Temperament Predicts the Quality of Social Interactions in Captive Female Rhesus Macaques (Macaca mulatta)
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Social Buffering and Fitness among Nonhuman-Primates Living in the Wild
1.2. Social Buffering and Welfare among Nonhuman-Primates Living in the Research Environment
1.3. Coping with Stressors—Implications for Research
1.4. Social Housing and the Quality of Social Interactions
2. Materials and Methods
2.1. Subjects and Housing
2.2. Social Introduction Procedure
2.3. Behavioral Data Collection
2.4. BioBehavioral Assessment
2.5. Statistical Analysis
3. Results
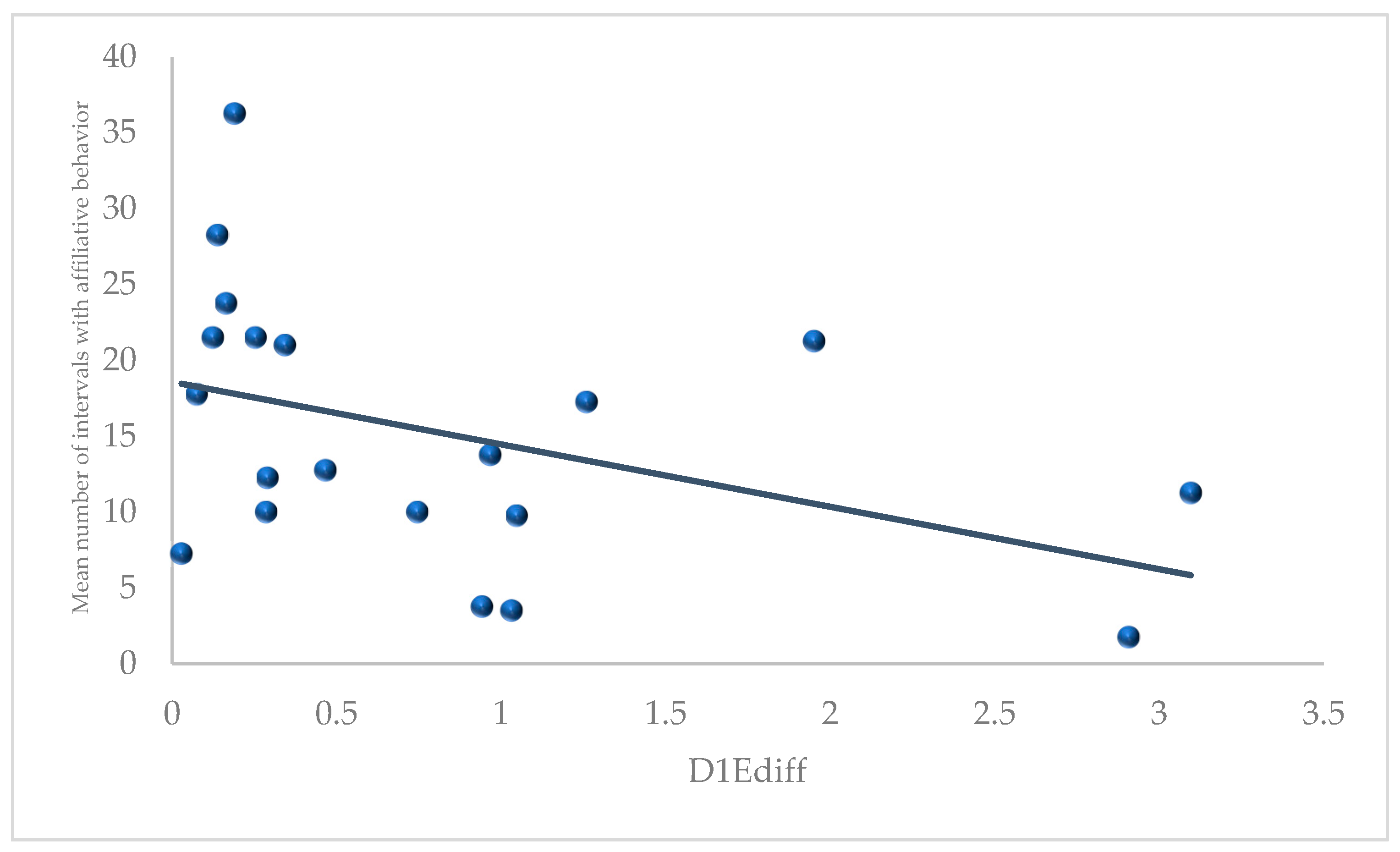
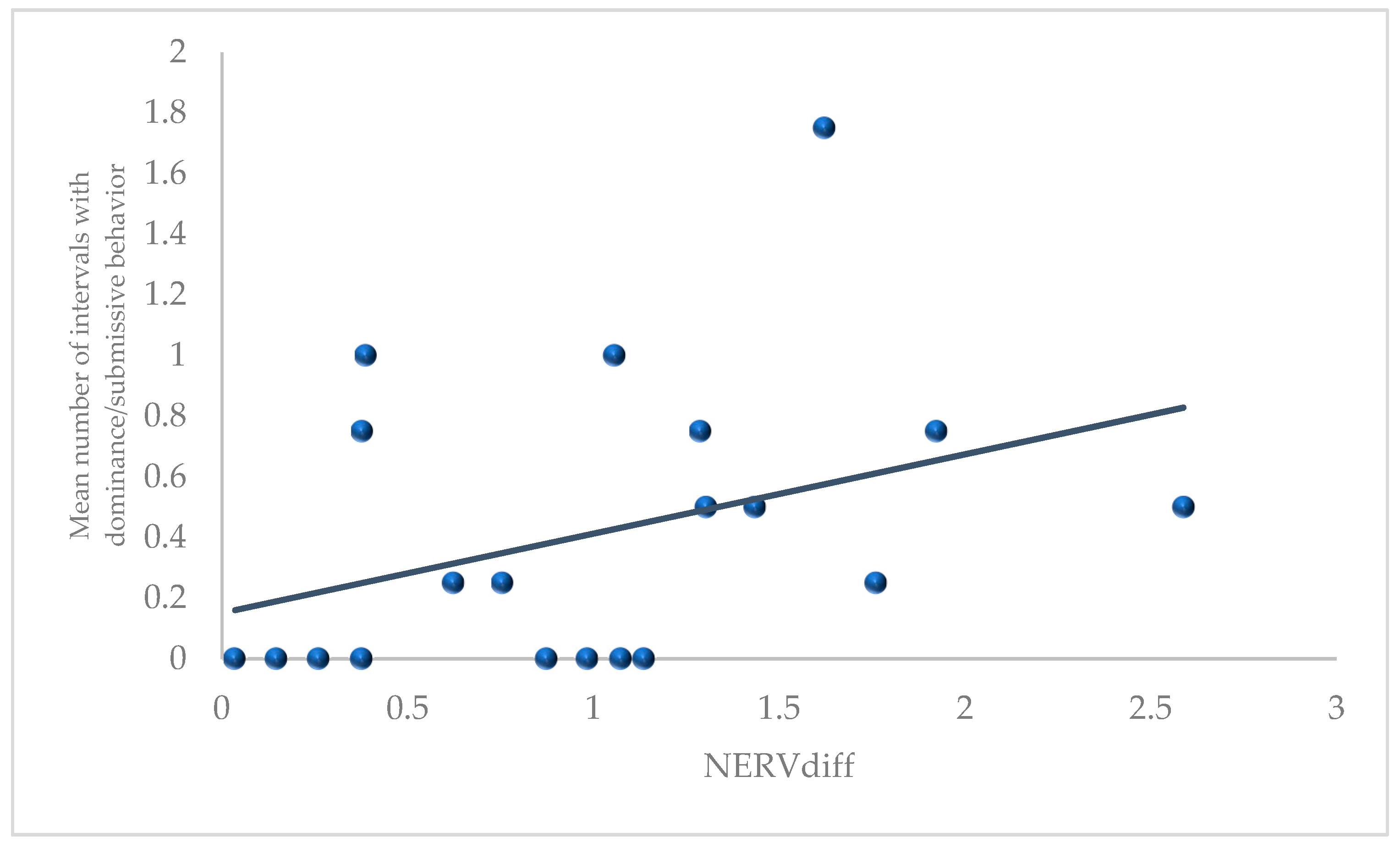
3.1. D1Ediff, HIEdiff, and NERVdiff with Behavioral Measures
3.2. NPmean and NPdiff with Behavioral Measures
4. Discussion
4.1. Using Levels of Behavior as the Response Variable
4.2. Day 1 Emotionality and Affiliative Behavior
4.3. Affiliative Behavior, Coping and Welfare
4.4. NERVdiff and Dominance/Submissive Behaviors
4.5. Social Information Processing and Anxious Behavior
4.6. Implications for Research
4.7. Implications for Welfare
