Canine Olfaction: Physiology, Behavior, and Possibilities for Practical Applications
Abstract
:Simple Summary
Abstract
1. Introduction
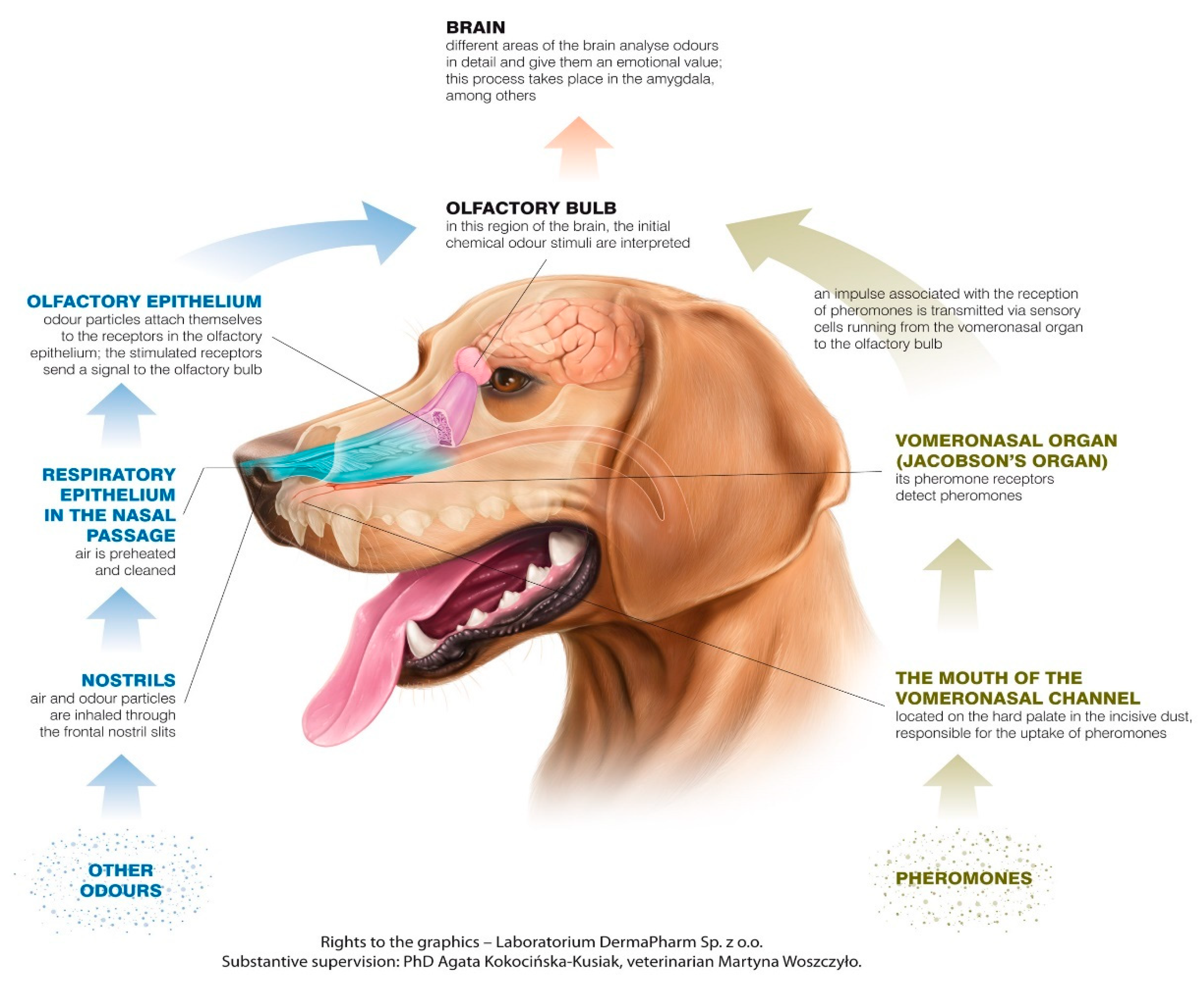
2. Anatomy and Physiology of Canine Olfaction
3. Internal and Environmental Factors Influencing Olfactory Skills: Olfaction Gene Polymorphism, Age, Sex, and Breed-Specific Olfactory Capacity
3.1. Genetic Implications
3.2. Breed
3.3. Age and Sex
3.4. Environmental Conditions
3.5. Diseases
3.6. Substances and Drugs Influencing Olfactory Abilities
4. Olfactory Behavior
4.1. Sniffing vs. Smelling
4.2. Sending and Receiving Olfactory Signals
4.3. Tracking Behavior
5. The Use of Canine Olfactory Skills
5.1. Detection of Dangerous and Illegal Substances
5.2. Detection of Biological Scents
5.3. Detection of Other Living Organisms
6. Recognition of the Physiological State by Olfaction
6.1. Detection of the Phase of Reproductive Cycle
6.2. Recognizing Emotional State
6.3. Dogs Detecting Diseases in Humans and Animals
7. Chemical Communication Influencing Animal Behavior
8. Novel Methods of Canine Olfaction Evaluation—fMRI Study
9. Limitations in Canine Detection—A Critical Assessment
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hepper, P.G.; Wells, D.L. Perinatal Olfactory Learning in the Domestic Dog. Chem. Senses 2005, 31, 207–212. [Google Scholar] [CrossRef] [Green Version]
- D’Aniello, B.; Semin, G.R.; Alterisio, A.; Aria, M.; Scandurra, A. Interspecies transmission of emotional information via chemosignals: From humans to dogs (Canis lupus familiaris). Anim. Cogn. 2018, 21, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Miklosi, A.; Topál, J.; Csányi, V. Big thoughts in small brains? Dogs as a model for understanding human social cognition. NeuroReport 2007, 18, 467–471. [Google Scholar] [CrossRef]
- Stitzel, S.E.; Aernecke, M.J.; Walt, D.R. Artificial Noses. Annu. Rev. Biomed. Eng. 2011, 13, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Dzięcioł, M.; Podgórski, P.; Stańczyk, E.; Szumny, A.; Woszczyło, M.; Pieczewska, B.; Niżański, W.; Nicpoń, J.; Wrzosek, M.A. MRI Features of the Vomeronasal Organ in Dogs (Canis familiaris). Front. Veter. Sci. 2020, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Salazar, I.; Cifuentes, J.M.; Sanchez-Quinteiro, P. Morphological and Immunohistochemical Features of the Vomeronasal System in Dogs. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 296, 146–155. [Google Scholar] [CrossRef]
- Jezierski, T.; Ensminger, J.; Papet, L.E. Canine Olfaction Science and Law: Advances in Forensic Science, Medicine, Conservation, and Environmental Remediation; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Craven, B.A.; Paterson, E.G.; Settles, G.S. The fluid dynamics of canine olfaction: Unique nasal airflow patterns as an explanation of macrosmia. J. R. Soc. Interface 2009, 7, 933–943. [Google Scholar] [CrossRef] [Green Version]
- Settles, G.S.; Kester, D.A.; Dodson-Dreibelbis, L.J. The External Aerodynamics of Canine Olfaction, in Sensors and Sensing in Biology and Engineering; Barth, F.G., Humphrey, J.A.C., Secomb, T.W., Eds.; Springer: Vienna, Austira, 2003; pp. 323–335. [Google Scholar]
- Patel, R.M.; Pinto, J.M. Olfaction: Anatomy, physiology, and disease. Clin. Anat. 2014, 27, 54–60. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Quaranta, A.; Rogers, L.J. Hemispheric Specialization in Dogs for Processing Different Acoustic Stimuli. PLoS ONE 2008, 3, e3349. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Sasso, R.; Pepe, A.M.; Vallortigara, G.; Quaranta, A. Dogs turn left to emotional stimuli. Behav. Brain Res. 2010, 208, 516–521. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Sasso, R.; Pepe, A.M.; Dimatteo, S.; Vallortigara, G.; Quaranta, A. Sniffing with the right nostril: Lateralization of response to odour stimuli by dogs. Anim. Behav. 2011, 82, 399–404. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L.; Bisazza, A. Possible evolutionary origins of cognitive brain lateralization. Brain Res. Rev. 1999, 30, 164–175. [Google Scholar] [CrossRef]
- Vallortigara, G.; Chiandetti, C.; Sovrano, V.A. Brain asymmetry (animal). Wiley Interdiscip. Rev Cogn. Sci. 2011, 2, 146–157. [Google Scholar] [CrossRef]
- Craig, A.D. Forebrain emotional asymmetry: A neuroanatomical basis? Trends Cogn. Sci. 2005, 9, 566–571. [Google Scholar] [CrossRef]
- Siniscalchi, M.; D’Ingeo, S.; Quaranta, A. The dog nose “KNOWS” fear: Asymmetric nostril use during sniffing at canine and human emotional stimuli. Behav. Brain Res. 2016, 304, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Webber, R.L.; Jeffcoat, M.K.; Harman, J.T.; Ruttimann, U.E. MR Demonstration of the Nasal Cycle in the Beagle Dog. J. Comput. Assist. Tomogr. 1987, 11, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Sobel, N.; Khan, R.M.; Saltman, A.; Sullivan, E.V.; Gabrieli, J.D. Olfaction The world smells different to each nostril. Nature 1999, 402, 35. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Fengyi, L. Sustentacular Cell Enwrapment of Olfactory Receptor Neuronal Dendrites: An Update. Genes 2020, 11, 493. [Google Scholar] [CrossRef]
- Jenkins, E.K.; DeChant, M.T.; Perry, E.B. When the Nose Doesn’t Know: Canine Olfactory Function Associated with Health, Management, and Potential Links to Microbiota. Front. Veter. Sci. 2018, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Sirotin, Y.B.; Shusterman, R.; Rinberg, D. Neural Coding of Perceived Odor Intensity. eNeuro 2015, 2, 0083. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, A.C.; Lindroos, A.K.; Lissner, L.; Torgerson, J.S.; Carlsson, B.; Carlsson, L.M.S.; Sjöström, L. Evidence for gender-specific associations between leptin and olfaction. J. Gender-Specific Med. Off. J. Partnersh. Women’s Health Columbia 2002, 5, 25–32. [Google Scholar]
