants with Antibacterial and Wound Healing Effects Used in Livestock (In Vitro Studies)
6. Plants with Antibacterial and Wound Healing Effects Used in Livestock (In Vivo Studies)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Montagna, W.; Parakkal, P.F. The Structure and Function of Skin, 3rd ed.; Elsevier: London, UK, 1974; Volume 3, pp. 1–17. [Google Scholar]
- Roth, R.R.; James, W.D. Microbial ecology of the skin. Annu. Rev. Microbiol. 1988, 42, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Lemieux-Labonté, V.; Tromas, N.; Shapiro, B.J.; Lapointe, F.-J. Environment and host species shape the skin microbiome of captive neotropical bats. Peer J. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Zeynalova, S.; Asadov, K.; Guliyev, F.; Vatani, M.; Aliyev, V. Epizootology and molecular diagnosis of lumpy skin disease among livestock in Azerbaijan. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Cogen, A.L.; Yamasaki, K.; Sanchez, K.M.; Dorschner, R.A.; Lai, Y.; MacLeod, D.T.; Torpey, J.W.; Otto, M.; Nizet, V.; Kim, J.E.; et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J. Investig. Dermatol. 2010, 130, 192–200. [Google Scholar] [CrossRef]
- Belden, L.K.; Harris, R.N. Infectious diseases in wildlife: The community ecology context. Front. Ecol. Environ. 2007, 5, 533–539. [Google Scholar] [CrossRef]
- Hoffmann, A.R.; Patterson, A.P.; Diesel, A.; Lawhon, S.D.; Ly, H.J.; Stephenson, C.E.; Mansell, J.; Steiner, J.M.; Dowd, S.E.; Olivry, T.; et al. The skin microbiome in healthy and allergic dogs. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Ross, A.A. The Mammalian Skin Microbiome. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 23 August 2018. [Google Scholar]
- Grice, E.A.; Kong, H.H.; Renaud, G.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Wolfsberg, T.G.; Turner, M.L.; Segre, J.A. A diversity profile of the human skin microbiota. Genome Res. 2008, 18, 1043–1050. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Capone, K.A.; Dowd, S.E.; Stamatas, G.N.; Nikolovski, J. Diversity of the human skin microbiome early in life. J. Investig. Dermatol. 2011, 131, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Nooruddin, M. Economic impact of leather defects in Bangladesh. J. Train. Dev. 1993, 6, 27–38. [Google Scholar]
- Foster, A.P. Staphylococcal skin disease in livestock. Vet. Dermatol. 2012, 23, 342–351. [Google Scholar] [CrossRef]
- Abrahamian, F.M.; Goldstein, E.J. Microbiology of animal bite wound infections. Clin. Microbiol. Rev. 2011, 24, 231–246. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microb. Biofilms 2015, 223–247. [Google Scholar] [CrossRef]
- Meyle, E.; Stroh, P.; Günther, F.; Hoppy-Tichy, T.; Wagner, C.; Hänsch, G.M. Destruction of bacterial biofilms by polymorphonuclear neutrophils: Relative contribution of phagocytosis, DNA release, and degranulation. Int. J. Artif. Organs 2010, 33, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Haaber, J.; Penadés, J.R.; Ingmer, H. Transfer of antibiotic resistance in Staphylococcus aureus. Trends Microbiol. 2017, 25, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, R.; Ren, S.; Szoboszlay, M.; Moe, L.A. Practical survey on antibiotic-resistant bacterial communities in livestock manure and manure-amended soil. J. Environ. Sci. Health 2016, 51, 14–23. [Google Scholar] [CrossRef]
- Voss, A.; Loeffen, F.; Bakker, J.; Klaassen, C.; Wulf, M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg. Infect. Dis. 2005, 11, 1965–1966. [Google Scholar] [CrossRef]
- Van Cleef, B.; van Benthem, B.; Verkade, E.J.; van Rijen, M.; Kluytmans-van den Bergh, M.; Graveland, H.; Bosch, T.; Verstappen, K.M.; Wagenaar, J.A.; Bos, M.E.; et al. Livestock-associated MRSA in household members of pig farmers: Transmission and dynamics of carriage, a prospective cohort study. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Verkade, E.; Kluytmans-van den Bergh, M.; van Benthem, B.; van Cleef, B.; van Rijen, M.; Bosch, T.; Schouls, L.; Kluytmans, J. Transmission of methicillin-resistant Staphylococcus aureus CC398 from livestock veterinarians to their household members. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Cuny, C.; Wieler, L.H.; Witte, W. Livestock-associated MRSA: The impact on humans. Antibiotics 2015, 4, 521–543. [Google Scholar] [CrossRef]
- Garcia-Alvarez, L.; Dawson, S.; Cookson, B.; Hawkey, P. Working across the veterinary and human health sectors. J. Antimicrob. Chemother. 2012, 67, 37–49. [Google Scholar] [CrossRef]
- Paterson, G.K.; Harrison, E.M.; Holmes, M.A. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2014, 22, 42–47. [Google Scholar] [CrossRef]
- Dhup, V.; Kearns, A.M.; Pichon, B.; Foster, H.A. First report of identification of livestock-associated MRSA ST9 in retail meat in England. Epidemiol. Infect. 2015, 143, 2989–2992. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Nunez-Garcia, J.; Kearns, A.M.; Doumith, M.; Butaye, P.R.; Argudín, M.A.; Lahuerta-Marin, A.; Pichon, B.; AbuOun, M.; Rogers, J.; et al. Livestock-associated methicillin resistant Staphylococcus aureus (LA-MRSA) clonal complex (CC) 398 isolated from UK animals belong to European lineages. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Wellington, E.M.H.; Boxall, A.B.A.; Cross, P.; Feil, E.J.; Gaze, W.H.; Hawkey, P.M.; Johnson-Rollings, A.S.; Jones, D.L.; Lee, N.M.; Otten, W.; et al. The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect. Dis. 2013, 13, 155–165. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1988, 339, 520–532. [Google Scholar] [CrossRef]
- Graveland, H.; Duim, B.; van Duijkeren, E.; Heederik, D.; Wagenaar, J.A. Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. Int. J. Med Microbiol. 2011, 301, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Daaloul-Jedidi, M.; Soudani, A.; Messadi, L. Nasal and rectal carriage of coagulase positive Staphylococcus in healthy goats. J. New Sci. 2016, 33, 1910–1913. [Google Scholar]
- Van Cleef, B.A.; Monnet, D.L.; Voss, A.; Krziwanek, K.; Allerberger, F.; Struelens, M.; Zemlickova, H.; Skov, R.L.; Vuopio-Varkila, J.; Cuny, C.; et al. Livestock-associated methicillin-resistant Staphylococcus aureus in humans, Europe. Emerg. Infect. Dis. 2011, 17. [Google Scholar] [CrossRef]
- Grace, D.; Fetsch, A. Staphylococcus aureus—A foodborne pathogen: Epidemiology, detection, characterization, prevention, and control: An overview. In Staphylococcus Aureus; Academic Press: Cambridge, MA, USA, 2018; pp. 3–8. [Google Scholar] [CrossRef]
- Cuny, C.; Friedrich, A.; Kozytska, S.; Layer, F.; Nübel, U.; Ohlsen, K.; Strommenger, B.; Walther, B.; Wieler, L.; Witte, W. Emergence of methicillin-resistant Staphylococcus aureus (MRSA) in different animal species. Int. J. Med Microbiol. 2010, 300, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.H.; Fox, L.K.; Middleton, J.R. Outbreak of mastitis caused by one strain of Staphylococcus aureus in a closed dairy herd. J. Am. Vet. Med Assoc. 1998, 212, 553–556. [Google Scholar] [PubMed]
- Peton, V.; Le Loir, Y. Staphylococcus aureus in veterinary medicine. Infect. Genet. Evol. 2014, 21, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaeghen, W.; Hermans, K.; Haesebrouck, F.; Butaye, P. Methicillin-resistant Staphylococcus aureus (MRSA) in food production animals. Epidemiol. Infect. 2010, 138, 606–625. [Google Scholar] [CrossRef]
- Acton, D.; Plat-Sinnige, M.J.T.; van Wamel, W.; de Groot, N.; van Belkum, A. Intestinal carriage of Staphylococcus aureus: How does its frequency compare with that of nasal carriage and what is its clinical impact? Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 115–127. [Google Scholar] [CrossRef]
- Erskine, R.; Cullor, J.; Schaellibaum, M.; Yancey, B.; Zecconi, A. Bovine mastitis pathogens and trends in resistance to antibacterial drugs. In National Mastitis Council Research Committee Report, Proceedings of the Annual Meeting, Charlotte, NC, USA, 1 January 2004; NMC Research Committee: New Prague, MN, USA, 2004. [Google Scholar]
- Todhunter, D.A.; Smith, K.L.; Hogan, J.S. Environmental streptococcal intramammary infections of the bovine mammary gland. J. Dairy Sci. 1995, 78, 2366–2374. [Google Scholar] [CrossRef]
- Calvinho, L.; Tirante, L. Prevalencia de Microorganismos Patógenos de Mastitis Bovina y Evolución del Estado de Salud de la Glándula Mamaria en Argentina en los Ultimos 25 Años. FAVE Sección Cienc. Vet. 2005, 4. [Google Scholar] [CrossRef][Green Version]
- Davies, P.L.; Leigh, J.A.; Bradley, A.J.; Archer, S.C.; Emes, R.D.; Green, M.J. Molecular Epidemiology of Streptococcus uberis clinical mastitis in dairy herds: Strain heterogeneity and transmission. J. Clin. Microbiol. 2016, 54, 68–74. [Google Scholar] [CrossRef]
- Ginn, P.; Mansell, L.; Rakich, P. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 5th ed.; Elsevier: Oxford, UK, 2007; pp. 553–781. [Google Scholar]
- Cooper, J.E. Veterinary Aspects of Captive Birds of Prey; Standfast Press: London, UK, 1973; pp. 1–256. [Google Scholar]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef]
- Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2020. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2018-trends-2010-2018-tenth-esvac-report_en.pdf (accessed on 3 March 2021).
