Quality of Reporting in Preclinical Urethral Tissue Engineering Studies: A Systematic Review to Assess Adherence to the ARRIVE Guidelines
Abstract
Simple Summary
Abstract
1. Introduction
2. Methodology
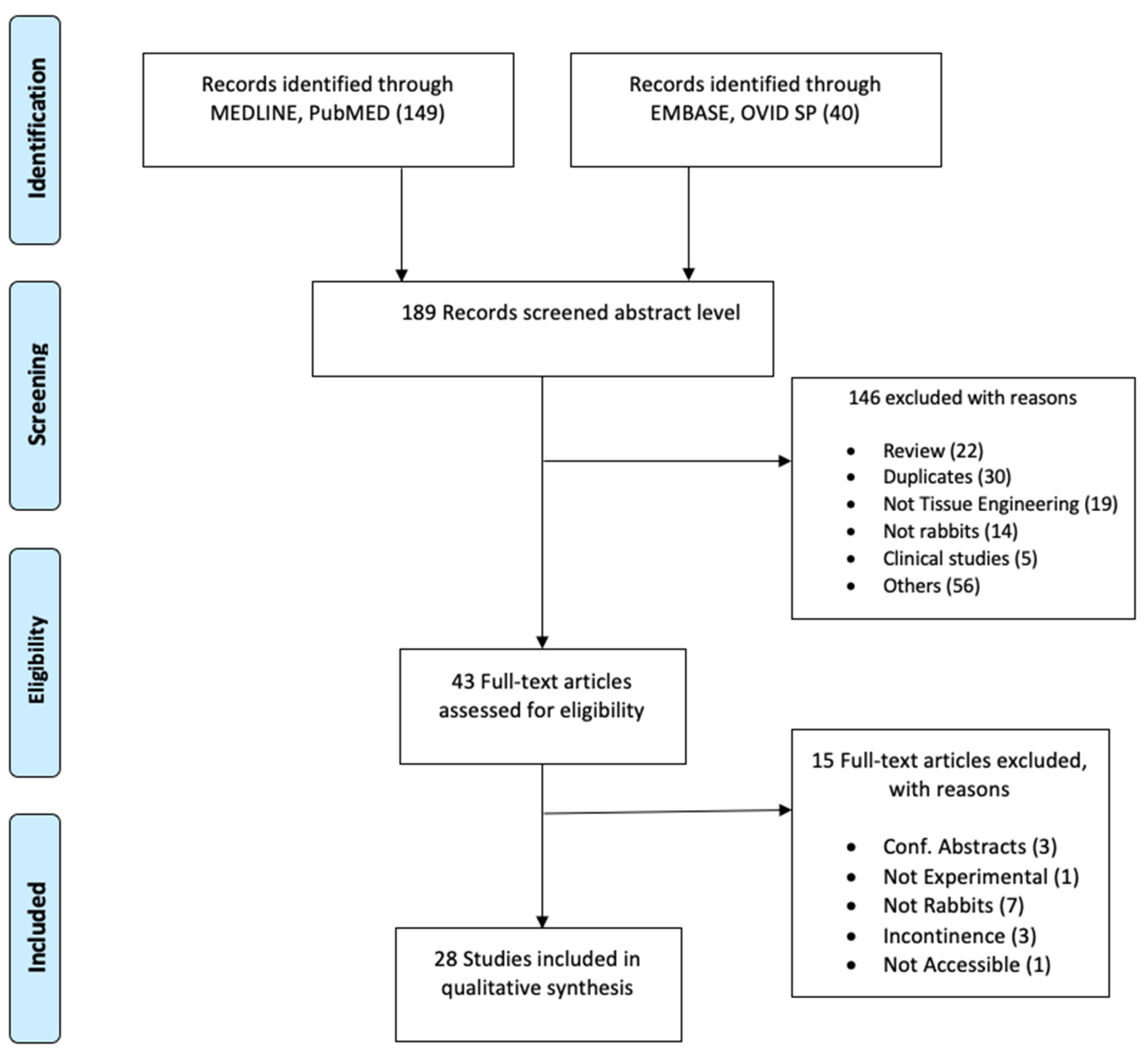
2.1. Literature Search
2.2. Screening
2.3. Data Extraction
2.4. Data Analysis
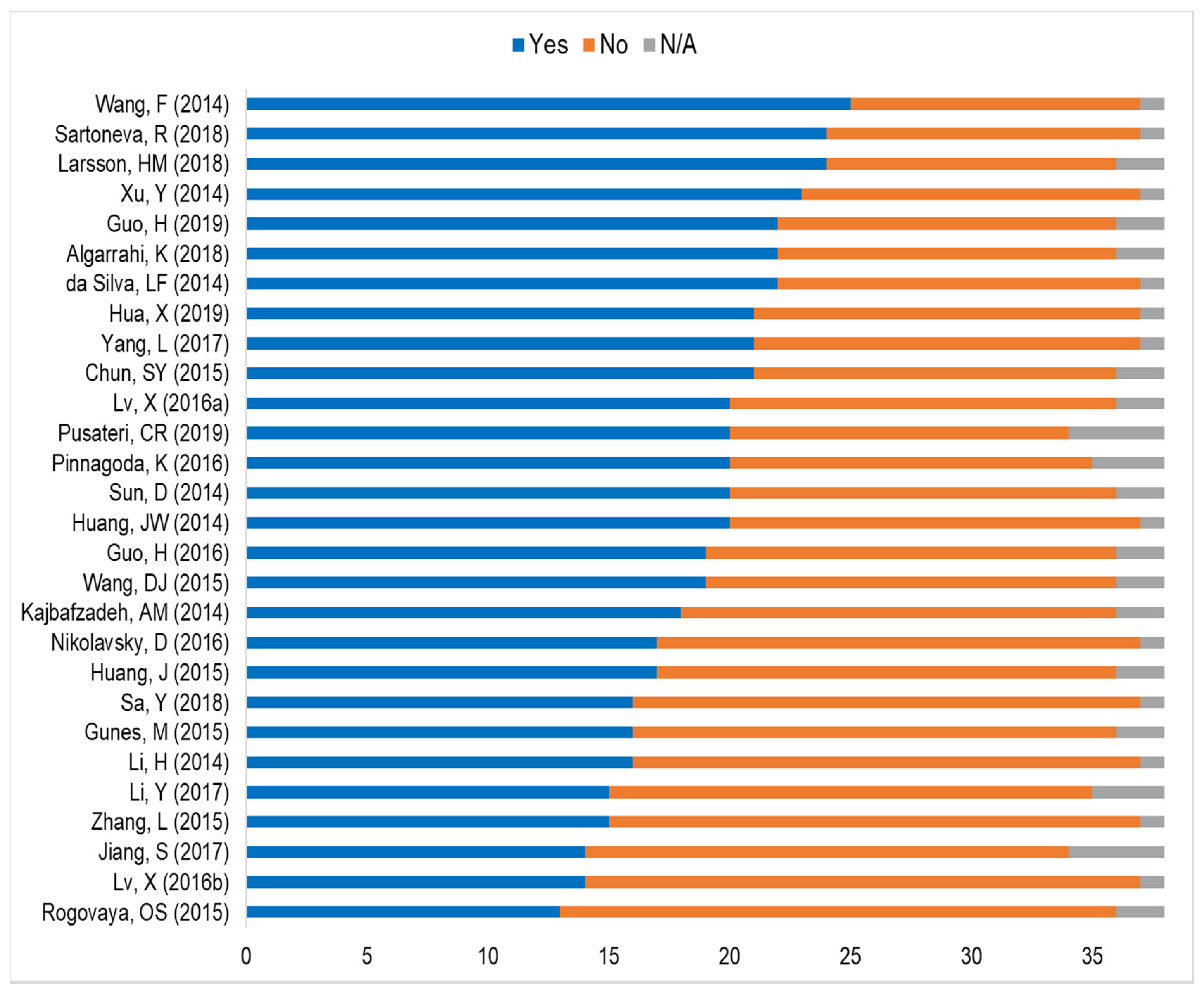
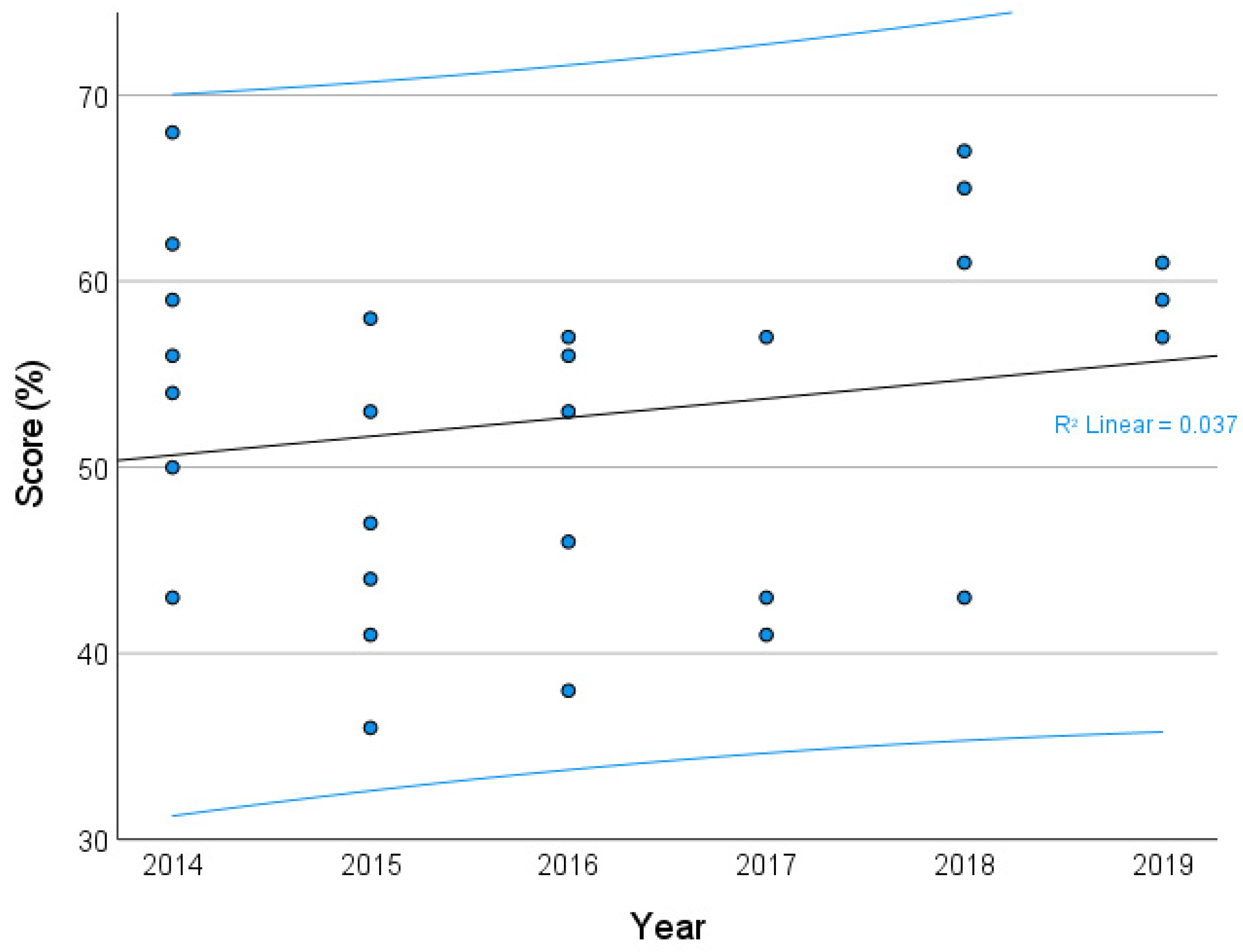
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santucci, R.A.; Joyce, G.F.; Wise, M. Male Urethral Stricture Disease. J. Urol. 2007, 177, 1667–1674. [Google Scholar] [CrossRef]
- Mangir, N.; Wilson, K.J.; Osman, N.I.; Chapple, C.R. Current state of urethral tissue engineering. Curr. Opin. Urol. 2019, 29, 385–393. [Google Scholar] [CrossRef]
- Keays, M.A.; Dave, S. Current hypospadias management: Diagnosis, surgical management, and long-term patient-centred outcomes. Can. Urol. Assoc. J. 2017, 11, S48–S53. [Google Scholar] [CrossRef]
- Chapple, C.; Andrich, D.; Atala, A.; Barbagli, G.; Cavalcanti, A.; Kulkarni, S.; Mangera, A.; Nakajima, Y. SIU/ICUD Consultation on Urethral Strictures: The Management of Anterior Urethral Stricture Disease Using Substitution Urethroplasty. Urology 2014, 83, S31–S47. [Google Scholar] [CrossRef]
- Snodgrass, W.; Bush, N. Primary hypospadias repair techniques: A review of the evidence. Urol. Ann. 2016, 8, 403–408. [Google Scholar] [CrossRef]
- Barbagli, G.; Sansalone, S.; Djinovic, R.; Romano, G.; Lazzeri, M. Current controversies in reconstructive surgery of the anterior urethra: A clinical overview. Int. Braz. J. Urol. 2012, 38, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Mundy, A.R.; Andrich, D.E. Urethral strictures. BJU Int. 2011, 107, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.O.; Mahdi, E.; Hasan, A.; AlAnsari, A.; Pennisi, C.P. Current Status of Tissue Engineering in the Management of Severe Hypospadias. Front. Pediatr. 