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez, M.M.; McCormack, K.M.; Howell, B.R. Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Soc. Neurosci. 2015, 10, 512–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostner, J.; Schülke, O. Chapter Four—Linking Sociality to Fitness in Primates: A Call for Mechanisms. In Advances in the Study of Behavior; Naguib, M., Barrett, L., Healy, S.D., Podos, J., Simmons, L.W., Zuk, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 50, pp. 127–175. [Google Scholar]
- Campbell, L.A.D.; Tkaczynski, P.J.; Lehmann, J.; Mouna, M.; Majolo, B. Social thermoregulation as a potential mechanism linking sociality and fitness: Barbary macaques with more social partners form larger huddles. Sci. Rep. 2018, 8, 6074. [Google Scholar] [CrossRef]
- Archie, E.A.; Tung, J.; Clark, M.; Altmann, J.; Alberts, S.C. Social affiliation matters: Both same-sex and opposite-sex relationships predict survival in wild female baboons. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141261. [Google Scholar] [CrossRef] [Green Version]
- Brent, L.J.N.; Heilbronner, S.R.; Horvath, J.E.; Gonzalez-Martinez, J.; Ruiz-Lambides, A.; Robinson, A.G.; Skene, J.H.P.; Platt, M.L. Genetic origins of social networks in rhesus macaques. Sci. Rep. 2013, 3, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, N.A. Understanding the links between social ties and fitness over the life cycle in primates. Behaviour 2019, 156, 859–908. [Google Scholar] [CrossRef] [Green Version]
- Pomerantz, O.; Meiri, S.; Terkel, J. Socio-ecological factors correlate with levels of stereotypic behavior in zoo-housed primates. Behav. Process. 2013, 98, 85–91. [Google Scholar] [CrossRef]
- Mason, G.J.; Latham, N.R. Can’t stop, won’t stop: Is stereotypy a reliable animal welfare indicator? Anim. Welf. 2004, 13, S57–S69. [Google Scholar]
- Baker, K.C. Survey of 2014 behavioral management programs for laboratory primates in the United States. Am. J. Primatol. 2016, 78, 780–796. [Google Scholar] [CrossRef] [Green Version]
- USDA. Annual Report Animal Usage by Fiscal Year. 2021. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjd6OvWwb7yAhUhwosBHW7mDhwQFnoECAIQAQ&url=https%3A%2F%2Fwww.aphis.usda.gov%2Fanimal_welfare%2Fannual-reports%2F2019%2Ffy19-summary-report-column-B.pdf&usg=AOvVaw3It_0HYBQsRGj365qBFvC6 (accessed on 23 July 2021).