- Johnson, A.P. Methicillin-resistant Staphylococcus aureus: The European landscape. J. Antimicrob. Chemother. 2011, 66, 43–48. [Google Scholar] [CrossRef]
- Michalova, E.; Schlegelova, J. Tetracyclines in veterinary medicine and bacterial resistance to them. Vet. Med. 2004, 49, 79. [Google Scholar] [CrossRef]
- Page, S.; Gautier, P. Use of antimicrobial agents in livestock. Rev. Sci. Tech. OIE 2012, 31, 145. [Google Scholar] [CrossRef]
- Apley, M.D.; Coetzee, J.F. Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 495–518. [Google Scholar]
- Rayner, C.; Munckhof, W. Antibiotics currently used in the treatment of infections caused by Staphylococcus aureus. Intern. Med. J. 2005, 35, 3–16. [Google Scholar] [CrossRef]
- Clark, D. The changing nature of farm systems research. In Proceedings of the New Zealand Society of Animal Production; New Zealand Society of Animal Production: Hamilton, New Zealand, 2013. [Google Scholar]
- World Health Statistics 2014. Available online: https://apps.who.int/iris/bitstream/handle/10665/112738/9789240692671_eng.pdf;jsessionid=B3BF04A1EE2E2AA0E05283475C89FE45?sequence=1 (accessed on 24 March 2021).
- Biswas, S.; Raoult, D.; Rolain, J.M. A bioinformatic approach to understanding antibiotic resistance in intracellular bacteria through whole genome analysis. Int. J. Antimicrob. Agents 2008, 32, 207–220. [Google Scholar] [CrossRef]
- D’Costa, V.M.; McGrann, K.M.; Hughes, D.W.; Wright, G.D. Sampling the antibiotic resistome. Science 2006, 311, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Rammelkamp, C.H.; Maxon, T. Resistance of Staphylococcus aureus to the action of penicillin. Exp. Biol. Med. 1942, 51, 386–389. [Google Scholar] [CrossRef]
- Andam, C.P.; Fournier, G.P.; Gogarten, J.P. Multilevel populations and the evolution of antibiotic resistance through horizontal gene transfer. FEMS Microbiol. Rev. 2011, 35, 756–767. [Google Scholar] [CrossRef] [PubMed]
- van Hoek, A.H.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Henk, J.M. Acquired antibiotic resistance genes: An overview. Front. Microbiol. 2011, 203. [Google Scholar] [CrossRef]
- Lindsay, J.A. Genomic variation and evolution of Staphylococcus aureus. Int. J. Med Microbiol. 2010, 300, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-resistant Staphylococcus aureus (MRSA): Antibiotic-resistance and the biofilm phenotype. MedChemComm 2019, 10, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Scherr, T.D.; Heim, C.E.; Morrison, J.M.; Kielian, T. Hiding in plain sight: Interplay between staphylococcal biofilms and host immunity. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955–1958. [Google Scholar] [CrossRef]
- De la Fuente-Núnez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E.W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Dortet, L.; Anguel, N.; Fortineau, N.; Richard, C.; Nordmann, P. In vivo acquired daptomycin resistance during treatment of methicillin-resistant Staphylococcus aureus endocarditis. Int. J. Infect. Dis. 2013, 17, 1076–1077. [Google Scholar] [CrossRef]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar]
- Chambers, H.F.; DeLeo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef]
- Appelbaum, P.C. Reduced glycopeptide susceptibility in methicillin-resistant Staphyloccocus aureus (MRSA). Int. J. Antimicrob. Agents 2007, 30, 398–408. [Google Scholar] [CrossRef]
- Francia, M.V.; Clewell, D.B. Transfer origins in the conjugative Enterococcus faecalis plasmids pAD1 and pAM373: Identification of the pAD1 nic site, a specific relaxase and a possible TraG-like protein. Mol. Microbiol. 2002, 45, 375–395. [Google Scholar] [CrossRef]
- Tenover, F.C.; Biddle, J.W.; Lancaster, M.V. Increasing resistance to vancomycin and other glycopeptides in Staphylococcus aureus. Emerg. Infect. Dis. 2001, 7, 327–332. [Google Scholar] [CrossRef]
- De Oliveira, L.P.; Barros, L.S.S.; Silva, V.C.; Cirquiera, M.G. Study of Staphylococcus aureus in raw and pasteurized milk consumed in the Reconcavo area of the State of Bahia, Brazil. J. Food Process. Technol. 2011. [Google Scholar] [CrossRef]
- Nöremark, M.; Frössling, J.; Lewerin, S.S. Application of routines that contribute to on-farm biosecurity as reported by Swedish livestock farmers. Transbound. Emerg. Dis. 2010, 57, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Bomba, A.; Jonecová, Z.; Koscova, J.; Nemcova, R. The improvement of probiotics efficacy by synergistically acting components of natural origin: A review. Biologia 2006, 61, 729–734. [Google Scholar] [CrossRef]
- Lemke, S.L.; Mayura, K.; Reeves, W.R.; Wang, N.; Fickey, C.; Phillips, T.D. Investigation of organophilic montmorillonite clay inclusion in zearalenonecontaminated diets using the mouse uterine weight bioassay. J. Toxicol. Environ. Health 2001, 62, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Toghyani, M.; Toghyani, M.; Gheisari, A.; Ghalamkari, G.; Eghbalsaied, S. Evaluation of cinnamon and garlic as antibiotic growth promoter substitutions on performance, immuneresponses, serum biochemical and haematological parameters in broiler chicks. Livest. Sci. 2011, 138, 167–173. [Google Scholar] [CrossRef]
- Bretaudeau, L.; Tremblais, K.; Aubrit, F.; Meichenin, M.; Arnaud, I. Good manufacturing practice (GMP) compliance for phage therapy medicinal products. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Ngassam-Tchamba, C.; Duprez, J.N.; Fergestad, M.; De Visscher, A.; L’Abee-Lund, T.; De Vliegher, S.; Wasteson, Y.; Touzain, F.; Blanchard, Y.; Lavigne, R.; et al. In Vitro and in Vivo assessment of phage therapy against Staphylococcus aureus causing bovine mastitis. J. Glob. Antimicrob. Resist. 2020, 22, 762–770. [Google Scholar] [CrossRef]
- Milho, C.; Silva, M.D.; Sillankorva, S.; Harper, D.R. Biofilm applications of bacteriophages. In Bacteriophages; Harper, D.R., Abedon, S.T., Burrowes, B.H., McConville, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–3. [Google Scholar]
- Islam, A.; Takagi, M.; Fukuyama, K.; Komatsu, R.; Albarracin, L.; Nochi, T.; Suda, Y.; Ikeda-Ohtsubo, W.; Rutten, V.; Eden, W.; et al. Transcriptome analysis of the inflammatory responses of bovine mammary epithelial cells: Exploring immunomodulatory target genes for bovine mastitis. Pathogens 2020, 9, 200. [Google Scholar] [CrossRef]
- Betts, J.W.; Hornsey, M.; La Ragione, R.M. 2018. Novel antibacterials: Alternatives to traditional antibiotics. Adv. Microb. Physiol. 2018, 73, 123–169. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Hirt, H.M.; M´Pia, B. Natural Medicine in the Tropics 1: Foundation Text: Tropical Plants as a Source of Health Care: Production Medicines and Cosmetics, 3rd ed.; Anamed: Winnenden, Germany, 2008. [Google Scholar]
- Ceasar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Barbosa, F.; Pinto, E.; Kijjoa, A.; Pinto, M.M.; Sousa, E. Targeting antimicrobial drug resistance with marine natural products. Int. J. Antimicrob. Agents 2020, 56. [Google Scholar] [CrossRef] [PubMed]
- Solecki, R.S. Shanidar IV, a Neanderthal flower burial in northern Iraq. Science 1975, 190, 880–881. [Google Scholar] [CrossRef]
- Mahady, G.B. Medicinal plants for the prevention and treatment of bacterial infections. Curr. Pharm. Des. 2005, 11, 2405–2427. [Google Scholar] [CrossRef]
- Watzl, B.; Leitzmann, C. Bioaktive Substanzen in Lebensmitteln; Georg Thieme Verlag: New York, NY, USA, 2005; pp. 1–254. [Google Scholar]