- Loch, D.; Breer, H.; Strotmann, J. Endocrine Modulation of Olfactory Responsiveness: Effects of the Orexigenic Hormone Ghrelin. Chem. Senses 2015, 40, 469–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavoi, B.M.; Jameela, H. Comparative Morphometry of the Olfactory Bulb, Tract and Stria in the Human, Dog and Goat. Int. J. Morphol. 2011, 29, 939–946. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Pustovyy, O.M.; Waggoner, P.; Beyers, R.J.; Schumacher, J.; Wildey, C.; Barrett, J.; Morrison, E.; Salibi, N.; Denney, T.; et al. Functional MRI of the Olfactory System in Conscious Dogs. PLoS ONE 2014, 9, e86362. [Google Scholar] [CrossRef] [Green Version]
- Swaney, W.; Keverne, E.B. The evolution of pheromonal communication. Behav. Brain Res. 2009, 200, 239–247. [Google Scholar] [CrossRef] [PubMed]
- McGann, J.P. Poor human olfaction is a 19th-century myth. Science 2017, 356, eaam7263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.M.; Trask, B.J. V2R gene families degenerated in primates, dog and cow, but expanded in opossum. Trends Genet. 2007, 23, 212–215. [Google Scholar] [CrossRef]
- Kociánová, I.; Gorošová, A.; Tichý, F.; Čížek, P.; Machálka, M. Structure of Masera’s Septal Olfactory Organ in Cat (Felis silvestris f. catus)—Light Microscopy in Selected Stages of Ontogeny. Acta Vet. Brno 2006, 75, 471–475. [Google Scholar]
- Ma, M.; Fleischer, J.; Breer, H.; Eisthen, H.L. The Septal Organ, Grueneberg Ganglion, and Terminal Nerve. In Handbook of Olfaction and Gustation; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1133–1150. [Google Scholar] [CrossRef]
- Barrios, A.W.; Quinteiro, P.S.; Salazar, I. Dog and mouse: Toward a balanced view of the mammalian olfactory system. Front. Neuroanat. 2014, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Jacquot, L.; Monnin, J.; Brand, G. Influence of nasal trigeminal stimuli on olfactory sensitivity. Comptes Rendus Biol. 2004, 327, 305–311. [Google Scholar] [CrossRef]
- Kobal, G.; Van Toller, S.; Hummel, T. Is there directional smelling? Cell. Mol. Life Sci. 1989, 45, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Tacher, S.; Quignon, P.; Rimbault, M.; Dreano, S.; André, C.; Galibert, F. Olfactory Receptor Sequence Polymorphism within and Between Breeds of Dogs. J. Hered. 2005, 96, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Robin, S.; Tacher, S.; Rimbault, M.; Vaysse, A.; Dréano, S.; André, C.; Hitte, C.; Galibert, F. Genetic diversity of canine olfactory receptors. BMC Genom. 2009, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Hall, N.J.; Smith, D.W.; Wynne, C.D. Pavlovian conditioning enhances resistance to disruption of dogs performing an odor discrimination. J. Exp. Anal. Behav. 2015, 103, 484–497. [Google Scholar] [CrossRef]
- Jezierski, T.; Adamkiewicz, E.; Walczak, M.; Sobczyńska, M.; Górecka-Bruzda, A.; Ensminger, J.; Papet, E. Efficacy of drug detection by fully-trained police dogs varies by breed, training level, type of drug and search environment. Forensic Sci. Int. 2014, 237, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, L.; Rogers, B.; Waggoner, L.P.; Katz, J.S. When the nose knows: Ontogenetic changes in detection dogs’ (Canis familiaris) responsiveness to social and olfactory cues. Anim. Behav. 2019, 153, 61–68. [Google Scholar] [CrossRef]
- Lazarowski, L.; Waggoner, L.P.; Krichbaum, S.; Singletary, M.; Haney, P.S.; Rogers, B.; Angle, C. Selecting Dogs for Explosives Detection: Behavioral Characteristics. Front. Veter. Sci. 2020, 7, 597. [Google Scholar] [CrossRef]
- Polgár, Z.; Kinnunen, M.; Újváry, D.; Miklosi, A.; Gácsi, M. A Test of Canine Olfactory Capacity: Comparing Various Dog Breeds and Wolves in a Natural Detection Task. PLoS ONE 2016, 11, e0154087. [Google Scholar] [CrossRef] [Green Version]
- Hirai, T.; Kojima, S.; Shimada, A.; Umemura, T.; Sakai, M.; Itakurat, C. Age-related changes in the olfactory system of dogs. Neuropathol. Appl. Neurobiol. 1996, 22, 531–539. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, H.; Ma, S.; Guo, D. Sex- and age-related differences in c-fos expression in dog olfactory bulbs. Acta Zool. 2017, 98, 370–376. [Google Scholar] [CrossRef]
- Gutzwiller, K.J. Minimizing Dog-Induced Biases in Game Bird Research. Wildl. Soc. Bull. (1973–2006) 1990, 18, 351–356. [Google Scholar]
- Majumder, S.S.; Bhadra, A. When Love Is in the Air: Understanding Why Dogs Tend to Mate when It Rains. PLoS ONE 2015, 10, e0143501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bräuer, J.; Blasi, D. Dogs display owner-specific expectations based on olfaction. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Shrestha, S.; Kamel, F.; Umbach, D.M.; Freeman, L.E.B.; Koutros, S.; Alavanja, M.; Blair, A.; Sandler, D.P.; Chen, H. High Pesticide Exposure Events and Olfactory Impairment among U.S. Farmers. Environ. Health Perspect. 2019, 127, 017005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, L.J.; Nusbaum, K.E.; Swango, L.J.; Hanrahan, L.N.; Sartin, E. Dysfunction of sense of smell caused by canine parainfluenza virus infection in dogs. Am. J. Veter. Res. 1988, 49, 188–190. [Google Scholar]
- Myers, L.J. Dysosmia of the dog in clinical veterinary medicine. Prog. Vet. Neurol. 1990, 1, 171–179. [Google Scholar]
- Asproni, P.; Cozzi, A.; Mainau, E.; Temple, D.; Manteca, X.; Bienboire-Frosini, C.; Pageat, P. First description of vomeronasal organ inflammatory changes in pigs. In Proceedings of the Annual Congress of the European College of Animal Welfare and Behavioural Medicine (ECAWBM), Apt, France, 19–22 November 2014. [Google Scholar]
- Asproni, P.; Cozzi, A.; Verin, R.; Lafont-Lecuelle, C.; Bienboire-Frosini, C.; Poli, A.; Pageat, P. Pathology and behaviour in feline medicine: Investigating the link between vomeronasalitis and aggression. J. Feline Med. Surg. 2016, 18, 997–1002. [Google Scholar] [CrossRef]
- Henkin, R.I. Drug-induced taste and smell disorders. Incidence, mechanisms and management related primarily to treatment of sensory receptor dysfunction. Drug Saf. 1994, 11, 318–377. [Google Scholar] [CrossRef]