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Buchanan-Smith, H.M. Environmental enrichment for primates in laboratories. Adv. Sci. Res. 2011, 5, 41–56. [Google Scholar] [CrossRef] [Green Version]
- Novak, M.A.; Hamel, A.F.; Kelly, B.J.; Dettmer, A.M.; Meyer, J.S. Stress, the HPA axis, and nonhuman primate well-being: A review. Appl. Anim. Behav. Sci. 2013, 143, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Clarke, A.S.; Wittwer, D.J.; Abbott, D.H.; Schneider, M.L. Long-term effects of prenatal stress on HPA axis activity in juvenile rhesus monkeys. Dev. Psychobiol. 1994, 27, 257–269. [Google Scholar] [CrossRef]
- Gilbert, M.H.; Baker, K.C. Social buffering in adult male rhesus macaques (Macaca mulatta): Effects of stressful events in single vs. pair housing. J. Med. Primatol. 2011, 40, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wooddell, L.J.; Hamel, A.F.; Murphy, A.M.; Byers, K.L.; Kaburu, S.S.K.; Meyer, J.S.; Suomi, S.J.; Dettmer, A.M. Relationships between affiliative social behavior and hair cortisol concentrations in semi-free ranging rhesus monkeys. Psychoneuroendocrinology 2017, 84, 109–115. [Google Scholar] [CrossRef]
- Winslow, J.T.; Noble, P.L.; Lyons, C.K.; Sterk, S.M.; Insel, T.R. Rearing Effects on Cerebrospinal Fluid Oxytocin Concentration and Social Buffering in Rhesus Monkeys. Neuropsychopharmacology 2003, 28, 910–918. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.P.; Broom, D.M. Measuring zoo animal welfare: Theory and practice. Zoo Biol. 2009, 28, 531–544. [Google Scholar] [CrossRef]
- Wechsler, B. Coping and coping strategies: A behavioural view. Appl. Anim. Behav. Sci. 1995, 43, 123–134. [Google Scholar] [CrossRef]
- Olsson, I.A.S.; Westlund, K. More than numbers matter: The effect of social factors on behaviour and welfare of laboratory rodents and non-human primates. Appl. Anim. Behav. Sci. 2007, 103, 229–254. [Google Scholar] [CrossRef]
- Lilly, A.A.; Mehlman, P.T.; Higley, J.D. Trait-like immunological and hematological measures in female rhesus across varied environmental conditions. Am. J. Primatol. 1999, 48, 197–223. [Google Scholar] [CrossRef]
- Rommeck, I.; Anderson, K.; Heagerty, A.; Cameron, A.; McCowan, B. Risk Factors and Remediation of Self-Injurious and Self-Abuse Behavior in Rhesus Macaques. J. Appl. Anim. Welf. Sci. 2009, 12, 61–72. [Google Scholar] [CrossRef]
- Guerrero-Martin, S.M.; Rubin, L.H.; McGee, K.M.; Shirk, E.N.; Queen, S.E.; Li, M.; Bullock, B.; Carlson, B.W.; Adams, R.J.; Gama, L.; et al. Psychosocial Stress Alters the Immune Response and Results in Higher Viral Load During Acute Simian Immunodeficiency Virus Infection in a Pigtailed Macaque Model of Human Immunodeficiency Virus. J. Infect. Dis. 2021, jiab252. [Google Scholar] [CrossRef]
- Capitanio, J.P.; Cole, S.W. Social instability and immunity in rhesus monkeys: The role of the sympathetic nervous system. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140104. [Google Scholar] [CrossRef] [Green Version]
- Pomerantz, O.; Baker, K.C.; Bellanca, R.U.; Bloomsmith, M.A.; Coleman, K.; Hutchinson, E.K.; Lutz, C.K.; Pierre, P.J.; Weed, J.L. Improving reproducibility—A call to include social housing information in research articles 1involving nonhumanprimates. Am. J. Primatol. 2021, submitted. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- NRC. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; pp. 127–175. [Google Scholar] [CrossRef]
- Baker, K.C.; Weed, J.L.; Crockett, C.M.; Bloomsmith, M.A. Survey of environmental enhancement programs for laboratory primates. Am. J. Primatol. 2007, 69, 377–394. [Google Scholar] [CrossRef] [PubMed]
- USDA. Animal Welfare Regulations. In Title 9—Animals and Animal Products; USDA: Washington, DC, USA, 2017. [Google Scholar]