- Kamboh, A.; Arain, M.A.; Mughal, M.J.; Zaman, A.; Arain, Z.M.; Soomro, A.H. Flavonoids: Health promoting phytochemicals for animal production-a review. J. Anim. Health Prod. 2015, 3, 6–13. [Google Scholar] [CrossRef]
- Maver, T.; Kurečič, M.; Smrke, D.M.; Kleinschek, K.S.; Maver, U. Herbal Medicine; IntechOpen: London, UK, 2018; pp. 121–150. [Google Scholar] [CrossRef]
- Assob, J.C.; Kamga, H.L.; Nsagha, D.S.; Njunda, A.L.; Nde, P.F.; Asongalem, E.A.; Njouendou, A.J.; Sandjon, B.; Penlap, V.B. Antimicrobial and toxicological activities of five medicinal plant species from Cameroon Traditional Medicine. BMC Complementary Altern. Med. 2011, 11, 1–11. [Google Scholar] [CrossRef]
- Schmid, K.; Ivemeyer, S.; Vogl, C.; Klarer, F.; Meier, B.; Hamburger, M.; Walkenhorst, M. Traditional use of herbal remedies in livestock by farmers in 3 Swiss cantons (Aargau, Zurich, Schaffhausen). Complementary Med. Res. 2012, 19, 125–136. [Google Scholar] [CrossRef]
- Poutaraud, A.; Michelot-Antalik, A.; Plantureux, S. Grasslands: A source of secondary metabolites for livestock health. J. Agric. Food Chem. 2017, 65, 6535–6553. [Google Scholar] [CrossRef] [PubMed]
- Hahn, N.I. Are phytoestrogens nature’s cure for what ails us? A look at the research. J. Acad. Nutr. Diet. 1998, 98, 974–976. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yang, M.; Or, K.H.; Yim, W.S.; Zuo, Z. Tissue accumulations of toxic Aconitum alkaloids after short-term and long-term oral administrations of clinically used radix Aconiti lateralis preparations in rats. Toxins 2019, 11, 353. [Google Scholar] [CrossRef]
- Bush, L.; Fannin, F. Alkaloids. In Tall Fescue for the Twenty-first Century, 3rd ed.; Fribourg, H.A., Hannaway, D.B., West, C.P., Eds.; Agronomy Monographs: Madison, WI, USA, 2009; Volume 53, pp. 229–249. [Google Scholar]
- Yang, L.; Stöckigt, J. Trends for diverse production strategies of plant medicinal alkaloids. Nat. Prod. Rep. 2010, 27, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.-X.; Huang, J.-L.; Yang, X.-Y.; Liu, J.-H.; Cao, H.-L.; Xiang, F.; Cheng, P.; Zeng, J.-G. Anticancer and reversing multidrug resistance activities of natural isoquinoline alkaloids and their structure-activity relationship. Curr. Med. Chem. 2018, 25, 5088–5114. [Google Scholar] [CrossRef] [PubMed]
- Ünsal, Ç.; Özbek, B.; Sariyar, G.; Mat, A. Antimicrobial activity of four annual Papaver species growing in Turkey. Pharm. Biol. 2009, 47, 4–6. [Google Scholar] [CrossRef]
- Kostic, D.A.; Mitic, S.S.; Mitić, M.; Zarubica, A.R.; Velickovic, J.M.; Dordevic, A.S.; Randelovic, S.S. Phenolic contents, antioxidant and antimicrobial activity of Papaver rhoeas L. extracts from Southeast Serbia. J. Med. Plants Res. 2010, 4, 1727–1732. [Google Scholar] [CrossRef]
- Zuo, G.Y.; Meng, F.Y.; Hao, X.Y.; Zhang, Y.L.; Wang, G.C.; Xu, G.L. Antibacterial alkaloids from Chelidonium majus Linn (Papaveraceae) against clinical isolates of methicillin-resistant Staphylococcus aureus. J. Pharm. Pharm. Sci. 2008, 11, 90–94. [Google Scholar] [CrossRef]
- Bhattacharjee, I.; Chatterjee, S.K.; Chandra, G. Isolation and identification of antibacterial components in seed extracts of Argemone mexicana L. (Papaveraceae). Asian Pac. J. Trop. Med. 2010, 3, 547–551. [Google Scholar] [CrossRef]
- Kim, M.G.; Lee, S.E.; Yang, J.Y.; Lee, H.S. Antimicrobial potentials of active component Isolated from Citrullus colocynthis fruits and structure-activity relationships of its analogues against foodborne bacteria. J. Sci. Food Agric. 2014, 94, 2529–2533. [Google Scholar] [CrossRef]
- Pan, X.; Bligh, S.W.; Smith, E. Quinolone alkaloids from fructus Euodiae show activity against methicillin-resistant Staphylococcus aureus. Phytother. Res. 2014, 28, 305–307. [Google Scholar] [CrossRef]
- Houdkova, M.; Rondevaldova, J.; Doskocil, I.; Kokoska, L. Evaluation of antibacterial potential and toxicity of plant volatile compounds using new broth microdilution volatilization method and modified MTT assay. Fitoterapia 2017, 118, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Hamoud, R.; Reichling, J.; Wink, M. Synergistic antibacterial activity of the combination of the alkaloid Aanguinarine with EDTA and the antibiotic streptomycin against multidrug resistant bacteria. J. Pharm. Pharmacol. 2015, 67, 264–273. [Google Scholar] [CrossRef]
- Tan, K.K.; Khoo, T.J.; Rajagopal, M.; Wiart, C. Antibacterial alkaloids from Artabotrys crassifolius Hook.f. & Thomson. Nat. Prod. Res. 2015, 29, 2346–2349. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.; Xu, M.; Dong, Q.; Zhang, Y.; Li, Y.; Ye, G.; Zhao, L. In vitro and In vivo bactericidal activity of Tinospora sagittate (Oliv.) Gagnep. var. craveniana (S.Y.Hu) Lo and its main effective component, palmatine, against porcine Helicobacter Pylori. BMC Complementary Altern. Med. 2016, 16, 331. [Google Scholar] [CrossRef]
- Azimi, G.; Hakakian, A.; Ghanadian, M.; Joumaa, A.; Alamian, S. Bioassay-directed isolation of quaternary benzylisoquinolines from Berberis integerrima with bactericidal activity against Brucella abortus. Res. Pharm. Sci. 2018, 13. [Google Scholar] [CrossRef]
- Yu, J.; Yin, T.P.; Wang, J.P.; Mei, R.F.; Cai, L.; Ding, Z.T. A new C20-diterpenoid alkaloid from the lateral roots of Aconitum carmichaeli. Nat. Prod. Res. 2017, 31, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement; CLSI: Wayne, PA, USA, 2015; pp. 1–240. [Google Scholar]
- Duffy, C.F.; Power, R.F. Antioxidant and antimicrobial properties of some Chinese plant extracts. Int. J. Antimicrob. Agents 2001, 17, 527–529. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, K.; Nishiyama, Y.; Ichimaru, M.; Moriyasu, M.; Kim, H.S.; Wataya, Y.; Yamori, T.; Takashi, T.; Lee, D.U. Structure-activity relationships of quaternary protoberberine alkaloids having an antimalarial activity. Eur. J. Med. Chem. 1999, 34, 1077–1083. [Google Scholar] [CrossRef]
- Tong, N.; Zhang, J.; Chen, Y.; Li, Z.; Luo, Y.; Zuo, H.; Zhao, X. Berberine sensitizes mutliple human cancer cells to the anticancer effects of doxorubicin in vitro. Oncol. Lett. 2012, 3, 1263–1267. [Google Scholar] [CrossRef]
- López, T.A.; Bianchini, M.L. Biochemistry ofhemlock (Conium maculatum L.) alkaloids and their acute and chronic toxicity in livestock. A review. Toxicon 1999, 37, 841–865. [Google Scholar] [CrossRef]
- Green, B.T.; Lee, S.T.; Gardner, D.R.; Welch, K.D.; Cook, D. Bioactive alkaloids from plants poisonous to livestock in North America. Isr. J. Chem. 2019, 59, 351–359. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Gupta, S. Extraction, characterization, stability and biological activity of flavonoids isolated from chamomile flowers. Mol. Cell. Pharmacol. 2009, 1, 138. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Saavedra, M.J.; Borges, A.; Dias, C.; Aires, A.; Bennett, R.N.; Rosa, E.S.; Simões, M. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med. Chem. 2010, 6, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Panichayupakaranant, P. Anti-Propionibacterium acnes assay-guided purification of Brazilin and preparation of Brazilin rich extract from Caesalpinia sappan heartwood. Pharm. Biol. 2014, 52, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Panichayupakaranant, P. Antioxidant, antibacterial, and antiinflammatory activities of standardized Brazilin-rich Caesalpinia sappan extract. Pharm. Biol. 2015, 53, 1339–1343. [Google Scholar] [CrossRef]