2018, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.O.; Yalcin, H.C.; Pennisi, C.P. From Acellular Matrices to Smart Polymers: Degradable Scaffolds that are Transforming the Shape of Urethral Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 1763. [Google Scholar] [CrossRef] [PubMed]
- Versteegden, L.R.M.; de Jonge, P.K.J.D.; IntHout, J.; van Kuppevelt, T.H.; Oosterwijk, E.; Feitz, W.F.J.; de Vries, R.B.M.; Daamen, W.F. Tissue Engineering of the Urethra: A Systematic Review and Meta-analysis of Preclinical and Clinical Studies. Eur. Urol. 2017, 72, 594–606. [Google Scholar] [CrossRef]
- Sievert, K.-D. Tissue Engineering of the Urethra: Solid Basic Research and Farsighted Planning are Required for Clinical Application. Eur. Urol. 2017, 72, 607–609. [Google Scholar] [CrossRef]
- Qi, N.; Li, W.; Tian, H. A Systematic Review of Animal and Clinical Studies on the Use of Scaffolds for Urethral Repair. Acta Acad. Med. Wuhan 2016, 36, 111–117. [Google Scholar] [CrossRef]
- Rosito, T.E.; Pires, J.A.S.; Delcelo, R.; Ortiz, V.; Macedo Jr, A. Macroscopic and histological evaluation of tunica vaginalis dorsal grafting in the first stage of Bracka’s urethroplasty: An experimental study in rabbits. BJU Int. 2011, 108, E17–E22. [Google Scholar] [CrossRef] [PubMed]