- Coleman, K. Individual differences in temperament and behavioral management practices for nonhuman primates. Appl. Anim. Behav. Sci. 2012, 137, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Truelove, M.A.; Martin, A.L.; Perlman, J.E.; Wood, J.S.; Bloomsmith, M.A. Pair housing of Macaques: A review of partner selection, introduction techniques, monitoring for compatibility, and methods for long-term maintenance of pairs. Am. J. Primatol. 2017, 79, e22485. [Google Scholar] [CrossRef]
- Robinson, L.M.; Waran, N.K.; Leach, M.C.; Morton, F.B.; Paukner, A.; Lonsdorf, E.; Handel, I.; Wilson, V.A.D.; Brosnan, S.F.; Weiss, A. Happiness is positive welfare in brown capuchins (Sapajus apella). Appl. Anim. Behav. Sci. 2016, 181, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Robinson, L.M.; Altschul, D.M.; Wallace, E.K.; Úbeda, Y.; Llorente, M.; Machanda, Z.; Slocombe, K.E.; Leach, M.C.; Waran, N.K.; Weiss, A. Chimpanzees with positive welfare are happier, extraverted, and emotionally stable. Appl. Anim. Behav. Sci. 2017, 191, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Rault, J.-L.; Hintze, S.; Camerlink, I.; Yee, J.R. Positive Welfare and the Like: Distinct Views and a Proposed Framework. Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Keverne, E.B.; Martensz, N.D.; Tuite, B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology 1989, 14, 155–161. [Google Scholar] [CrossRef]
- Capitanio, J.P.; Blozis, S.A.; Snarr, J.; Steward, A.; McCowan, B.J. Do “birds of a feather flock together” or do “opposites attract”? Behavioral responses and temperament predict success in pairings of rhesus monkeys in a laboratory setting. Am. J. Primatol. 2017, 79, e22464. [Google Scholar] [CrossRef] [Green Version]
- MacAllister, R.P.; Heagerty, A.; Coleman, K. Behavioral predictors of pairing success in rhesus macaques (Macaca mulatta). Am. J. Primatol. 2020, 82, e23081. [Google Scholar] [CrossRef]
- Pomerantz, O.; Baker, K.C. Higher levels of submissive behaviors at the onset of the pairing process of rhesus macaques (Macaca mulatta) are associated with lower risk of wounding following introduction. Am. J. Primatol. 2017, 79. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, V.; Del Rosso, L.A.; Seil, S.K.; Calonder, L.A.; Madrid, J.E.; Bone, K.J.; Sherr, E.H.; Garner, J.P.; Capitanio, J.P.; Parker, K.J. Early Predictors of Impaired Social Functioning in Male Rhesus Macaques (Macaca mulatta). PLoS ONE 2016, 11, e0165401. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, J.P. Variation in biobehavioral organization. In Handbook of Primate Behavioral Management; CRC Press: Boca Raton, FL, USA, 2017; pp. 55–73. [Google Scholar]
- Martin, P.; Bateson, P.P.G. Measuring Behaviour: An Introductory Guide; Cambridge University Press: New York, NY, USA, 2013; pp. 48–60. [Google Scholar]
- Capitanio, J.P. Knowledge of biobehavioral organization can facilitate better science: A review of the BioBehavioral Assessment Program at the California National Primate Research Center. Animals 2021, 11, 2445. [Google Scholar]
- Golub, M.S.; Hogrefe, C.E.; Widaman, K.F.; Capitanio, J.P. Iron deficiency anemia and affective response in rhesus monkey infants. Dev. Psychobiol. 2009, 51, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, D.H.; Capitanio, J.P. Latent variables affecting behavioral response to the human intruder test in infant rhesus macaques (Macaca mulatta). Am. J. Primatol. 2013, 75, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Hannibal, D.L.; Cassidy, L.C.; Vandeleest, J.; Semple, S.; Barnard, A.; Chun, K.; Winkler, S.; McCowan, B. Intermittent pair-housing, pair relationship qualities, and HPA activity in adult female rhesus macaques. Am. J. Primatol. 2018, 80, e22762. [Google Scholar] [CrossRef]