- Dey, D.; Ray, R.; Hazra, B. Antimicrobial activity of pomegranate fruit constituents against drug-resistant Mycobacterium tuberculosis and Beta-lactamase producing Klebsiella pneumoniae. Pharm. Biol. 2015, 53, 1474–1480. [Google Scholar] [CrossRef]
- Shahzad, M.; Millhouse, E.; Culshaw, S.; Edwards, C.A.; Ramage, G.; Combet, E. Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2015, 6, 719–729. [Google Scholar] [CrossRef]
- Yuan, M.; Shi, D.Z.; Wang, T.Y.; Zheng, S.Q.; Liu, L.J.; Sun, Z.X.; Wang, R.F.; Ding, Y. Transformation of trollioside and isoquercetin by human intestinal flora in vitro. Chin. J. Nat. Med. 2016, 14, 220–226. [Google Scholar] [CrossRef]
- De Freitas, V.A.; Glories, Y.; Monique, A. Developmental changes of procyanidins in grapes of red Vitis vinifera varieties and their composition in respective wines. Am. J. Enol. Vitic. 2000, 51, 397–403. [Google Scholar]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Dorman, H.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Babu, K.S.; Babu, T.H.; Srinivas, P.V.; Sastry, B.S.; Kishore, K.H.; Murty, U.S.N.; Rao, J.M. Synthesis and in vitro study of novel 7-O-acyl derivatives of Oroxylin A as antibacterial agents. Bioorganic Med. Chem. Lett. 2005, 15, 3953–3956. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant natural products targeting bacterial virulence factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef] [PubMed]
- Manner, S.; Skogman, M.; Goeres, D.; Vuorela, P.; Fallarero, A. Systematic exploration of natural and synthetic flavonoids for the inhibition of Staphylococcus aureus biofilms. Int. J. Mol. Sci. 2013, 14, 19434–19451. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Fialová, S.; Hupková, H.; Grančai, D. Rosmarinic acid interaction with planktonic and biofilm Staphylococcus aureus. Nat. Prod. Commun. 2013, 8. [Google Scholar] [CrossRef]
- Wallock-Richards, D.J.; Marles-Wright, J.; Clarke, D.J.; Maitra, A.; Dodds, M.; Hanley, B.; Campopiano, D.J. Molecular basis of Streptococcus mutans sortase A inhibition by the flavonoid natural product trans-chalcone. Chem. Commun. 2015, 51, 10483–10485. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell–cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef]
- Ding, T.; Gu, L.; Liu, X. Influence of steam pressure on chemical changes of heat-treated mongolian pine wood. BioResources 2011, 6, 1880–1889. [Google Scholar]
- Lin, R.-D.; Chin, Y.-P.; Hou, W.C.; Lee, M.-H. The effects of antibiotics combined with natural polyphenols against clinical methicillin-resistant Staphylococcus aureus (MRSA). Planta Med. 2008, 74, 840–846. [Google Scholar] [CrossRef]
- Hu, Z.-Q.; Zhao, W.H.; Asano, N.; Yoda, Y.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 558–560. [Google Scholar] [CrossRef]
- Fan, P.; Lou, H. Effects of polyphenols from grape seeds on oxidative damage to cellular DNA. Mol. Cell. Biochem. 2004, 267, 67–74. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Touriño, S.; Torres, J.L. Fractions from grape and pine. Chem. Res. Toxicol. 2007, 20, 1543–1548. [Google Scholar] [CrossRef]
- Fu, L.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Kang, N.J.; Shin, S.H.; Lee, H.J.; Lee, K.W. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol. Ther. 2011, 130, 310–324. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini-Rev. Med. Chem. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- McLeod, M. Plant tannins-their role in forage quality. Nutr. Abstr. Rev. 1974, 44, 803–815. [Google Scholar]
- Smith, A.H.; Mackie, R.I. Effect of condensed tannins on bacterial diversity and metabolic activity in the rat gastrointestinal tract. Appl. Environ. Microbiol. 2004, 70, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T.; Lu, Z.; Chou, M. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem. Toxicol. 1998, 36, 1053–1060. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Widsten, P.; Cruz, C.D.; Fletcher, G.C.; Pajak, M.A.; McGhie, T.K. Tannins and extracts of fruit byproducts: Antibacterial activity against foodborne bacteria and antioxidant capacity. J. Agric. Food Chem. 2014, 62, 11146–11156. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial activity of punicalagin against Staphylococcus aureus and its effect on biofilm formation. Foodborne Pathog. Dis. 2017, 14, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Salih, E.Y.; Julkunen-Tiitto, R.; Lampi, A.M.; Kanninen, M.; Luukkanen, O.; Sipi, M.; Lehtonen, M.; Vuorela, H.; Fyhrquist, P. Terminalia laxiflora and Terminalia brownii contain a broad spectrum of antimycobacterial compounds including ellagitannins, ellagic acid derivatives, triterpenes, fatty acids and fatty alcohols. J. Ethnopharmacol. 2018, 227, 82–96. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Glibota, N.; Frías, M.P.; Altarejos, J.; Gálvez, A.; Ortega-Morente, E.; Salido, S. Antimicrobial and antibiofilm activities of procyanidins extracted from laurel wood against a selection of foodborne microorganisms. Int. J. Food Sci. Technol. 2017, 52, 679–686. [Google Scholar] [CrossRef]
- Chan, C.L.; Gan, R.Y.; Shah, N.P.; Corke, H. Polyphenols from selected dietary spices and medicinal herbs differentially affect common food-borne pathogenic bacteria and lactic acid bacteria. Food Control 2018, 92, 437–443. [Google Scholar] [CrossRef]
- Chung, K.T.; Lu, Z.; Chou, M.W. Growth inhibition of selected food-borne bacteria by tannic acid, propyl gallate and related compounds. Lett. Appl. Microbiol. 1993, 17, 29–32. [Google Scholar] [CrossRef]
- Xiao, X.-N.; Wang, F.; Yuan, Y.-T.; Liu, J.; Liu, Y.-Z.; Yi, X. Antibacterial activity and mode of action of dihydromyricetin from Ampelopsis grossedentata leaves against food-borne bacteria. Molecules 2019, 24, 2831. [Google Scholar] [CrossRef] [PubMed]
- Hancock, V.; Dahl, M.; Vejborg, R.M.; Klemm, P. Dietary plant components ellagic acid and tannic acid inhibit Escherichia coli biofilm formation. J. Med. Microbiol. 2010, 59, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.E.; Martin, N.R.; Parzych, K.R.; Rickard, A.H.; Underwood, A.; Boles, B.R. Tannic acid inhibits Staphylococcus aureus surface colonization in an IsaA-dependent manner. Infect. Immun. 2013, 81, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Farha, A.K.; Yang, Q.-Q.; Kim, G.; Zhang, D.; Mavumengwana, V.; Habimana, O.; Li, H.-B.; Corke, H.; Gan, R.-Y. Inhibition of multidrug-resistant foodborne Staphylococcus aureus biofilms by a natural terpenoid (+)-nootkatone and related molecular mechanism. Food Control 2020, 112. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330–335. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Pisseri, F.; Bertoli, A.; Pistelli, L. Essential oils in medicine: Principles of therapy. Parassitologia 2008, 50, 89–91. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Lambert, R.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Baydar, H.; Sagdic, O.; Ozkan, G.; Karadogan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Nurdin, E.; Amelia, T.; Makin, M. The effects of herbs on milk yield and milk quality of mastitis dairy cow. J. Indones. Trop. Anim. Agric. 2011, 36, 104–108. [Google Scholar] [CrossRef][Green Version]
- Giannenas, I.; Bonos, E.; Christaki, E.; Florou-Paneri, P.C. Essential oils and their applications in animal nutrition. Med. Aromat. Plants 2013, 2, 1–12. [Google Scholar] [CrossRef]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.-G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef]