- Keller, M.; Douhard, Q.; Baum, M.J.; Bakker, J. Destruction of the Main Olfactory Epithelium Reduces Female Sexual Behavior and Olfactory Investigation in Female Mice. Chem. Senses 2006, 31, 315–323. [Google Scholar] [CrossRef]
- Ramaihgari, B.; Pustovyy, O.M.; Waggoner, P.; Beyers, R.J.; Wildey, C.; Morrison, E.; Salibi, N.; Katz, J.S.; Denney, T.S.; Vodyanoy, V.J.; et al. Zinc Nanoparticles Enhance Brain Connectivity in the Canine Olfactory Network: Evidence from an fMRI Study in Unrestrained Awake Dogs. Front. Veter. Sci. 2018, 5, 127. [Google Scholar] [CrossRef]
- Essler, J.L.; Smith, P.G.; Berger, D.; Gregorio, E.; Pennington, M.R.; McGuire, A.; Furton, K.G.; Otto, C.M. A Randomized Cross-Over Trial Comparing the Effect of Intramuscular Versus Intranasal Naloxone Reversal of Intravenous Fentanyl on Odor Detection in Working Dogs. Animals 2019, 9, 385. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, E.K.; Lee-Fowler, T.M.; Angle, T.C.; Behrend, E.N.; Moore, G.E. Effects of oral administration of metronidazole and doxycycline on olfactory capabilities of explosives detection dogs. Am. J. Vet. Res. 2016, 77, 906–912. [Google Scholar] [CrossRef]
- Schaefer, M.; Iravani, B.; Arshamian, A.; Lundström, J.N. No Evidence That Hormonal Contraceptives Affect Chemosensory Perception. i-Perception 2021, 12, 2041669520983339. [Google Scholar] [CrossRef]
- Ezeh, P.I.; Myers, L.J.; Hanrahan, L.A.; Kemppainen, R.J.; Cummins, K.A. Effects of steroids on the olfactory function of the dog. Physiol. Behav. 1992, 51, 1183–1187. [Google Scholar] [CrossRef]
- Kepecs, A.; Uchida, N.; Mainen, Z.F. The Sniff as a Unit of Olfactory Processing. Chem. Senses 2005, 31, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, J.; Charbonneau, G.; Collignon, O.; Lepore, F. Odor Localization and Sniffing. Chem. Senses 2008, 34, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Sobel, N.; Prabhakaran, V.T.; Desmond, J.E.; Glover, G.H.; Goode, R.L.; Sullivan, E.V.; Gabrieli, J.D.E. Sniffing and smelling: Separate subsystems in the human olfactory cortex. Nature 1998, 392, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Berns, G.S.; Brooks, A.M.; Spivak, M. Scent of the familiar: An fMRI study of canine brain responses to familiar and unfamiliar human and dog odors. Behav. Process. 2015, 110, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preti, G.; Muetterties, E.L.; Furman, J.M.; Kennelly, J.J.; Johns, B.E. Volatile constituents of dog (Canis familiaris) and coyote (Canis latrans) anal sacs. J. Chem. Ecol. 1976, 2, 177–186. [Google Scholar] [CrossRef]
- Dunbar, I.F. Olfactory preferences in dogs: The response of male and female beagles to conspecific odors. Behav. Biol. 1977, 20, 471–481. [Google Scholar] [CrossRef]
- Horowitz, A. Being a Dog: Following the Dog into a World of Smell; Scribner: New York, NY, USA, 2016. [Google Scholar]
- Gadbois, S.; Reeve, C. Canine Olfaction: Scent, Sign, and Situation. In Domestic Dog Cognistion and Behaviour: The Scientific Study of Canis familiaris; Horowitz, A., Ed.; Springer: Heidelberg/Berlin, Germany, 2014; pp. 3–29. [Google Scholar]
- Hart, B. Environmental and hormonal influences on urine marking behavior in the adult male dog. Behav. Biol. 1974, 11, 167–176. [Google Scholar] [CrossRef]
- McGuire, B.; Bemis, K.E. Scent marking in shelter dogs: Effects of body size. Appl. Anim. Behav. Sci. 2017, 186, 49–55. [Google Scholar] [CrossRef]
- McGuire, B.; Olsen, B.; Bemis, K.E.; Orantes, D. Urine marking in male domestic dogs: Honest or dishonest? J. Zool. 2018, 306, 163–170. [Google Scholar] [CrossRef]
- Harrington, F.H. Double Marking in Arctic Wolves, Canis lupus arctos: Influence of Order on Posture. Can. Field-Nat. 2006, 120, 471–473. [Google Scholar] [CrossRef] [Green Version]
- McGuire, B. Scent marking in shelter dogs: Effects of sex and age. Appl. Anim. Behav. Sci. 2016, 182, 15–22. [Google Scholar] [CrossRef]
- Ferkin, M.H.; Pierce, A. Perspectives on over-marking: Is it good to be on top? J. Ethol. 2007, 25, 107–116. [Google Scholar] [CrossRef]
- Lisberg, A.E.; Snowdon, C.T. Effects of sex, social status and gonadectomy on countermarking by domestic dogs, Canis familiaris. Anim. Behav. 2011, 81, 757–764. [Google Scholar] [CrossRef]
- Kaufmann, C.A.; Forndran, S.; Stauber, C.; Woerner, K.; Gansloßer, U. The Social Behaviour of Neutered Male Dogs Compared to Intact Dogs (Canis lupus familiaris): Video Analyses, Questionnaires and Case Studies. Veter. Med. Open J. 2017, 2, 22–37. [Google Scholar] [CrossRef]
- Cafazzo, S.; Natoli, E.; Valsecchi, P. Scent-Marking Behaviour in a Pack of Free-Ranging Domestic Dogs. Ethology 2012, 118, 955–966. [Google Scholar] [CrossRef]
- McGuire, B.; Fry, K.; Orantes, D.; Underkofler, L.; Parry, S. Sex of Walker Influences Scent-marking Behavior of Shelter Dogs. Animals 2020, 10, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, M.L.; Gunther, M.S.; Wilmers, C.C. The scent of your enemy is my friend? The acquisition of large carnivore scent by a smaller carnivore. J. Ethol. 2016, 35, 13–19. [Google Scholar] [CrossRef]
- Jezierski, T.; Walczak, M.; Górecka, A. Information-seeking behaviour of sniffer dogs during match-to-sample training in the scent lineup. Pol. Psychol. Bull. 2008, 39, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Thesen, A.; Steen, J.B.; Døving, K.B. Behaviour of dogs during olfactory tracking. J. Exp. Biol. 1993, 180, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Hepper, P.G.; Wells, D.L. How Many Footsteps Do Dogs Need to Determine the Direction of an Odour Trail? Chem. Senses 2005, 30, 291–298. [Google Scholar] [CrossRef]
- Taslitz, A.E. Does the Cold Nose Know—The Unscientific Myth of the Dog Scent Lineup. Hastings Law J. 1990, 42, 15. [Google Scholar]