- Faydacı, G.; Tarhan, F.; Tuncer, M.; Eryıldırım, B.; Celik, O.; Keser, S.H.; Özgül, A. Comparison of Two Experimental Models for Urethral Stricture in the Anterior Urethra of the Male Rabbit. Urology 2012, 80, 225.e7–225.e11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Theodorescu, D.; Balcom, A.; Smith, C.; McLorie, G.; Churchill, B.; Khoury, A. Urethral replacement with vascularized tunica vaginalis: Defining the optimal form of use. J. Urol. 1998, 159, 1708–1711. [Google Scholar] [CrossRef]
- Kemppainen, E.; Talja, M.; Riihelä, M.; Pohjonen, T.; Törmälä, P.; Alfthan, O. A bioresorbable urethral stent. An experimental study. Urol. Res. 1993, 21, 235–238. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8342257 (accessed on 17 February 2021). [CrossRef]
- Bradley, J.E.; Anderson, U.A.; Woolsey, S.M.; Thornbury, K.D.; McHale, N.G.; Hollywood, M.A. Characterization of T-type calcium current and its contribution to electrical activity in rabbit urethra. Am. J. Physiol. Cell Physiol. 2004, 286, C1078–C1088. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F.W. The choice of animal model and reduction. Altern. Lab. Anim. 2004, 32 (Suppl. 2), 59–64. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15601228 (accessed on 17 February 2021). [CrossRef] [PubMed]
- Mignini, L.E.; Khan, K.S. Methodological quality of systematic reviews of animal studies: A survey of reviews of basic research. BMC Med. Res. Methodol. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, D.A.; Hooft, L.; Ter Riet, G. Systematic reviews and meta-analyses of preclinical studies: Publication bias in laboratory animal experiments. Lab. Anim. 2011, 45, 225–230. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O’Collins, V.; Macleod, M.R. Can Animal Models of Disease Reliably Inform Human Studies? PLoS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef]