- Cox, S.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef]
- Denyer, S.; Hugo, W. Biocide-induced damage to the bacterial cytoplasmic membrane. In Mechanism of Action of Chemical Biocides; Deyner, S.P., Hugo, W., Eds.; Blackwell Scientific Publications: Oxford, UK, 1991; Volume 27, pp. 171–187. [Google Scholar]
- Chauhan, A.K.; Kang, S.C. Thymol disrupts the membrane integrity of Salmonella ser. typhimurium in vitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res. Microbiol. 2014, 165, 559–565. [Google Scholar] [CrossRef]
- Hippenstiel, F.; Abdel-Wareth, A.A.A.; Kehraus, S.; Südekum, K.-H. Effects of selected herbs and essential oils, and their active components on feed intake and performance of broilers-a review. Arch. Für Geflügelkunde 2011, 75, 226–234. [Google Scholar]
- Mourey, A.; Canillac, N. Anti-Listeria monocytogenes activity of essential oils components of conifers. Food Control 2002, 13, 289–292. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Rosato, A.; Piarulli, M.; Corbo, F.; Muraglia, M.; Carone, A.; Vitali, M.; Vitali, C. In vitro synergistic action of certain combinations of gentamicin and essential oils. Curr. Med. Chem. 2010, 17, 3289–3295. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Horky, P.; Skalickova, S.; Smerkova, K.; Skladanka, J. Essential oils as a feed additives: Pharmacokinetics and potential toxicity in monogastric animals. Animals 2019, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Al-Azem, D.A.; Malik Al-Saadi, S.A.A.; Al-Derawi, K.H. The protective effects of Syzygium aromaticum essential oil extract against methotrexate induced hepatic and renal toxicity in rats. J. Pure Appl. Microbiol. 2019, 13, 505–515. [Google Scholar] [CrossRef]
- Fateh, A.H.; Mohamed, Z.; Chik, Z.; Alsalahi, A.; Md Zin, S.R.; Alshawsh, M.A. Prenatal developmental toxicity evaluation of Verbena officinalis during gestation period in female Sprague-Dawley rats. Chem. Biol. Interact. 2019, 304, 28–42. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Et Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef]
- Harborne, J.B. The chemical basis of plant defense. In Plant Defenses against Mammalian Herbivory; CRC Press: Boca Raton, FL, USA, 1991; pp. 45–59. [Google Scholar]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. Found. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the role of plant natural products in antibiotic drug discovery. Chem. Rev. 2020, 121, 3495–3560. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Romero, J.C.; Gonzáles-Ríos, H.; Borges, A.; Simões, M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid. Based Complementary Altern. Med. 2015. [Google Scholar] [CrossRef]
- Sparg, S.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Carelli, M.; Biazzi, E.; Panara, F.; Tava, A.; Scaramelli, L.; Porceddu, A.; Graham, N.; Odoardi, M.; Piano, E.; Arcioni, S.; et al. Medicago truncatula CYP716A12 is a multifunctional oxidase involved in the biosynthesis of hemolytic saponins. Plant Cell 2011, 23, 3070–3081. [Google Scholar] [CrossRef]
- Chaieb, I. Saponins as insecticides: A review. Tunis. J. Plant Prot. 2010, 5, 39–50. [Google Scholar]
- Mbaveng, A.T.; Ndontsa, B.L.; Kuete, V.; Nguekeu, Y.M.M.; Celik, I.; Mbouangouere, R.; Tane, P.; Efferth, T. A naturally occuring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death. Phytomedicine 2018, 43, 78–85. [Google Scholar] [CrossRef]
- Lanzotti, V. Bioactive polar natural compounds from garlic and onions. Phytochem. Rev. 2012, 11, 179–196. [Google Scholar] [CrossRef]
- Nabinejad, A. Antibacterial effects of Saponaria officinalis extracts against avian pathogenic Escherichia coli (APEC). Afr. J. Agric. Res. 2013, 8, 2068–2071. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.; Ramos, L.; Moreno, C.; Zúñiga-Paredes, J.C.; Carlosama-Yepez, M.; Ruales, P. Antimicrobial activity of plant-food by-products: A review focusing on the tropics. Livest. Sci. 2016, 189, 32–49. [Google Scholar] [CrossRef]
- Qin, X.-J.; Sun, D.-J.; Chen, C.X.; Hua, Y.; He, L.; Liu, H.-Y. Steroidal saponins with antimicrobial activity fromstems and leaves of Paris polyphylla var. yunnanensis. Steroids 2012, 77, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Fouedjou, R.T.; Teponno, R.B.; Quassinti, L.; Bramucci, M.; Petrelli, D.; Vitali, L.A.; Fiorini, D.; Tapondjou, L.A.; Barboni, L. Steroidal saponins from the leaves of Cordyline fruticose (L.) A. Chev. And their cytotoxic and antimicrobial activity. Phytochem. Lett. 2014, 7, 62–68. [Google Scholar] [CrossRef]
- Srivastava, G.; Jain, R.; Vyas, N.; Mehta, A.; Kachhwaha, S.; Kotharim, S.L. Antimicrobial activity of the methanolic extract, fractions and isolated compounds from Citrullus colocynthis (L.) Schrad. Int. J. Pharma Bio Sci. 2013, 4, 825–833. [Google Scholar]
- Fomogne-Fodjo, M.C.; Ndinteh, D.T.; Olivier, D.K.; Kempgens, P.; van Vuuren, S.; Krause, R.W. Secondary metabolites from Tetracera potatoria stem bark with anti-mycobacterial activity. J. Ethnopharmacol. 2017, 195, 238–245. [Google Scholar] [CrossRef]
- Tiam, E.R.; Ngono Bikobo, D.S.; Abouem, A.Z.A.; MbabiNyemeck, N.; Moni Ndedi, E.D.F.; Betote Diboue, P.H.; Nyegue, M.A.; Atchade, A.T.; Emmanuel Pegnyemb, D.; Bochet, C.G.; et al. Secondary metabolites from Triclisia gilletii (De Wild) staner (Menispermaceae) with antimycobacterial activity against Mycobacterium tuberculosis. Nat. Prod. Res. 2019, 33, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Saboora, A.; Sajjadi, S.-T.; Mohammadi, P.; Fallahi, Z. Antibacterial activity of different composition of aglycone and glycosidic saponins from tuber of Cyclamen coum Miller. Ind. Crop. Prod. 2019, 140. [Google Scholar] [CrossRef]
- Korchowiec, B.; Gorczyca, M.; Wojszko, K.; Janikowska, M.; Henry, M.; Rogalska, E. Impact of two different saponins on the organization of model lipid membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 1963–1973. [Google Scholar] [CrossRef]
- Avato, P.; Bucci, R.; Tava, A.; Vitali, C.; Rosato, A.; Bialy, Z.; Jurzysta, M. Antimicrobial activity of saponins from Medicago sp.: Structure-activity relationship. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 454–457. [Google Scholar] [CrossRef]
- Khan, A.A.; Naqvi, T.S.; Naqvi, M.S. Identification of phytosaponins as novel biodynamic agents: An updated overview. Asian J. Exp. Biol. Sci. 2012, 3, 459–467. [Google Scholar]
- Martins, A.; Andrea, V.; Viveiros, M.; Molnar, J.; Hohmann, J.; Amaral, L. Antibacterial properties of compounds isolated from Carpobrotus edulis. Int. J. Antimicrob. Agents 2011, 37, 438–444. [Google Scholar] [CrossRef]
- Grudniak, A.M.; Kurek, A.; Szarlak, J.; Woslak, K. Oleanolic and ursolic acids influence affect the expression of the cysteine regulon and the stress response in Escherichia coli. Curr. Microbiol. 2011, 62, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Grudniak, A.M.; Szwed, M.; Klicka, A.; Samluk, L.; Wolska, K.; Janiszowska, W.; Popowska, M. Oleanolic acid and ursolic acid affect peptidoglycan metabolism in Listeria monocytogenes. Antonie Van Leeuwenhoek 2010, 97, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ablat, A.; Mohamad, J.; Awang, K.; Shilpi, J.A.; Arya, A. Evaluation of antidiabetic and antioxidant properties of Brucea javanica seed. Sci. World J. 2014, 1. [Google Scholar] [CrossRef]