- Beebe, S.C.; Howell, T.J.; Bennett, P.C. Using Scent Detection Dogs in Conservation Settings: A Review of Scientific Literature Regarding Their Selection. Front. Veter. Sci. 2016, 3, 96. [Google Scholar] [CrossRef] [Green Version]
- Jinn, J.; Connor, E.G.; Jacobs, L.F. How Ambient Environment Influences Olfactory Orientation in Search and Rescue Dogs. Chem. Senses 2020, 45, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Cablk, M.E.; Sagebiel, J.C.; Heaton, J.S.; Valentin, C. Olfaction-based Detection Distance: A Quantitative Analysis of How Far Away Dogs Recognize Tortoise Odor and Follow It to Source. Sensors 2008, 8, 2208–2222. [Google Scholar] [CrossRef]
- Gsell, A.; Innes, J.; De Monchy, P.; Brunton, D. The success of using trained dogs to locate sparse rodents in pest-free sanctuaries. Wildl. Res. 2010, 37, 39–46. [Google Scholar] [CrossRef]
- Jezierski, T. Psy w Służbie Policji, Wojska i Ratownictwa. 2011. Available online: http://ph.ptz.icm.edu.pl/wp-content/uploads/2016/12/2-Jezierski.pdf (accessed on 12 June 2021).
- Rolland, R.M.; Hamilton, P.K.; Kraus, S.D.; Davenport, B.; Gillett, R.M.; Wasser, S.K. Faecal sampling using detection dogs to study reproduction and health in North Atlantic right whales (Eubalaena glacialis). J. Cetacean Res. Manag. 2006, 8, 121–125. [Google Scholar]
- Karp, D. Detecting small and cryptic animals by combining thermography and a wildlife detection dog. Sci. Rep. 2020, 10, 5220. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.E.; Bidlack, A.L.; Hurt, A.; Getz, W.M. Detection distance and environmental factors in conservation detection dog surveys. J. Wildl. Manag. 2011, 75, 243–251. [Google Scholar] [CrossRef]
- Dahlgren, D.; Elmore, R.D.; Smith, D.A.; Hurt, A.; Arnett, E.B.; Connelly, J.W. Use of Dogs in Wildlife Research and Management. Wildl. Tech. Man. 2012, 1, 140–153. [Google Scholar]
- Hussein, A.K.; Sullivan, M.; Penderis, J. Effect of brachycephalic, mesaticephalic, and dolichocephalic head conformations on olfactory bulb angle and orientation in dogs as determined by use of in vivo magnetic resonance imaging. Am. J. Veter. Res. 2012, 73, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Meola, S.D. Brachycephalic Airway Syndrome. Top. Companion Anim. Med. 2013, 28, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Hall, N.; Glenn, K.; Smith, D.W.; Wynne, C.D.L. Performance of Pugs, German Shepherds, and Greyhounds (Canis lupus familiaris) on an odor-discrimination task. J. Comp. Psychol. 2015, 129, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Schoon, G. The effect of the ageing of crime scene objects on the results of scent identification line-ups using trained dogs. Forensic Sci. Int. 2005, 147, 43–47. [Google Scholar] [CrossRef]
- Williams, M.; Johnston, J.M. Training and maintaining the performance of dogs (Canis familiaris) on an increasing number of odor discriminations in a controlled setting. Appl. Anim. Behav. Sci. 2002, 78, 55–65. [Google Scholar] [CrossRef]
- Lazarowski, L.; Krichbaum, S.; DeGreeff, L.E.; Simon, A.; Singletary, M.; Angle, C.; Waggoner, L.P. Methodological Considerations in Canine Olfactory Detection Research. Front. Veter. Sci. 2020, 7, 408. [Google Scholar] [CrossRef]
- Rygg, A.D.; Van Valkenburgh, B.; A Craven, B. The Influence of Sniffing on Airflow and Odorant Deposition in the Canine Nasal Cavity. Chem. Senses 2017, 42, 683–698. [Google Scholar] [CrossRef] [Green Version]
- Concha, A.; Mills, D.S.; Feugier, A.; Zulch, H.; Guest, C.; Harris, R.; Pike, T.W. Using sniffing behavior to differentiate true negative from false negative responses in trained scent-detection dogs. Chem. Senses 2014, 39, 749–754. [Google Scholar] [CrossRef] [Green Version]
- Lasa, J.; Browarska-Walczowska, A. Zmysł Węchu, Kryminalistyka, Metody Analityczne; Raport Nr 2/POP; INSTYTUT FIZYKI JĄDROWEJ im. Henryka Niewodniczańskiego: Kraków, Poland, 2003. [Google Scholar]
- Kokocinska-Kusiak, A.; Matalińska, J.; Sacharczuk, M.; Sobczyńska, M.; Góral-Radziszewska, K.; Wileńska, B.; Misicka, A.; Jezierski, T. Can mice be trained to discriminate urine odor of conspecifics with melanoma before clinical symptoms appear? J. Veter. Behav. 2020, 39, 64–76. [Google Scholar] [CrossRef]
- Gazit, I.; Terkel, J. Explosives detection by sniffer dogs following strenuous physical activity. Appl. Anim. Behav. Sci. 2003, 81, 149–161. [Google Scholar] [CrossRef]
- Poling, A.; Weetjens, B.J.; Cox, C.; Beyene, N.; Bach, H.; Sully, A. Teaching Giant African Pouched Rats to Find Landmines: Operant Conditioning with Real Consequences. Behav. Anal. Pract. 2010, 3, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, I. What the Dog’s Nose Knows. J. Mine Action 2001, 5, 108–109. [Google Scholar]
- Phelan, J.M. Chemical Sensing for Buried Landmines—Fundamental Processes Influencing Trace Chemical Detection; DOE Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 2002. [Google Scholar]
- Phelan, J.; Webes, S. Chemical Sensing for Buried Landmines: Fundamental Processes Influencing Trace Chemical Detection. In Mine Detection Dogs: Training Operations and Odour Detection; McLea, I.G., Ed.; Geneva International Centre for Humanitarian Demining (GICHD): Geneva, Switzerland, 2003; pp. 209–286. [Google Scholar]
- Fjellanger, R. The REST Concept. In Mine Detection Dogs Training, Operations and Odour Detection; McLea, I.G., Ed.; Geneva International Centre for Humanitarian Demining (GICHD): Geneva, Switzerland, 2003; pp. 53–105. [Google Scholar]
- Hayter, D. Training dogs to detect tripwires. In Mine Detection Dogs T raining, Operations and Odour Detection; McLea, I.G., Ed.; Geneva International Centre for Humanitarian Demining (GICHD): Geneva, Switzerland, 2003; pp. 109–138. [Google Scholar]
- Bach, H.; McLean, I. Remote Explosive Scent Tracing: Genuine or a Paper Tiger? J. Conv. Weapons Destr. 2003, 7, 24. [Google Scholar]