- McMillan, J.; Maier, A.; Tully, L.; Coleman, K. The effects of temperament on pairing success in female rhesus macaques. Am. J. Primatol. 2003, 60 (Suppl. S1), 95. [Google Scholar]
- Weinstein, T.A.R.; Capitanio, J.P. Individual differences in infant temperament predict social relationships of yearling rhesus monkeys, Macaca mulatta. Anim. Behav. 2008, 76, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Morton, F.B.; Weiss, A.; Buchanan-Smith, H.M.; Lee, P.C. Capuchin monkeys with similar personalities have higher-quality relationships independent of age, sex, kinship and rank. Anim. Behav. 2015, 105, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Bassett, L.; Buchanan-Smith, H.M. Effects of predictability on the welfare of captive animals. Appl. Anim. Behav. Sci. 2007, 102, 223–245. [Google Scholar] [CrossRef] [Green Version]
- Pomerantz, O.; Terkel, J. Effects of Positive Reinforcement Training Techniques on the Psychological Welfare of Zoo-Housed Chimpanzees (Pan troglodytes). Am. J. Primatol. 2009, 71, 687–695. [Google Scholar] [CrossRef]
- Kummrow, M. Diagnostic and Therapeutic Guidelines to Abnormal Behavior in Captive Nonhuman Primates. Vet. Clin. Exot. Anim. Pract. 2021, 24, 253–266. [Google Scholar] [CrossRef]
- Mellor, D.J.; Beausoleil, N. Extending the ‘Five Domains’ model for animal welfare assessment to incorporate positive welfare states. Anim. Welf. 2015, 24, 241. [Google Scholar] [CrossRef]
- Edgar, J.L.; Mullan, S.M.; Pritchard, J.C.; McFarlane, U.J.C.; Main, D.C.J. Towards a ‘Good Life’ for Farm Animals: Development of a Resource Tier Framework to Achieve Positive Welfare for Laying Hens. Animals 2013, 3, 584–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarland, R.; Majolo, B. Grooming Coercion and the Post-Conflict Trading of Social Services in Wild Barbary Macaques. PLoS ONE 2011, 6, e26893. [Google Scholar] [CrossRef]
- Bracke, M.B.M.; Hopster, H. Assessing the Importance of Natural Behavior for Animal Welfare. J. Agric. Environ. Ethics 2006, 19, 77–89. [Google Scholar] [CrossRef]
- Wittig, R.M.; Crockford, C.; Lehmann, J.; Whitten, P.L.; Seyfarth, R.M.; Cheney, D.L. Focused grooming networks and stress alleviation in wild female baboons. Horm. Behav. 2008, 54, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Graves, F.C.; Wallen, K.; Maestripieri, D. Opioids and attachment in rhesus macaque (Macaca mulatta) abusive mothers. Behav. Neurosci. 2002, 116, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Basile, B.; Hampton, R.; Chaudry, A.; Murray, E. Presence of a privacy divider increases proximity in pair-housed rhesus monkeys. Animal Welfare. 2007, 16, 37–40. [Google Scholar]
- Cooper, M.A.; Bernstein, I.S. Evaluating dominance styles in Assamese and rhesus macaques. Int. J. Primatol. 2008, 29, 225–243. [Google Scholar] [CrossRef]
- Beisner, B.A.; McCowan, B. Signaling context modulates social function of silent bared-teeth displays in rhesus macaques (Macaca mulatta). Am. J. Primatol. 2014, 76, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendl, M.; Burman, O.H.P.; Parker, R.M.A.; Paul, E.S. Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. Appl. Anim. Behav. Sci. 2009, 118, 161–181. [Google Scholar] [CrossRef]
- Mathews, A.; MacLeod, C. Induced processing biases have causal effects on anxiety. Cogn. Emot. 2002, 16, 331–354. [Google Scholar] [CrossRef]
- Reinhardt, V.; Reinhardt, A. Impact of a privacy panel on the behavior of caged female rhesus monkeys living in pairs. J. Exp. Anim. Sci. 1991, 34, 55–58. [Google Scholar] [PubMed]