- Coleman, J.J.; Okoli, I.; Tegos, G.P.; Holson, E.B.; Wagner, F.F.; Hamblin, M.R.; Mylonakis, E. Characterization of plant-derived saponin natural products against Candida albicans. ACS Chem. Biol. 2010, 5, 321–332. [Google Scholar] [CrossRef]
- Tamokou, J.; Mbaveng, A.T.; Kuete, V. Antimicrobial activities of African medicinal spices and vegetables. Med. Spices Veg. Afr. 2017, 207–237. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Tamokou, J.-. de-D.; Kengne, I.Ch.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of saponins from Melanthera elliptica and their synergistic effects with antibiotics against pathogenic phenotypes. Chem. Cent. J. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Nakamura, S.; Li, N.; Li, X.; Matsuda, H. Bioactive saponins and glycosides. XXV. Acylated oleanane- type triterpene saponins from the seeds of tea plant (Camellia sinensis). Chem. Pharm. Bull. 2007, 55, 57–63. [Google Scholar] [CrossRef]
- Poojary, M.M.; Putnik, P.; Kovačević, D.B.; Barba, F.J.; Lorenzo, J.M.; Dias, D.A.; Shpigelman, A. Stability and extraction of bioactive sulfur compounds from Allium genus processed by traditional and innovative technologies. J. Food Compos. Anal. 2017, 61, 28–39. [Google Scholar] [CrossRef]
- Stoewsand, G. Bioactive organosulfur phytochemicals in Brassica oleracea vegetables—A review. Food Chem. Toxicol. 1995, 33, 537–543. [Google Scholar] [CrossRef]
- Sagdic, O.; Tornuk, F. Antimicrobial properties of organosulfur compounds. In Dietary Phytochemicals and Microbes; Patra, A.K., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 127–156. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Neachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Cavallito, C.J.; Bailey, J.H. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Leontiev, R.; Hohaus, N.; Gruhlke, M.C.H.; Slusarenko, A.J. A comparison of the antibacterial and antifungal activities of thiosulfinate analogues of allicin. Sci. Rep. 2018, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Halkier, B.A. Biosynthesis of glucosinolates in the developing silique walls and seeds of Sinapis alba. Phytochemistry 1998, 48, 1145–1150. [Google Scholar] [CrossRef]
- Brabban, A.; Edwards, C. The effects of glucosinolates and their hydrolysis products on microbial growth. J. Appl. Bacteriol. 1995, 79, 171–177. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Sanches-Silva, A.; Daglia, M. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci. Technol. 2016, 52. [Google Scholar] [CrossRef]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Mohammed, S.A.; Banerjee, S.K.; Sharopov, F.; Rigano, D.; Sharifi-Rad, J.; et al. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Shaikh, H.; Shaikh, S. Phytochemistry and neuroprotective effect of Alium sativum: An exhaustive review. World J. Adv. Sci. Res. 2020, 3, 155–168. [Google Scholar]
- Wallock-Richards, D.; Doherty, C.J.; Doherty, L.; Clarke, D.J.; Place, M.; Govan, J.R.; Campopiano, D.J. Garlic revisited: Antimicrobial activity of allicin-containing garlic extracts against Burkholderia cepacian complex. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Hameed, H.M.A.; Islam, M.M.; Chhotaray, C.; Wang, C.; Liu, Y.; Tan, Y.; Li, X.; Tan, S.; Delorme, V.; Yew, W.W.; et al. Molecular targets related drug resistance mechanisms in MDR-, XDR-, and TDR-Mycobacterium tuberculosis strains. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Ariza-Romero, J.J.; Banos-Arjona, A.; Exposito-Ruiz, M.; Gutierrez-Fernandez, J. In vitro antibacterial activity of propyl-propane-thiosulfinate and propyl-propane-thiosulfonate derived from Allium spp. against gram-negative and gram-positive multi-drug-resistant bacteria isolated from human samples. BioMed Res. Int. 2018. [Google Scholar] [CrossRef]
- Da Cruz, R.C.; Denardi, L.B.; Mossmann, N.J.; Piana, M.; Alves, S.H.; de Campos, M.M. Antimicrobial activity and chromatographic analysis of extracts from Tropaeolum pentaphyllum Lam. tubers. Molecules 2016, 21, 566. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Watanabe, K.; Suma, K.; Origuchi, K.; Matsufuji, H.; Seki, T.; Ariga, T. Antibacterial potential of garlic-derived allicin and its cancellation by sulfhydryl compounds. Biosci. Biotechnol. Biochem. 2009, 73, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Huh, J.E.; Kyung, S.H.; Kyung, K.H. Antimicrobial activity of alk (en) yl sulfides found in essential oils of garlic and onion. Food Sci. Biotechnol. 2004, 13, 235–239. [Google Scholar]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Feldberg, R.; Chang, S.; Kotik, A.; Nadler, M.; Neuwirth, Z.; Sundstrom, D.; Thompson, N. In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrob. Agents Chemother. 1988, 32, 1763–1768. [Google Scholar] [CrossRef]
- Tsao, R.; Peterson, C.J.; Coats, J.R. Glucosinolate breakdown products as insect fumigants and their effect on carbon dioxide emission of insects. BMC Ecol. 2002, 2, 1–7. [Google Scholar] [CrossRef]
- Choo, S.; Chin, V.K.; Wong, E.H.; Madhavan, P.; Tay, S.T.; Yong, P.V.C.; Chong, P.P. Review: Antimicrobial properties of allicin used alone or in combination with other medications. Folia Microbiol. 2020, 65, 451–465. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, R.; Pei, F.; Liang, B.-B. Antibacterial activity of allicin alone and in combination with β-lactams against Staphylococcus spp. and Pseudomonas Aeruginosa. J. Antibiot. 2007, 60, 335–338. [Google Scholar] [CrossRef]
- Tajima, H.; Kimoto, H.; Taketo, A. Specific antimicrobial synergism of synthetic hydroxy isothiocyanates with aminoglycoside antibiotics. Biosci. Biotechnol. Biochem. 2001, 65, 1886–1888. [Google Scholar] [CrossRef]
- Tajima, H.; Kimoto, H.; Taketo, A. Paradoxical effect of synthetic hydroxy isothiocyanates on antimicrobial action of aminoglycosides. Biosci. Biotechnol. Biochem. 2003, 67, 1844–1846. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Mukherjee, P.K.; Maulik, S.K. Garlic as an antioxidant: The good, the bad and the ugly. Phytother. Res. 2003, 17, 97–106. [Google Scholar] [CrossRef]
- Alnaqeeb, M.A.; Thomson, M.; Bordia, T.; Ali, M. Histopathological effects of garlic on liver and lung of rats. Toxicol. Lett. 1996, 85, 157–164. [Google Scholar] [CrossRef]
- McGaw, L.J.; Eloff, J.N. Ethnoveterinary use of southern African plants and scientific evaluation of their medicinal properties. J. Ethnopharmacol. 2008, 119, 559–574. [Google Scholar] [CrossRef]
- Hamadani, A.; Ganai, N.A.; Shanaz, S.; Khan, N.; Bukhari, S.S.; Iqbal, Z.; Ayaz, A. Usage of phytochemicals in veterinary practice. J. Entomol. Zool. Stud. 2018, 6, 1997–2000. [Google Scholar]
- Ökmen, G.; Cantekin, Z.; Alam, M.I.; Türkcan, O.; Ergün, Y. Antibacterial and antioxidant activities of Liquidambar orientalis Mill. various extracts against bacterial pathogens causing mastitis. Turk. J. Agric. 2017, 5, 883. [Google Scholar] [CrossRef][Green Version]
- Disler, M.; Schmid, K.; Ivemeyer, S.; Hamburger, M.; Walkenhorst, M. Traditional homemade herbal remedies used by farmers of northern Switzerland to treat skin alterations and wounds in livestock. Planta Med. 2013, 79. [Google Scholar] [CrossRef]
- Bartha, S.G.; Quave, C.L.; Balogh, L.; Papp, N. Ethnoveterinary practices of Covasna County, Transylvania, Romania. J. Ethnobiol. Ethnomedicine 2015, 11. [Google Scholar] [CrossRef]