- Leung, V.; Rousseau-Blass, F.; Beauchamp, G.; Pang, D.S.J. ARRIVE has not ARRIVEd: Support for the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines does not improve the reporting quality of papers in animal welfare, analgesia or anesthesia. PLoS ONE 2018, 13, e0197882. [Google Scholar] [CrossRef] [PubMed]
- Bezdjian, A.; Klis, S.F.L.; Peters, J.P.M.; Grolman, W.; Stegeman, I. Quality of reporting of otorhinolaryngology articles using animal models with the ARRIVE statement. Lab. Anim. 2018, 52, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ting, K.H.J.; Hill, C.L.; Whittle, S.L. Quality of reporting of interventional animal studies in rheumatology: A systematic review using the ARRIVE guidelines. Int. J. Rheum. Dis. 2015, 18, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Altman, D.G.; Avey, M.T.; Baker, M.; Browne, W.; Clark, A.; Cuthill, I.C.; et al. Revision of the ARRIVE guidelines: Rationale and scope. BMJ Open Sci. 2018, 2, e000002. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Vet. Res. 2020, 16, 242. [Google Scholar] [CrossRef]
- Collins, F.S.; Tabak, L.A. Policy: NIH plans to enhance reproducibility. Nature 2014, 505, 612–613. [Google Scholar] [CrossRef]
- Žiaran, S.; Galambošová, M.; Danišovič, L. Tissue engineering of urethra: Systematic review of recent literature. Exp. Biol. Med. 2017, 242, 1772–1785. [Google Scholar] [CrossRef]
- De Kemp, V.; De Graaf, P.; Fledderus, J.O.; Bosch, J.L.H.R.; De Kort, L.M.O. Tissue engineering for human urethral reconstruction: Systematic review of recent literature. PLoS ONE 2015, 10, e0118653. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Huang, J.-W.; Xie, M.-K.; Zhang, Y.; Wei, G.