- Honess, P.E.; Marin, C.M. Enrichment and aggression in primates. Neurosci. Biobehav. Rev. 2006, 30, 413–436. [Google Scholar] [CrossRef]
- Silk, J.B.; Kappeler, P.M. Sociality in primates. In Comparative Social Evolution; Rubenstein, D.R., Abbot, P., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 253–283. [Google Scholar]
- Schülke, O.; Ostner, J. Chapter 9 Ecological and Social Influences on Sociality. In The Evolution of Primate Societies; Mitani, J.C., Call, J., Kappeler, P.M., Palombit, R.A., Silk, J.B., Eds.; University of Chicago Press: Chicago, IL, USA, 2021; pp. 195–219. [Google Scholar] [CrossRef]
- Silk, J.B. The adaptive value of sociality. In The Evolution of Primate Societies; University of Chicago Press: Chicago, IL, USA, 2012; pp. 552–564. [Google Scholar]
- Maninger, N.; Capitanio, J.P.; Mason, W.A.; Ruys, J.D.; Mendoza, S.P. Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology 2010, 35, 1055–1062. [Google Scholar] [CrossRef] [Green Version]
- Maninger, N.; Hinde, K.; Mendoza, S.P.; Mason, W.A.; Larke, R.H.; Ragen, B.J.; Jarcho, M.R.; Cherry, S.R.; Rowland, D.J.; Ferrer, E.; et al. Pair bond formation leads to a sustained increase in global cerebral glucose metabolism in monogamous male titi monkeys (Callicebus cupreus). Neuroscience 2017, 348, 302–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhardt, V. Common husbandry-related variables in biomedical research with animals. Lab. Anim. 2004, 38, 213–235. [Google Scholar] [CrossRef] [Green Version]
- Garner, J.P. Stereotypies and Other Abnormal Repetitive Behaviors: Potential Impact on Validity, Reliability, and Replicability of Scientific Outcomes. ILAR J. 2005, 46, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Debray, R.; Snyder-Mackler, N.; Kohn, J.N.; Wilson, M.E.; Barreiro, L.B.; Tung, J. Social affiliation predicts mitochondrial DNA copy number in female rhesus macaques. Biol. Lett. 2019, 15, 20180643. [Google Scholar] [CrossRef] [Green Version]
- Tasker, L. Linking welfare and quality of scientific output in cynomolgus macaques (Macaca fascicularis) used for regulatory toxicology. Ph.D. Thesis, School of Natural Sciences, Psychology, University of Stirling, Stirling, UK, 2012. [Google Scholar]
- Grandi, L.C.; Ishida, H. The Physiological Effect of Human Grooming on the Heart Rate and the Heart Rate Variability of Laboratory Non-Human Primates: A Pilot Study in Male Rhesus Monkeys. Front. Vet. Sci. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Friedman, H.; Ator, N.; Haigwood, N.; Newsome, W.; Allan, J.S.; Golos, T.G.; Kordower, J.H.; Shade, R.E.; Goldberg, M.E.; Bailey, M.R. The critical role of nonhuman primates in medical research. Pathog. Immun. 2017, 2, 352. [Google Scholar] [CrossRef] [Green Version]
- Subbaraman, N. The US is boosting funding for research monkeys in the wake of COVID. Nature 2021, 595, 633–634. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, J.P.; Kyes, R.C.; Fairbanks, L.A. Considerations in the Selection and Conditioning of Old World Monkeys for Laboratory Research: Animals from Domestic Sources. ILAR J. 2006, 47, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, M.B.; Mendoza, S.P.; Kaplan, J.N. Behavior and plasma cortisol following brief peer separation in juvenile squirrel monkeys. Am. J. Primatol. 1982, 3, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, R.I.M. The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neurosci. Biobehav. Rev. 2010, 34, 260–268. [Google Scholar] [CrossRef]
- Honess, P.E.; Marin, C.M. Behavioural and physiological aspects of stress and aggression in nonhuman primates. Neurosci. Biobehav. Rev. 2006, 30, 390–412. [Google Scholar] [CrossRef] [PubMed]