- Chusri, S.; Tongrod, S.; Saising, J.; Mordmuang, A.; Limsuwan, S.; Sanpinit, S.; Voravuthikunchai, S.P. Antibacterial and anti-biofilm effects of polyherbal formula and its constituents against coagulase-negative -positive staphylococci isolated from bovine mastitis. J. Appl. Anim. Res. 2017, 45, 364–372. [Google Scholar] [CrossRef]
- Bruschi, P.; Urso, V.; Solazzo, D.; Tonini, M.; Signorini, M.A. Traditional knowledge on ethno-veterinary and fodder plants in South Angola: An ethnobotanic field survey in Mopane woodlands in Bibala, Namibe province. J. Agric. Environ. Int. Dev. 2017, 111, 105–121. [Google Scholar] [CrossRef]
- Kalayou, S.; Haileselassie, M.; Gebre-egziabher, G.; Tiku´e, T.; Sahle, S.; Taddele, G.H.; Ghezu, M. In-vitro antimicrobial activity screening of some ethnoveterinary medicinal plants traditionally used against mastitis, wound and gastrointestinal tract complication in Tigray Region, Ethiopia. Asian Pac. J. Trop. Biomed. 2012, 2, 516–522. [Google Scholar] [CrossRef]
- Pattanayak, S.; Dutta, M.K.; Debnath, P.K.; Bandyopadhyay, S.K.; Saha, B.; Maity, D. A study on ethno-medicinal use of some commonly available plants for wound healing and related activities in three southern districts of West Bengal, India. Explor. Anim. Med Res. 2012, 2, 97–110. [Google Scholar]
- Mishra, D. Cattle wounds and ethnoveterinary medicine: A study in Polasara block, Ganjam district, Orissa, India. Indian J. Tradit. Knowl. 2013, 12, 62–65. [Google Scholar]
- Parthiban, R.; Vijayakumar, S.; Prabhu, S.; Gnanaselvam, E.; Yabesh, M. Quantitative traditional knowledge of medicinal plants used to treat livestock diseases from Kudavasal taluk of Thiruvarur district, Tamil Nadu, India. Rev. Bras. Farmacogn. 2015, 26, 109–121. [Google Scholar] [CrossRef]
- Mubarack, H.M.; Doss, A.; Dhanabalan, R.; Venkataswamy, R. Activity of some selected medicinal plant extracts against bovine mastitis pathogens. J. Anim. Vet. Adv. 2011, 10, 738–741. [Google Scholar] [CrossRef]
- Tamilselvan, N.; Thirumalai, T.; Elumalai, E.K.; Balaji, R.; David, E. Pharmacognosy of Coccinia grandis: A review. Asian Pac. J. Trop. Biomed. 2011, 1, 299–302. [Google Scholar] [CrossRef]
- Migliato, K.F.; Chiosini, M.A.; Mendonca, F.A.; Esquisatto, M.A.; Salgado, H.R.; Santos, G.M. Effect of glycolic extract of Dillenia indica L. combined with microcurrent stimulation on experimental lesions in Wistar Rats. Wounds: A Compend. Clin. Res. Pract. 2011, 23, 111–120. [Google Scholar]
- Wenbin, L.; Kandhare, A.D.; Mukherjee, A.A.; Bodhankar, S.L. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: Role of TGF-β/Smads and Ang-1/Tie-2 signaling pathways. Excli J. 2018, 17, 399–419. [Google Scholar] [CrossRef]
- Chakraborty, T.; Gupta, S.; Nair, A.; Chauhan, S.; Saini, V. Wound healing potential of insulin-loaded nanoemulsion with Aloe vera gel in diabetic rats. J. Drug Deliv. Sci. Technol. 2021, 64. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Eskandari, M.H. Kefir accelerates burn wound healing through inducing fibroblast cell migration in vitro and modulating the expression of IL-1β, TGF-β1, and bFGF. Probiotics Antimicrob. Proteins 2018, 11, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Marcelline, A.N.; Timothée, O.A.; Martial, S.V.; Aminata, A.O.; Armand, K.A.; Claude, K.A.L. In vivo antistaphylococcal activity evaluation of Ocimum gratissimum Linn. (Lamiaceae) ophthalmic ointment. J. Adv. Med. Med Res. 2020, 44–57. [Google Scholar] [CrossRef]
- Hase, P.; Digraskar, S.; Ravikanth, K.; Dandale, M.; Maini, S. Management of subclinical mastitis with mastilep gel and herbal spray (AV/AMS/15). Int. J. Pharm. Pharmacol. 2013, 2, 64–67. [Google Scholar]
- Abboud, M.; Rammouz, R.; Jammal, B.; Sleiman, M. In vitro and in vivo antimicrobial activity of two essential oils Thymus vulgaris and Lavandula angustifolia against bovine Staphylococcus and Streptococcus mastitis pathogen. Middle East J. Agric. 2015, 4, 975–983. [Google Scholar]
- Cho, B.-W.; Cha, C.N.; Lee, S.-O.; Kim, M.-J.; Park, J.-Y.; Yoo, C.Y.; Son, S.-E.; Kim, S.; Lee, H.-J. Therapeutic effect of oregano essential oil on subclinical bovine mastitis caused by Staphylococcus aureus and Escherichia coli. Korean J. Vet. Res. 2015, 55, 253–257. [Google Scholar] [CrossRef]
- Kebede, B.; Negese, T. Evaluation of acaricidal effect of ethnoveterinary medicinal plant by in vivo and in vitro against Sarcoptes scabiei var. caprae of infected goats in North Shoa, Oromia regional state, Ethiopia. J. Tradit. Med. Clin. Naturop. 2017, 6. [Google Scholar] [CrossRef]
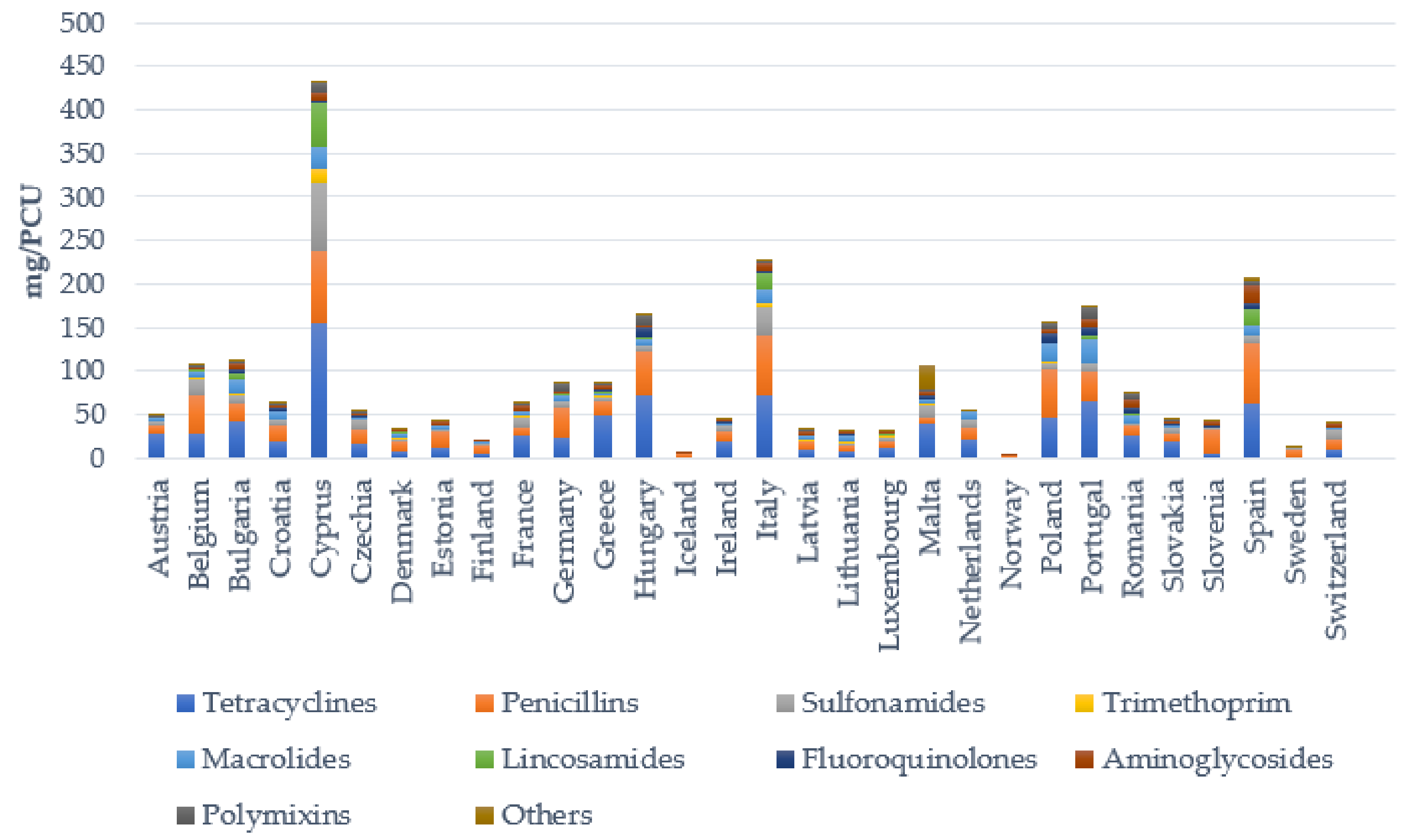
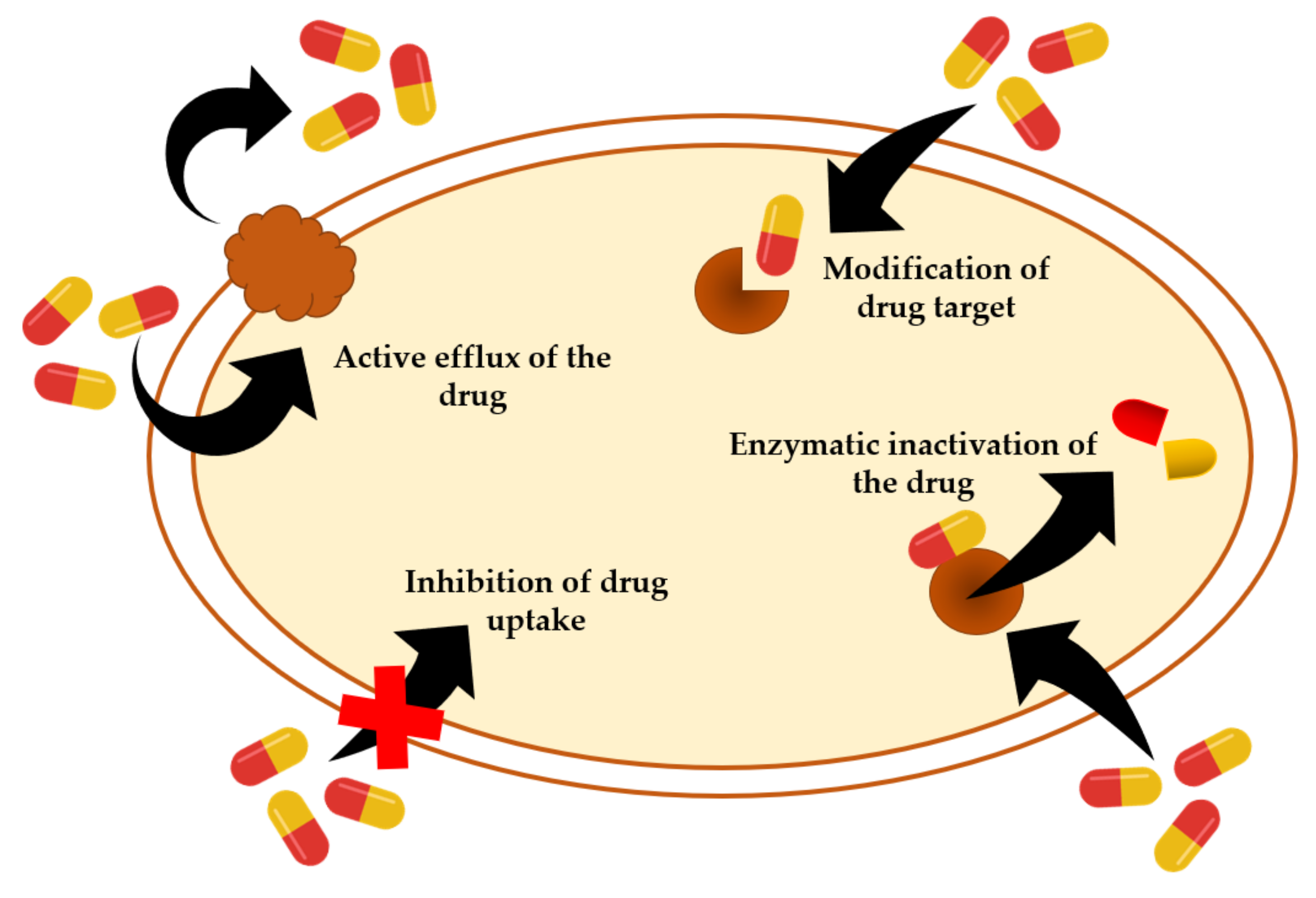
| Species of Livestock | Bacterial Pathogens |
|---|---|
| Cattle | Actinomyces bovis, Bacteroides melaninogenicus, Staphylococcus aureus, S. hyicus, Streptococcus dysgalactiae, Fusobacterium necrophorum, Moraxella bovis, Trueperella pyogenes |
| Pigs | Dermatophylus congolensis, S. hyicus, S. intermedius, S. chromogenes, S. sciuri |
| Goats | Dermatophylus congolensis, S. aureus, S. hyicus, S. haemolyticus, S. warneri, S. epidermidis, S. chromogenes, S. caprae, S. simulans |
| Sheep | Dermatophylus congolensis, Corynebacterium pseudotuberculosis, Pithomyces fungus, S. aureus, S. xylosus, S. epidermidis, Str. dysgalactiae |
| Poultry | S. aureus, S. hyicus |
| References | Phytochemicals | Activity |
|---|---|---|
| [92] | Alkaloids | Antimicrobial, anti-inflammatory |
| [91] | Polyphenols | Anticarcinogenic, antimicrobial, antioxidative, antithrombic, immunomodulatory properties, anti-inflammatory, influence on blood pressure |
| [91] | Saponins | Anticarcinogenic, antimicrobial, immunomodulatory properties, influence on blood pressure |
| [92] | Tannins | Antimicrobial, anti-inflammatory, antioxidative |
| [98] | Essential oils | Antimicrobial, anti-inflammatory |
| [91] | Terpenoids | Anticarcinogenic, antimicrobial, anti-inflammatory, cholesterol-lowering effect |
| [91] | Carotenoids | Anticarcinogenic, antioxidative, immunomodulatory properties, cholesterol-lowering effect |
| [91] | Organosulfur compounds | Anticarcinogenic, antimicrobial, antioxidative, antithrombic, immunomodulatory properties, anti-inflammatory, influence on blood pressure |
| [91] | Phytosterols | Anticarcinogenic, cholesterol-lowering effect |
| [91] | Protease inhibitors | Anticarcinogenic, antioxidative, modulate blood glucose levels |
| [91] | Phytoestrogens | Anticarcinogenic, antioxidative, immunomodulatory properties |
| References | Alkaloids | MIC (µg/mL) | ||
|---|---|---|---|---|
| Classes of Alkaloids | Specific Representatives | G+ | G− | |
| [109,110,111] | Quinolines alkaloids | 4-methyl quinolone, 8-hydroxyquinolone, evocarpine | 2–50 | 8–100 |
| [112,113,114,115] | Isoquinolines, aporphines, phenanthrenes | Lysicamine, artabotrine, liridine, sanguinarine, berberine, jatrorhizine, columbamine, buesgenine, palmitine | 0.5–2.5 | 0.78–32 |
| [116] | Other alkaloids | Carmichaedine | 8 | - |
| References | Phenolic Compounds | MIC (µg/mL) | ||
|---|---|---|---|---|
| Classes of Phenolics | Specific Representatives | G+ | G− | |
| [128,129,130,131,132] | Flavonoids | Quercetin, myricetin, brazilin, neobavaisoflavonde, lupinifolin, 6,8-diprenyleriodictyol, pseudarflavone A | 0.5–62.5 | 4–32 |
| [128,129,130,131,132] | Nonflavonoids | 3´-demethoxy-6-O-demethylisoguaiacin, 4-epi-larreatricin, dihydroguaiaretic acid, resveratrol | 12.5–>1000 | 25–1280 |
| References | Tannins | MIC (µg/mL) | ||
|---|---|---|---|---|