-J.; Li, X.; Li, H.-B.; Wang, J.-H.; Zhu, W.-D.; Li, C.; Xu, Y.-M.; et al. Reconstruction of Penile Urethra With the 3-Dimensional Porous Bladder Acellular Matrix in a Rabbit Model. Urology 2014, 84, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, Y.; Xie, H.; Li, C.; Song, L.; Feng, C.; Zhang, Q.; Xie, M.; Wang, Y.; Lv, X. Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: An animal model. Tissue Eng. Part A 2014, 20, 774–784. [Google Scholar] [CrossRef]
- Wang, F.; Liu, T.; Yang, L.; Zhang, G.; Liu, H.; Yi, X.; Yang, X.; Lin, T.; Qin, W.; Yuan, J. Urethral reconstruction with tissue-engineered human amniotic scaffold in rabbit urethral injury models. Med. Sci. Monit. 2014, 20, 2430–2438. [Google Scholar] [CrossRef] [PubMed]
- Kajbafzadeh, A.-M.; Sabetkish, S.; Tourchi, A.; Amirizadeh, N.; Afshar, K.; Abolghasemi, H.; Elmi, A.; Talab, S.S.; Eshghi, P.; Mohseni, M.J. The application of tissue-engineered preputial matrix and fibrin sealant for urethral reconstruction in rabbit model. Int. Urol. Nephrol. 2014, 46, 1573–1580. [Google Scholar] [CrossRef]
- Sun, D.; Yang, Y.; Wei, Z.; Xu, Y.; Zhang, X.; Hong, B. Engineering of pre-vascularized urethral patch with muscle flaps and hypoxia-activated hUCMSCs improves its therapeutic outcome. J. Cell. Mol. Med. 2014, 18, 434–443. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, D.C.; Wei, Z.T.; Hong, B.F.; Yang, Y. Experimental study on transplantation of autologous minced muscle with human umbilical cord mesenchymal stem cells for urethral reconstruction. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3412–3419. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25491616 (accessed on 17 February 2021).