- Katz, S.R.; Midkiff, C.R. Unconfirmed Canine Accelerant Detection: A Reliability Issue in Court. J. Forensic Sci. 1998, 43, 16142J. [Google Scholar] [CrossRef]
- Kurz, M.E.; Billard, M.; Rettig, M.; Augustiniak, J.; Lange, J.; Larsen, M.; Warrick, R.; Mohns, T.; Bora, R.; Broadus, K. Evaluation of canines for accelerant detection at fire scenes. J. Forensic Sci. 1994, 39, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.E.; Schultz, S.; Griffith, J.; Broadus, K.; Sparks, J.; Dabdoub, G.; Brock, J. Effect of background interference on accelerant detection by canines. J. Forensic Sci. 1996, 41, 868–873. [Google Scholar] [CrossRef]
- Tindall, R.; Lothridge, K. An Evaluation of 42 Accelerant Detection Canine Teams. J. Forensic Sci. 1995, 40, 13825J. [Google Scholar] [CrossRef]
- Arner, L.; Johnson, G.; Skovronek, H. Delineating toxic areas by canine olfaction. J. Hazard. Mater. 1986, 13, 375–381. [Google Scholar] [CrossRef]
- Crook, A. Use of odour detection dogs in residue management programs. Asian-Australas. J. Anim. Sci. 2000, 13, 219. [Google Scholar]
- Adams, G.; Johnson, K. Sleep, work, and the effects of shift work in drug detector dogs Canis familiaris. Appl. Anim. Behav. Sci. 1994, 41, 115–126. [Google Scholar] [CrossRef]
- Lorenzo, N.; Wan, T.; Harper, R.J.; Hsu, Y.-L.; Chow, M.; Rose, S.; Furton, K.G. Laboratory and field experiments used to identify Canis lupus var. familiaris active odor signature chemicals from drugs, explosives, and humans. Anal. Bioanal. Chem. 2003, 376, 1212–1224. [Google Scholar] [CrossRef]
- Rouhi, A.M. Detecting Illegal Substances. Chem. Eng. News Arch. 1997, 75, 24–29. [Google Scholar] [CrossRef]
- Kalmus, H. The discrimination by the nose of the dog of individual human odours and in particular of the odours of twins. Br. J. Anim. Behav. 1955, 3, 25–31. [Google Scholar] [CrossRef]
- Schoon, G.; De Bruin, J. The ability of dogs to recognize and cross-match human odours. Forensic Sci. Int. 1994, 69, 111–118. [Google Scholar] [CrossRef]
- Settle, R.H.; Sommerville, B.A.; McCormick, J.; Broom, D.M. Human scent matching using specially trained dogs. Anim. Behav. 1994, 48, 1443–1448. [Google Scholar] [CrossRef] [Green Version]
- Schoon, G. Scent identification lineups by dogs (Canis familiaris): Experimental design and forensic application. Appl. Anim. Behav. Sci. 1996, 49, 257–267. [Google Scholar] [CrossRef]
- Harvey, L.M.; Harvey, J.W. Reliability of bloodhounds in criminal investigations. J. Forensic Sci. 2003, 48, 2002118. [Google Scholar] [CrossRef]
- Fenton, V. The use of dogs in search, rescue and recovery. J. Wilderness Med. 1992, 3, 292–300. [Google Scholar] [CrossRef]
- Hebard, C. Use of search and rescue dogs. J. Am. Vet. Med. Assoc. 1993, 203, 999–1001. [Google Scholar] [PubMed]
- Lasseter, A.E.; Jacobi, K.P.; Farley, R.; Hensel, L. Cadaver dog and handler team capabilities in the recovery of buried human remains in the southeastern United States. J. Forensic Sci. 2003, 48, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Komar, D. The use of cadaver dogs in locating scattered, scavenged human remains: Preliminary field test results. J. Forensic Sci. 1999, 44, 14474J–408. [Google Scholar] [CrossRef]
- Engeman, R.M.; Rodriquez, D.V.; Linnell, M.A.; Pitzler, M.E. A review of the case histories of the brown tree snakes (Boiga irregularis) located by detector dogs on Guam. Int. Biodeterior. Biodegrad. 1998, 42, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Engeman, R.M.; Vice, D.S.; Rodriguez, D.V.; Gruver, K.S.; Santos, W.S.; Pitzler, M.E. Effectiveness of the detector dogs used for deterring the dispersal of Brown Tree Snakes. Pac. Conserv. Biol. 1998, 4, 256. [Google Scholar] [CrossRef]
- Engeman, R.M.; Vice, D.S.; York, D.; Gruver, K.S. Sustained evaluation of the effectiveness of detector dogs for locating brown tree snakes in cargo outbound from Guam. Int. Biodeterior. Biodegrad. 2002, 49, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Nakash, J.; Osem, Y.; Kehat, M. A suggestion to use dogs for detecting red palm weevil (Rhynchophorus ferrugineus) infestation in date palms in Israel. Phytoparasitica 2000, 28, 153–155. [Google Scholar] [CrossRef]
- Wallner, W.E.; Ellis, T.L. Olfactory Detection of Gypsy Moth Pheromone and Egg Masses by Domestic Canines. Environ. Entomol. 1976, 5, 183–186. [Google Scholar] [CrossRef]
- Brooks, S.E.; Oi, F.M.; Koehler, P.G. Ability of Canine Termite Detectors to Locate Live Termites and Discriminate Them from Non-Termite Material. J. Econ. Entomol. 2003, 96, 1259–1266. [Google Scholar] [CrossRef]
- Culliney, T.; Grace, J. Prospects for the biological control of subterranean termites (Isoptera: Rhinotermitidae), with special reference to Coptotermes formosanus. Bull. Entomol. Res. 2000, 90, 9–21. [Google Scholar] [CrossRef]
- Lewis, V.; Fouche, C.F.; Lemaster, R.L. Evaluation of dog-assisted searches and electronic odor devices for detecting the western subterranean termite. For. Prod. J. 1997, 47, 79–84. [Google Scholar]
- Welch, J.B. A Detector Dog for Screwworms (Diptera: Calliphoridae). J. Econ. Entomol. 1990, 83, 1932–1934. [Google Scholar] [CrossRef] [Green Version]
- Shelby, R.A.; Schrader, K.K.; Tucker, A.; Klesius, P.H.; Myers, L.J. Detection of catfish off-flavour compounds by trained dogs. Aquac. Res. 2004, 35, 888–892. [Google Scholar] [CrossRef]
- Hanson, T.R. Economic Impact of Off-Flavor to the U.S. Catfish Industry. In ACS Symposium Series; American Chemical Society (ACS): Washington, DC, USA, 2003; pp. 13–29. [Google Scholar]
- Kauhanen, E.; Harri, M.; Nevalainen, A.; Nevalainen, T. Validity of detection of microbial growth in buildings by trained dogs. Environ. Int. 2002, 28, 153–157. [Google Scholar] [CrossRef]
- Wasser, S.K.; Davenport, B.; Ramage, E.R.; E Hunt, K.; Parker, M.; Clarke, C.; Stenhouse, G. Scat detection dogs in wildlife research and management: Application to grizzly and black bears in the Yellowhead Ecosystem, Alberta, Canada. Can. J. Zool. 2004, 82, 475–492. [Google Scholar] [CrossRef]