| Classes of Tannins | Specific Representatives | G+ | G− | |
| [153,154,155] | Gallotannins | Tannic acid, hexa-O-galloylglucose, hepta-O-galloylglucose, 1,2,6-tri-O-galloyl-β-D-glucopyranose | 0.16–1000 | 5–3200 |
| [154,156,157] | Ellagitannins | Punicalagin, corilagin, tellimagrandin I, tercatain, chebulagic acid, isorugosin A, davidiinm castalagin | 0.25–1000 | 4–3200 |
| [154,158,159] | Proanthocyanidins | Procyanidin A1, procyanidin B1, procyanidin B2, Procyanidin B3, procyanidin B4, rhodonidin A, prodelphidin, epicatechin | 0.1–100 | 2–800 |
| References | Terpenoids | MIC (µg/mL) | ||
|---|---|---|---|---|
| Classes of Terpenoids | Specific Representatives | G+ | G− | |
| [193] | Monoterpenoids | Carvacrol, thymol, linalool, citronellol, α-terpineol | 0.007–32 | 0.015–55 |
| [193] | Sesquiterpenoids | Xanthorrhizol, onopordopicrin | 0.5–86.2 | 2.2–6.8 |
| [193] | Diterpenoids | Carnosol, carnosic acid, rosmanol, lasiodin, bafoudiosbulbin C, (-)-copalic acid, dehydrobietic acid | 0.5–25 | 3.1–64 |
| [193] | Polyterpenoids | Nimbolide | 8 | - |
| References | Saponins | MIC (µg/mL) | ||
|---|---|---|---|---|
| Classes of Saponins | Specific Representatives | G+ | G− | |
| [202,203] | Steroidal saponins | Progenin II, diosgenin, spirosta-5,25(27)-diene-1β,3β-diol-1-O-α-l-rhamnopyranosyl- (1→ 2)-β-d-fucopyranoside (fruticoside H) | 7.8–>256 | 128–>256 |
| [204,205,206] | Triterpenoid saponins | Oleanolic acid, betulinic acid, moronic acid, ursolic acid, friedelane-3,11-dione | 1.52–64 | 1–100 |
| References | Organosulfur Compounds | MIC (µg/mL) | ||
|---|---|---|---|---|
| Classes of Organosulfur Compounds | Specific Representatives | G+ | G− | |
| [230,231,232] | Thiosulfinates | Ajoene, Z-ajoene, allicin, propyl-propane thiosulfinate | 4–20 | 0.5–>500 |
| [233] | Glucosinolates | Benzyl-isothiocyanate, allyl-isothiocyanate | 4–40 | 10–40 |
| References | Family | Botanical Name | Treatment | Part of Plant |
|---|---|---|---|---|
| [247] | Altingiaceae | Liquidambar orientalis | Bovine mastitis | Leaves |
| [248,249] | Apiaceae | Eryngium planum, Conium maculatum, Sanicula europaea | Bovine mastitis, wound healing | Herb |
| [250] | Arecaceae | Areca catechu | Bovine mastitis | Seeds |
| [251] | Asphodelaceae | Aloe species | Bacterial skin diseases, wound healing | Leaves |
| [252] | Asparagaceae | Achyranthes aspera, Drimia maritima | Bovine mastitis, other bacterial skin diseases | Leaves |
| [248,252,253,254,255,256] | Asteraceae | Achillea millefolium, Arnica montana, Artemisia nilagirica, Calendula officinalis, Eclipta prostrata, Eupatorium triplinerve, Blumea lacera, Cyanthillium cinereum, Haplocarpha scaposa, Helianthus annuus, Matricaria recutita, Mikania scandens, Saussurea costus, Solidago virgaurea, Stevia rebaudiana, Tagetes erecta, T. patula, Tridax procumbens, Vernonia species, Wedelia chinensis | Bovine mastitis, wound healing | Flowers, leaves, roots |
| [248] | Boraginaceae | Bourreria orbicularis, Heliotropium indicum, Symphitum officinale | Wound healing | Barks, leaves, roots |
| [256] | Bignoniaceae | Spathodea campanulate | Bovine mastitis | Leaves |
| [253] | Capparaceae | Capparis zeylanica | Wound healing | Leaves |
| [250] | Clusiaceae | Garcinia mangostana | Bovine mastitis | Pericarp |
| [253,257] | Cucurbitaceae | Coccinia grandis | Bacterial skin diseases, wound healing | Fruits, leaves, roots |
| [253,258] | Dilleniaceae | Dillenia indica | Bacterial skin diseases, wound healing | Fruits |
| [253] | Ebenaceae | Diospyros malabarica | Wound healing | Leaves |
| [248,252,253,254,255,256] | Euphorbiaceae | Acalipha indica, Euphorbia hirta, Croton bonplandianum, C. macrostachyus, Jatropha zeyheri, Ricinus communis | Wound healing | Leaves, roots |
| [248] | Fabaceae | Acacia nilotica, Aeschynomene indica, Butanea monosperma, Calpurnia aurea, Cullen corylifolium, Crocosmia aurea, Glycyrrhiza glabra, Pterocarpus marsupium, Rhynchosia capitate, Saraca indica, Senna alata, S. sophera, S. alexandria, Schotia latifolia, Vigna unguiculata | Bovine mastitis, other bacterial skin diseases, wound healing | Fruits, leaves |
| [256] | Hypericaceae | Hypericum perforatum, H. revolutum | Wound healing | Flowers, roots |
| [253] | Chenopodiaceae | Chenopodium bonus-henricus | Wound healing | Leaves |
| [250] | Lamiaceae | Anisomeles indica, Leucas aspera, Lavandula angustifolia, Mentha, arvensis, Minthostachys verticillata, Ocimum sanctum, Ocimum tenuiflorum, Origanum vulgare, Plectranthus amboinicus, P. ambiguous, Tectona grandis, Thymus vulgaris, Vitex negundo | Bovine mastitis, other bacterial skin diseases, wound healing | Flowers, leaves |
| [253,257] | Lauraceae | Litsea glutinosa | Wound healing | Barks, leaves |
| [253,258] | Malvaceae | Gossypium herbaceium, Malva neglecta, M. sylvestris, Sida cordifolia | Bacterial skin diseases, wound healing | Herbs, leaves |
| [253] | Menispermaceae | Tinospora sinensis | Wound healing | Stem |
| [248,252,253,256] | Molluginaceae | Glinus lotoides | Wound healing | Latex, leaves |
| [248] | Moraceae | Ficus benghalensis, F. caria, F. racemosa, F.thonningi, Morus nigrai | Bacterial skin diseases, wound healing | Latex, leaves, roots |
| [256] | Myrtaceae | Eucalyptus globulus, Syzygium cumini | Bacterial skin diseases | Leaves |
| [253] | Papaveraceae | Fumaria indica, Papaver somniferum, Chelidonium majus | Bovine mastitis, wound healing | Leaves |
| [250] | Pandaceae | Pandanus foetidus | Wound healing | Leaves |
| [253,257] | Pinaceae | Cedrus deodara, Picea abies | Bovine mastitis, other bacterial skin diseases | Bark |
| [253,258] | Poaceae | Bambusa bambos, Cynodon dactylon | Bovine mastitis, wound healing | Leaves, shoots |
| [253] | Polygonaceae | Rumex obtusifolius | Bacterial skin diseases | Leaves, roots |
| [248,252,253,254,255] | Rhamnaceae | Ziziphus mucronata, Z. spina-christi | Bovine mastitis | Leaves, roots |
| [248] | Rubiaceae | Morinda citrifolia | Bovine mastitis | Leaves |
| [256] | Salvadoraceae | Salvadora persica | Bacterial skin diseases, wound healing | Leaves |
| [253] | Solanaceae | Atropa belladonna, Datura metel, Nicotiana tabacum, Solanum hastifolium, S. americanum, S. sodomeum, S. virginianum, Withania somnifera | Bovine mastitis, other bacterial skin diseases, wound healing | Leaves, roots |
| [250] | Symplocaceae | Symplocos racemose | Bacterial skin diseases | Bark |
| [253,257] | Vitaceae | Cissus quandrangularis | Bacterial skin diseases | Aerial parts |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mala, L.; Lalouckova, K.; Skrivanova, E. Bacterial Skin Infections in Livestock and Plant-Based Alternatives to Their Antibiotic Treatment. Animals 2021, 11, 2473. https://doi.org/10.3390/ani11082473
Mala L, Lalouckova K, Skrivanova E. Bacterial Skin Infections in Livestock and Plant-Based Alternatives to Their Antibiotic Treatment. Animals. 2021; 11(8):2473. https://doi.org/10.3390/ani11082473
Chicago/Turabian StyleMala, Lucie, Klara Lalouckova, and Eva Skrivanova. 2021. "Bacterial Skin Infections in Livestock and Plant-Based Alternatives to Their Antibiotic Treatment" Animals 11, no. 8: 2473. https://doi.org/10.3390/ani11082473
APA StyleMala, L., Lalouckova, K., & Skrivanova, E. (2021). Bacterial Skin Infections in Livestock and Plant-Based Alternatives to Their Antibiotic Treatment. Animals, 11(8), 2473. https://doi.org/10.3390/ani11082473