- Arenas da Silva, L.F.; Micol, L.; Tiemessen, D.; van Kuppevelt, T.H.; Frey, P.; Oosterwijk, E.; Geutjes, P.; Feitz, W.F. Is There a Need for Smooth Muscle Cell Transplantation in Urethral Reconstruction? Tissue Eng. Part A 2014, 20, 1542–1549. [Google Scholar] [CrossRef]
- Chun, S.Y.; Kim, B.S.; Kwon, S.Y.; Park, S.I.; Song, P.H.; Yoo, E.S.; Kim, B.W.; Kwon, T.G.; Kim, H.T. Urethroplasty using autologous urethral tissue-embedded acellular porcine bladder submucosa matrix grafts for the management of long-segment urethral stricture in a rabbit model. J. Korean Med. Sci. 2015, 30, 301–307. [Google Scholar] [CrossRef]
- Guo, H.; Sa, Y.; Huang, J.; Wang, Z.; Wang, L.; Xie, M.; Lv, X. Urethral Reconstruction with Small Intestinal Submucosa Seeded with Oral Keratinocytes and TIMP-1 siRNA Transfected Fibroblasts in a Rabbit Model. Urol. Int. 2016, 96, 223–230. [Google Scholar] [CrossRef]
- Huang, J.-W.; Lv, X.-G.; Li, Z.; Song, L.-J.; Feng, C.; Xie, M.-K.; Li, C.; Li, H.-B.; Wang, J.-H.; Zhu, W.-D.; et al. Urethral reconstruction with a 3D porous bacterial cellulose scaffold seeded with lingual keratinocytes in a rabbit model. Biomed. Mater. 2015, 10, 055005. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, X.; Zhao, W.; Niu, G.; Mo, X.; Fu, Q. Application of Wnt Pathway Inhibitor Delivering Scaffold for Inhibiting Fibrosis in Urethra Strictures: In Vitro and in Vivo Study. Int. J. Mol. Sci. 2015, 16, 27659–27676. [Google Scholar] [CrossRef]
- Wang, D.; Li, M.; Huang, W.; Lu, M.; Hu, C.; Li, K.; Qiu, J.; Gao, X. Repair of urethral defects with polylactid acid fibrous membrane seeded with adipose-derived stem cells in a rabbit model. Connect. Tissue Res. 2015, 56, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Rogovaya, O.S.; Fayzulin, A.K.; Vasiliev, A.V.; Kononov, A.V.; Terskikh, V. V Reconstruction of rabbit urethral epithelium with skin keratinocytes. Acta Naturae 2015, 7, 70–77. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25927003 (accessed on 17 February 2021). [CrossRef]
- Lv, X.; Li, Z.; Chen, S.; Xie, M.; Huang, J.; Peng, X.; Yang, R.; Wang, H.; Xu, Y.; Feng, C. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials 2016, 84, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Guo, Q.; Han, F.; Chen, C.; Ling, C.; Chen, W.; Li, B. Electrospun Poly(l-lactide)/Poly(ethylene glycol) Scaffolds Seeded with Human Amniotic Mesenchymal Stem Cells for Urethral Epithelium Repair. Int. J. Mol. Sci. 2016, 17, 1262. [Google Scholar] [CrossRef] [PubMed]
- Pinnagoda, K.; Larsson, H.M.; Vythilingam, G.; Vardar, E.; Engelhardt, E.-M.; Thambidorai, R.C.; Hubbell, J.A.; Frey, P. Engineered acellular collagen scaffold for endogenous cell guidance, a novel approach in urethral regeneration. Acta Biomater. 2016, 43, 208–217. [Google Scholar] [CrossRef]
- Güneş, M.; Altok, M.; Güneş, A.; Umul, M.; Özyıldız, Z.; Sönmez, T.T.; Orhan, H.; Özmen, Ö. A Novel Approach to Penile Augmentation Urethroplasty Using Buccal Mucosa and Amniotic Membrane: A Pilot Study in a Rabbit Model. Urology 2016, 87, 210–215. [Google Scholar] [CrossRef]