- Coleman, K.; Tully, L.A.; McMillan, J.L. Temperament correlates with training success in adult rhesus macaques. Am. J. Primatol. 2005, 65, 63–71. [Google Scholar] [CrossRef] [PubMed]

| Abnormal Behavior | |
|---|---|
| Collapses self-hair plucking, self-injurious behavior, repetitive abnormal behavior, and any abnormal idiosyncrasies. | |
| Aggressive Behavior | |
| Aggressive contact/biting | Physical contact involving biting or biting attempts. Includes “mouth fight” in protected contact. |
| Aggressive contact/no biting | Physical contact without involvement of the mouth (e.g., pushing, pulling, grabbing, and scratching). |
| Threatening | At least one of the following partner-directed gestures: ears flattened against the head, brow retracted, open-mouth stare, head bobbing, slap surface or slap at the partner without making contact, and lunging (high-speed aggressive intention movement toward another animal). |
| Anxious Behavior | |
| Body shuddering | A quick shake of the body. |
| Scratching | Vigorous back-and-forth movement of nails on one’s own body. |
| Yawning | Monkey opens mouth wide, often exposing teeth. The head is usually tossed back, and this is sometimes accompanied by a flexing of the open mouth. |
| Teeth grinding | Clenching teeth with noise from moving jaw. |
| Dominance/Submissive behavior | |
| Displaying | Vigorous shaking, slamming, or bouncing off the cage. |
| Fear grimacing/Silent bared teeth | Grin-like facial expression involving retraction of the lips, exposing teeth. |
| Affiliative Behavior | |
| Co-threatening/solicit co-threat | Alternating threats and glancing at the partner, that may or may not join in the threatening. |
| Grooming | Manipulating, brushing, or licking of fur (or eyes, wounds) of another animal with the mouth and/or both hands. Includes both groomer and animal receiving grooming. |
| Lip-smacking | Bringing the lips together rapidly, resulting in a smacking sound; teeth are covered. Directed at potential partner. |
| Mounting | With or without pelvic thrusting and with or without foot clasp. Includes both mounter and animal being mounted. |
| Playing/play soliciting | Non-aggressive, lively actions performed with another individual with or without direct physical contact (e.g., chasing), without pilo-erection, but with relaxed facial expressions. |
| Rump presenting | A posture involving a stance on all fours with the hind quarters elevated and the tail raised. In some animals the tail may be lifted to the side rather than raised. In some instances, animals may place their heads between their legs. |
| Proximity | Sitting or lying in the same cage on the same level (both on floor of cage or both on perch) and not engaging in aggressive interaction (i.e., threatening or aggressive contact) for at least 5 s. |
| Touch Grate | Animal touching grate, excluding hands, hands in a relaxed position. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomerantz, O.; Capitanio, J.P. Temperament Predicts the Quality of Social Interactions in Captive Female Rhesus Macaques (Macaca mulatta). Animals 2021, 11, 2452. https://doi.org/10.3390/ani11082452
Pomerantz O, Capitanio JP. Temperament Predicts the Quality of Social Interactions in Captive Female Rhesus Macaques (Macaca mulatta). Animals. 2021; 11(8):2452. https://doi.org/10.3390/ani11082452
Chicago/Turabian StylePomerantz, Ori, and John P. Capitanio. 2021. "Temperament Predicts the Quality of Social Interactions in Captive Female Rhesus Macaques (Macaca mulatta)" Animals 11, no. 8: 2452. https://doi.org/10.3390/ani11082452
APA StylePomerantz, O., & Capitanio, J. P. (2021). Temperament Predicts the Quality of Social Interactions in Captive Female Rhesus Macaques (Macaca mulatta). Animals, 11(8), 2452. https://doi.org/10.3390/ani11082452