- Kohn, M.H.; Wayne, R.K. Facts from feces revisited. Trends Ecol. Evol. 1997, 12, 223–227. [Google Scholar] [CrossRef]
- Mills, L.; Citta, J.J.; Lair, K.P.; Schwartz, M.K.; Tallmon, D.A. Estimating Animal Abundance Using Noninvasive DNA Sampling: Promise and Pitfalls. Ecol. Appl. 2000, 10, 283–294. [Google Scholar] [CrossRef]
- Wasser, S.K.; Hunt, K.E.; Brown, J.L.; Cooper, K.; Crockett, C.M.; Bechert, U.; Millspaugh, J.J.; Larson, S.; Monfort, S.L. A Generalized Fecal Glucocorticoid Assay for Use in a Diverse Array of Nondomestic Mammalian and Avian Species. Gen. Comp. Endocrinol. 2000, 120, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Akenson, J.J.; Henjum, M.G.; Wertz, T.L.; Craddock, T.J. Use of Dogs and Mark-Recapture Techniques to Estimate American Black Bear Density in Northeastern Oregon. Ursus 2001, 12, 203–209. [Google Scholar]
- Smith, D.A.; Ralls, K.; Hurt, A.; Adams, B.; Parker, M.; Davenport, B.; Smith, M.C.; Maldonado, J. Detection and accuracy rates of dogs trained to find scats of San Joaquin kit foxes (Vulpes macrotis mutica). Anim. Conserv. 2003, 6, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.A.; Ralls, K.; Davenport, B.; Adams, B.; Maldonado, J.E. Canine Assistants for Conservationists. Science 2001, 291, 435. [Google Scholar] [CrossRef] [PubMed]
- Kerley, L.L.; Salkina, G.P. Using Scent-Matching Dogs to Identify Individual Amur Tigers from Scats. J. Wildl. Manag. 2007, 71, 1349–1356. [Google Scholar] [CrossRef]
- Robert, M.; Laporte, P. Field Techniques for Studying Breeding Yellow Rails (Técnicas de Campo para Estudiar a Individuos Reproductivos de Coturnicops Noveboracensis). J. Field Ornithol. 1997, 68, 56–63. [Google Scholar]
- Shute, N. Dogging Rare Geese to Save Them. Natl. Wildl. 1990, 28, 22. [Google Scholar]
- Browne, C.M. The Use of Dogs to Detect New Zealand Reptile Scents: A Thesis Presented in Partial Fulfilment of the Requirements for the Degree of Master of Science in Zoology at Massey University, Palmerston North, New Zealand. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2005. [Google Scholar]
- Colbourne, R. Little spotted kiwi (Apteryx owenii): Recruitment and behaviour of juveniles on Kapiti Island, New Zealand. J. R. Soc. N. Z. 1992, 22, 321–328. [Google Scholar] [CrossRef]
- Dzieciol, M.; Niżański, W.; Ochota, M.; Kozdrowski, R.; Stańczyk, E. Observation on Possibility to Identify by the Stud Dogs the Signs of the Fertile Period in Bitches. J. Anim. Veter. Adv. 2012, 11, 962–967. [Google Scholar] [CrossRef] [Green Version]
- Dzięcioł, M.; Stańczyk, E.; Noszczyk-Nowak, A.; Niżański, W.; Ochota, M.; Kozdrowski, R. Influence of bitches sex pheromones on the heart rate and other chosen parameters of blood flow in stud dogs (Canis familiaris). Res. Veter. Sci. 2012, 93, 1241–1247. [Google Scholar] [CrossRef]
- Dzięcioł, M.; Niżański, W.; Stańczyk, E.; Kozdrowski, R.; Najder-Kozdrowska, L.; Twardoń, J. The influence of antibiotic treatment of bitches in oestrus on their attractiveness to males during mating. Pol. J. Veter. Sci. 2013, 16, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Kiddy, C.A.; Mitchell, D.S.; Bolt, D.J.; Hawk, H.W.; Haney, A.F.; Schomberg, D.W. Detection of Estrus-Related Odors in Cows by Trained Dogs. Biol. Reprod. 1978, 19, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Kiddy, C.; Mitchell, D.; Hawk, H. Estrus-Related Odors in Body Fluids of Dairy Cows. J. Dairy Sci. 1984, 67, 388–391. [Google Scholar] [CrossRef]
- Hawk, H.; Conley, H.; Kiddy, C. Estrus-Related Odors in Milk Detected by Trained Dogs. J. Dairy Sci. 1984, 67, 392–397. [Google Scholar] [CrossRef]
- D’Aniello, B.; Fierro, B.; Scandurra, A.; Pinelli, C.; Aria, M.; Semin, G.R. Sex differences in the behavioral responses of dogs exposed to human chemosignals of fear and happiness. Anim. Cogn. 2021, 24, 299–309. [Google Scholar] [CrossRef]
- Semin, G.R.; Scandurra, A.; Baragli, P.; Lanatà, A.; D’Aniello, B. Inter- and Intra-Species Communication of Emotion: Chemosignals as the Neglected Medium. Animals 2019, 9, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakeman, U.; Eilam, H.; Schild, C.M.; Grinstein, D.; Eshed, Y.; Laster, M.; Fride, E.; Anavi-Goffer, S. Detection of Impending Aggressive Outbursts in Patients with Psychiatric Disorders: Violence Clues from Dogs. Sci. Rep. 2019, 9, 17228. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography–mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angle, C.; Waggoner, L.P.; Ferrando, A.; Haney, P.; Passler, T. Canine Detection of the Volatilome: A Review of Implications for Pathogen and Disease Detection. Front. Veter. Sci. 2016, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.W.; Goldstein, L.H. Can seizure-alert dogs predict seizures? Epilepsy Res. 2011, 97, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Catala, A.; Grandgeorge, M.; Schaff, J.-L.; Cousillas, H.; Hausberger, M.; Cattet, J. Dogs demonstrate the existence of an epileptic seizure odour in humans. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Dominguez-Ortega, L.; Díaz-Gállego, E.; Pozo, F.; García-Armenter, S.C.; Comino, M.S.; Dominguez-Sanchez, E. Narcolepsy and odor: Preliminary report. SEMERGEN Med. De Fam. 2013, 39, e41–e46. [Google Scholar] [CrossRef]
- Wells, D.L.; Lawson, S.W.; Siriwardena, A. Canine Responses to Hypoglycemia in Patients with Type 1 Diabetes. J. Altern. Complement. Med. 2008, 14, 1235–1241. [Google Scholar] [CrossRef]
- Weber, K.S.; Roden, M.; Müssig, K. Do dogs sense hypoglycaemia? Diabet. Med. 2015, 33, 934–938. [Google Scholar] [CrossRef]
- Reeve, C.; Cummings, E.; McLaughlin, E.; Smith, S.; Gadbois, S. An Idiographic Investigation of Diabetic Alert Dogs’ Ability to Learn from a Small Sample of Breath Samples from People with Type 1 Diabetes. Can. J. Diabetes 2020, 44, 37.e1–43.e1. [Google Scholar] [CrossRef] [Green Version]