- Nikolavsky, D.; Manwaring, J.; Bratslavsky, G.; Caza, T.; Landas, S.; Hryniewicz-Jankowska, A.; Kotula, L. Novel Concept and Method of Endoscopic Urethral Stricture Treatment Using Liquid Buccal Mucosal Graft. J. Urol. 2016, 196, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, W.; Liu, B.; Wang, Y.; Chu, J.; Xiong, G.; Shen, L.; Long, C.; Lin, T.; He, D.; et al. Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem Cell Res. Ther. 2017, 8, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xu, Z.; Zhao, Y.; Yan, L.; Zhou, Z.; Gu, G. Urethral Reconstruction Using Mesothelial Cell-Seeded Autogenous Granulation Tissue Tube: An Experimental Study in Male Rabbits. Biomed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Larsson, H.M.; Vythilingam, G.; Pinnagoda, K.; Vardar, E.; Engelhardt, E.M.; Sothilingam, S.; Thambidorai, R.C.; Kamarul, T.; Hubbell, J.A.; Frey, P. Fiber density of collagen grafts impacts rabbit urethral regeneration. Sci. Rep. 2018, 8, 10057. [Google Scholar] [CrossRef]
- Algarrahi, K.; Affas, S.; Sack, B.S.; Yang, X.; Costa, K.; Seager, C.; Estrada, C.R.; Mauney, J.R. Repair of injured urethras with silk fibroin scaffolds in a rabbit model of onlay urethroplasty. J. Surg. Res. 2018, 229, 192–199. [Google Scholar] [CrossRef]
- Sa, Y.; Wang, L.; Shu, H.; Gu, J. Post-transcriptional suppression of TIMP-1 in epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts reduces urethral scar formation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Sartoneva, R.; Nordback, P.H.; Haimi, S.; Grijpma, D.W.; Lehto, K.; Rooney, N.; Seppänen-Kaijansinkko, R.; Miettinen, S.; Lahdes-Vasama, T. Comparison of Poly(l-lactide-co-ε-caprolactone) and Poly(trimethylene carbonate) Membranes for Urethral Regeneration: An In Vitro and In Vivo Study. Tissue Eng. Part A 2018, 24, 117–127. [Google Scholar] [CrossRef]
- Pusateri, C.R.; Doudt, A.D.; Gauerke, S.; McCammon, K.; Qin, X.; Ork, B.; Khoury, J.M.; May, A.D.; Zuckerman, J.M. Placental membrane grafts for urethral replacement in a rabbit model: A pilot study. World J. Urol. 2019, 38, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-L.; Jia, Z.-M.; Wang, L.; Bao, X.-Q.; Huang, Y.-C.; Zhou, J.-M.; Xie, H.; Yang, X.-J.; Chen, F. Tubularized urethral reconstruction using a prevascularized capsular tissue prelaminated with buccal mucosa graft in a rabbit model. Asian J. Androl. 2019, 21, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Chen, J.; Xu, Y.; Li, B. Combined Dorsal Plus Ventral Double Tunica Vaginalis Graft Urethroplasty: An Experimental Study in Rabbits. Urology 2019, 126, 209–216. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Feng, F.; Men, C.; Yang, D.; Gao, Z.; Zhu, Z.; Cui, Y.; Zhao, H. A Preclinical Study of Cell-seeded Tubularized Scaffolds Specially Secreting LL37 for Reconstruction of Long Urethral Defects. Anticancer Res. 2017, 37, 4295–4301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Landis, S.C.; Amara, S.G.; Asadullah, K.; Austin, C.P.; Blumenstein, R.; Bradley, E.W.; Crystal, R.G.; Darnell, R.B.; Ferrante, R.J.; Fillit, H.; et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nat. Cell Biol. 2012, 490, 187–191. [Google Scholar] [CrossRef]