- Guest, C.; Pinder, M.; Doggett, M.; Squires, C.; Affara, M.; Kandeh, B.; Dewhirst, S.; Morant, S.V.; D’Alessandro, U.; Logan, J.G.; et al. Trained dogs identify people with malaria parasites by their odour. Lancet Infect. Dis. 2019, 19, 578–580. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, M.; Jezierski, T.; Broffman, M.; Hubbard, A.; Turner, K.; Janecki, T. Diagnostic Accuracy of Canine Scent Detection in Early- and Late-Stage Lung and Breast Cancers. Integr. Cancer Ther. 2006, 5, 30–39. [Google Scholar] [CrossRef]
- Ehmann, R.; Boedeker, E.; Friedrich, U.; Sagert, J.; Dippon, J.; Friedel, G.; Walles, T. Canine scent detection in the diagnosis of lung cancer: Revisiting a puzzling phenomenon. Eur. Respir. J. 2012, 39, 669–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jezierski, T.; Walczak, M.; Ligor, T.; Rudnicka, J.; Buszewski, B. Study of the art: Canine olfaction used for cancer detection on the basis of breath odour. Perspectives and limitations. J. Breath Res. 2015, 9, 027001. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Jezierski, T.; Górecka-Bruzda, A.; Sobczyńska, M.; Ensminger, J. Impact of individual training parameters and manner of taking breath odor samples on the reliability of canines as cancer screeners. J. Veter. Behav. 2012, 7, 283–294. [Google Scholar] [CrossRef]
- Amundsen, T.; Sundstrøm, S.; Buvik, T.; Gederaas, O.A.; Haaverstad, R. Can dogs smell lung cancer? First study using exhaled breath and urine screening in unselected patients with suspected lung cancer. Acta Oncol. 2013, 53, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.T.; Schatz, C.B.; Myers, L.J.; Kosty, M.; Gonczy, C.; Kroener, J.; Tran, M.; Kurtzhals, P.; Heath, S.; Koziol, J.A.; et al. The Use of Canines in the Detection of Human Cancers. J. Altern. Complement. Med. 2008, 14, 61–67. [Google Scholar] [CrossRef]
- Cornu, J.-N.; Cancel-Tassin, G.; Ondet, V.; Girardet, C.; Cussenot, O. Olfactory Detection of Prostate Cancer by Dogs Sniffing Urine: A Step Forward in Early Diagnosis. Eur. Urol. 2011, 59, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Elliker, K.R.; A Sommerville, B.; Broom, D.M.; Neal, D.E.; Armstrong, S.; Williams, H.C. Key considerations for the experimental training and evaluation of cancer odour detection dogs: Lessons learnt from a double-blind, controlled trial of prostate cancer detection. BMC Urol. 2014, 14, 22. [Google Scholar] [CrossRef] [Green Version]
- Horvath, G.; Järverud, G.A.K.; Järverud, S.; Horváth, I. Human Ovarian Carcinomas Detected by Specific Odor. Integr. Cancer Ther. 2008, 7, 76–80. [Google Scholar] [CrossRef]
- Willis, C.M.; Church, S.M.; Guest, C.M.; Cook, W.A.; McCarthy, N.; Bransbury, A.J.; Church, M.R.T.; Church, J.C.T. Olfactory detection of human bladder cancer by dogs: Proof of principle study. BMJ 2004, 329, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoda, H.; Kohnoe, S.; Yamazato, T.; Satoh, Y.; Morizono, G.; Shikata, K.; Morita, M.; Watanabe, A.; Kakeji, Y.; Inoue, F.; et al. Colorectal cancer screening with odour material by canine scent detection. Gut 2011, 60, 814–819. [Google Scholar] [CrossRef]
- Williams, H.; Pembroke, A. Sniffer Dogs in the Melanoma Clinic? Lancet 1989, 333, 734. [Google Scholar] [CrossRef]
- Lamote, K.; Janssens, E.; Schillebeeckx, E.; Lapperre, T.S.; De Winter, B.Y.; Van Meerbeeck, J.P. The scent of COVID-19: Viral (semi-)volatiles as fast diagnostic biomarkers? J. Breath Res. 2020, 14, 042001. [Google Scholar] [CrossRef]
- Steppert, C.; Steppert, I.; Sterlacci, W.; Bollinger, T. Rapid detection of SARS-CoV-2 infection by multicapillary column coupled ion mobility spectrometry (MCC-IMS) of breath. A proof of concept study. J. Breath Res. 2021, 5, 027105. [Google Scholar] [CrossRef]
- Jendrny, P.; Schulz, C.; Twele, F.; Meller, S.; Von Köckritz-Blickwede, M.; Osterhaus, A.D.M.E.; Ebbers, J.; Pilchová, V.; Pink, I.; Welte, T.; et al. Scent dog identification of samples from COVID-19 patients—A pilot study. BMC Infect. Dis. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, D.; Sarkis, R.; Tourtier, J.P.; Julien-Lecocq, C.; Benard, A.; Roger, V.; Levesque, E.; Bernes-Luciani, E.; Maestracci, B.; Morvan, P.; et al. Detection dogs as a help in the detection of COVID-19 Can the dog alert on COVID-19 positive persons by sniffing axillary sweat samples? Proof-of-concept study. PLoS ONE 2020, 15, e0243122. [Google Scholar] [CrossRef]
- Mendel, J.; Frank, K.; Edlin, L.; Hall, K.; Webb, D.; Mills, J.; Holness, H.K.; Furton, K.G.; Mills, D. Preliminary accuracy of COVID-19 odor detection by canines and HS-SPME-GC-MS using exhaled breath samples. Forensic Sci. Int. Synerg. 2021, 3, 100155. [Google Scholar] [CrossRef]
- Aksenov, A.A.; Sandrock, C.E.; Zhao, W.; Sankaran, S.; Schivo, M.; Harper, R.; Cardona, C.J.; Xing, Z.; Davis, C.E. Cellular Scent of Influenza Virus Infection. ChemBioChem 2014, 15, 1040–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Qader, A.A.; Lieberman, D.; Avni, Y.S.; Svobodin, N.; Lazarovitch, T.; Sagi, O.; Zeiri, Y. Volatile organic compounds generated by cultures of bacteria and viruses associated with respiratory infections. Biomed. Chromatogr. 2015, 29, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Mashir, A.; Paschke, K.M.; Van Duin, D.; Shrestha, N.K.; Laskowski, D.; Storer, M.; Yen-Lieberman, B.; Gordon, S.M.; Aytekin, M.; A Dweik, R. Effect of the influenza A (H1N1) live attenuated intranasal vaccine on nitric oxide (FE NO) and other volatiles in exhaled breath. J. Breath Res. 2011, 5, 037107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alasaad, S.; Permunian, R.; Gakuya, F.; Mutinda, M.; Soriguer, R.C.; Rossi, L. Sarcoptic-mange detector dogs used to identify infected animals during outbreaks in wildlife. BMC Veter. Res. 2012, 8, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, K.M.; Cotton, S.J.; Sandeman, R.M. The use of detector dogs in the diagnosis of nematode infections in sheep feces. J. Veter. Behav. 2008, 3, 25–31. [Google Scholar] [CrossRef]
- Jezierski, T.; Dzięcioł, M.; Szumny, A.; Niżański, W.; Woszczyło, M.; Pieczewska, B.; Godzińska, E.J. Discrimination of estrus odor in urine by male dogs in different experimental settings. J. Veter. Behav. 2018, 29, 25–30. [Google Scholar] [CrossRef]
- Samuel, L.; Arnesen, C.; Zedrosser, A.; Rosell, F. Fears from the past? The innate ability of dogs to detect predator scents. Anim. Cogn. 2020, 23, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Hirano, Y.; Oosawa, T.; Tonosaki, K. Electroencephalographic olfactometry (EEGO) analysis of odour responses in dogs. Res. Veter. Sci. 2000, 69, 263–265. [Google Scholar] [CrossRef]
- Prichard, A.; Chhibber, R.; King, J.; Athanassiades, K.; Spivak, M.; Berns, G.S. Decoding Odor Mixtures in the Dog Brain: An Awake fMRI Study. Chem. Senses 2020, 45, 833–844. [Google Scholar] [CrossRef]
- Szabó, D.; Czeibert, K.; Kettinger, Á.; Gácsi, M.; Andics, A.; Miklosi, A.; Kubinyi, E. Resting-state fMRI data of awake dogs (Canis familiaris) via group-level independent component analysis reveal multiple, spatially distributed resting-state networks. Sci. Rep. 2019, 9, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Dzięcioł, M. Influence of human putative sex pheromones on brainactivity monitored by rsfMRI. In Proceedings of the Dog Olfactory Conference, Worldwide Online Conference, Wrocław, Poland, 17 November 2020. [Google Scholar]
- Lorig, T.S. Beyond Self-report: Brain Imaging at the Threshold of Odor Perception. Chemosens. Percept. 2012, 5, 46–54. [Google Scholar] [CrossRef]
- Polgár, Z.; Miklósi, Á.; Gácsi, M. Strategies Used by Pet Dogs for Solving Olfaction-Based Problems at Various Distances. PLoS ONE 2015, 10, e0131610. [Google Scholar] [CrossRef] [Green Version]
- Hare, B.; Tomasello, M. Domestic dogs (Canis familiaris) use human and conspecific social cues to locate hidden food. J. Comp. Psychol. 1999, 113, 173–177. [Google Scholar] [CrossRef]
- Szetei, V.; Miklósi, Á.; Topál, J.; Csányi, V. When dogs seem to lose their nose: An investigation on the use of visual and olfactory cues in communicative context between dog and owner. Appl. Anim. Behav. Sci. 2003, 83, 141–152. [Google Scholar] [CrossRef]
- Bomers, M.K.; A Van Agtmael, M.; Luik, H.; Van Veen, M.C.; E Vandenbroucke-Grauls, C.M.J.; Smulders, Y.M. Using a dog’s superior olfactory sensitivity to identify Clostridium difficile in stools and patients: Proof of principle study. BMJ 2012, 345, e7396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Los, E.A.; Ramsey, K.L.; Guttmann-Bauman, I.; Ahmann, A.J. Reliability of Trained Dogs to Alert to Hypoglycemia in Patients with Type 1 Diabetes. J. Diabetes Sci. Technol. 2016, 11, 506–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonder-Frederick, L.A.; Grabman, J.H.; Shepard, J.A. Diabetes Alert Dogs (DADs): An assessment of accuracy and implications. Diabetes Res. Clin. Pract. 2017, 134, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M. Diabetes alert dogs: A narrative critical overview. Clin. Chem. Lab. Med. 2019, 57, 452–458. [Google Scholar] [CrossRef]
- Hall, N.J.; Wynne, C.D. Odor mixture training enhances dogs’ olfactory detection of Home-Made Explosive precursors. Heliyon 2018, 4, e00947. [Google Scholar] [CrossRef] [Green Version]
- Dorman, D.; Foster, M.; Lazarowski, L. Training with Multiple Structurally Related Odorants Fails to Improve Generalization of Ammonium Nitrate Detection in Domesticated Dogs (Canis familiaris). Animals 2021, 11, 213. [Google Scholar] [CrossRef]
- Lazarowski, L.; Dorman, D.C. Explosives detection by military working dogs: Olfactory generalization from components to mixtures. Appl. Anim. Behav. Sci. 2014, 151, 84–93. [Google Scholar] [CrossRef]
- Johnen, D.; Heuwieser, W.; Fischer-Tenhagen, C. Canine scent detection—Fact or fiction? Appl. Anim. Behav. Sci. 2013, 148, 201–208. [Google Scholar] [CrossRef]
- Hayes, J.; McGreevy, P.; Forbes, S.; Laing, G.; Stuetz, R. Critical review of dog detection and the influences of physiology, training, and analytical methodologies. Talanta 2018, 185, 499–512. [Google Scholar] [CrossRef] [PubMed]
- LitJulie, L.; Schweitzer, J.B.; Oberbauer, A.M. Handler beliefs affect scent detection dog outcomes. Anim. Cogn. 2011, 14, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Johnen, D.; Heuwieser, W.; Fischer-Tenhagen, C. An approach to identify bias in scent detection dog testing. Appl. Anim. Behav. Sci. 2017, 189, 1–12. [Google Scholar] [CrossRef]
- Edwards, T.L. Automated Canine Scent-Detection Apparatus: Technical Description and Training Outcomes. Chem. Senses 2019, 44, 449–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackner, K.; Pleil, J. Canine olfaction as an alternative to analytical instruments for disease diagnosis: Understanding ‘dog personality’ to achieve reproducible results. J. Breath Res. 2017, 11, 012001. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokocińska-Kusiak, A.; Woszczyło, M.; Zybala, M.; Maciocha, J.; Barłowska, K.; Dzięcioł, M. Canine Olfaction: Physiology, Behavior, and Possibilities for Practical Applications. Animals 2021, 11, 2463. https://doi.org/10.3390/ani11082463
Kokocińska-Kusiak A, Woszczyło M, Zybala M, Maciocha J, Barłowska K, Dzięcioł M. Canine Olfaction: Physiology, Behavior, and Possibilities for Practical Applications. Animals. 2021; 11(8):2463. https://doi.org/10.3390/ani11082463
Chicago/Turabian StyleKokocińska-Kusiak, Agata, Martyna Woszczyło, Mikołaj Zybala, Julia Maciocha, Katarzyna Barłowska, and Michał Dzięcioł. 2021. "Canine Olfaction: Physiology, Behavior, and Possibilities for Practical Applications" Animals 11, no. 8: 2463. https://doi.org/10.3390/ani11082463