- von der Behrens, W. Animal Models of Subjective Tinnitus. Neural Plast. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Prager, E.M.; Bergstrom, H.C.; Grunberg, N.E.; Johnson, L.R. The Importance of Reporting Housing and Husbandry in Rat Research. Front. Behav. Neurosci. 2011, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Kirk, R.G.W. Care in the Cage: Materializing Moral Economies of Animal Care in the Biomedical Sciences, c.1945. In Animal Housing and Human-Animal Relations: Politics, Practices and Infrastructures; Bjørkdahl, K., Druglitrø, T., Eds.; Routledge: Oxon, UK, 2018. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30933457 (accessed on 17 February 2021).
- Abbas, T.O.; Ali, M.; Moog, R. Double-Lumen Valve-Controlled Intra-Operative Pyeloplasty Stent (VIPs): A New Technology for Post-Pyeloplasty Stenting - Proof of Concept Study in a Preclinical Large Animal Model. Res. Rep. Urol. 2020, 12, 61–74. [Google Scholar] [CrossRef]
- Festing, M.F.W.; Altman, D.G. Guidelines for the Design and Statistical Analysis of Experiments Using Laboratory Animals. ILAR J. 2002, 43, 244–258. [Google Scholar] [CrossRef]
- Hirst, J.A.; Howick, J.; Aronson, J.K.; Roberts, N.; Perera, R.; Koshiaris, C.; Heneghan, C. The Need for Randomization in Animal Trials: An Overview of Systematic Reviews. PLoS ONE 2014, 9, e98856. [Google Scholar] [CrossRef]
- The NPQIP Collaborative Group. Did a change in Nature journals’ editorial policy for life sciences research improve reporting? BMJ Open Sci. 2019, 3, e000035. [Google Scholar] [CrossRef]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef]
- Baker, D.; Lidster, K.; Sottomayor, A.; Amor, S. Two Years Later: Journals Are Not Yet Enforcing the ARRIVE Guidelines on Reporting Standards for Pre-Clinical Animal Studies. PLoS Biol. 2014, 12, e1001756. [Google Scholar] [CrossRef]
- Flórez-Vargas, O.; Brass, A.; Karystianis, G.; Bramhall, M.; Stevens, R.; Cruickshank, S.; Nenadic, G. Bias in the reporting of sex and age in biomedical research on mouse models. Elife 2016, 5, e13615. [Google Scholar] [CrossRef]
- Hair, K.; Macleod, M.R.; Sena, E.S. A randomised controlled trial of an Intervention to Improve Compliance with the ARRIVE guidelines (IICARus). Res. Integr. Peer Rev. 2019, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2018, 52, 135–141. [Google Scholar] [CrossRef] [PubMed]
- The EQUATOR Network | Enhancing the QUAlity and Transparency Of Health Research. Available online: https://www.equator-network.org/ (accessed on 17 February 2021).
- Avey, M.T.; Moher, D.; Sullivan, K.J.; Fergusson, D.; Griffin, G.; Grimshaw, J.M.; Hutton, B.; Lalu, M.M.; Macleod, M.; Marshall, J.; et al. The Devil Is in the Details: Incomplete Reporting in Preclinical Animal Research. PLoS ONE 2016, 11, e0166733. [Google Scholar] [CrossRef]

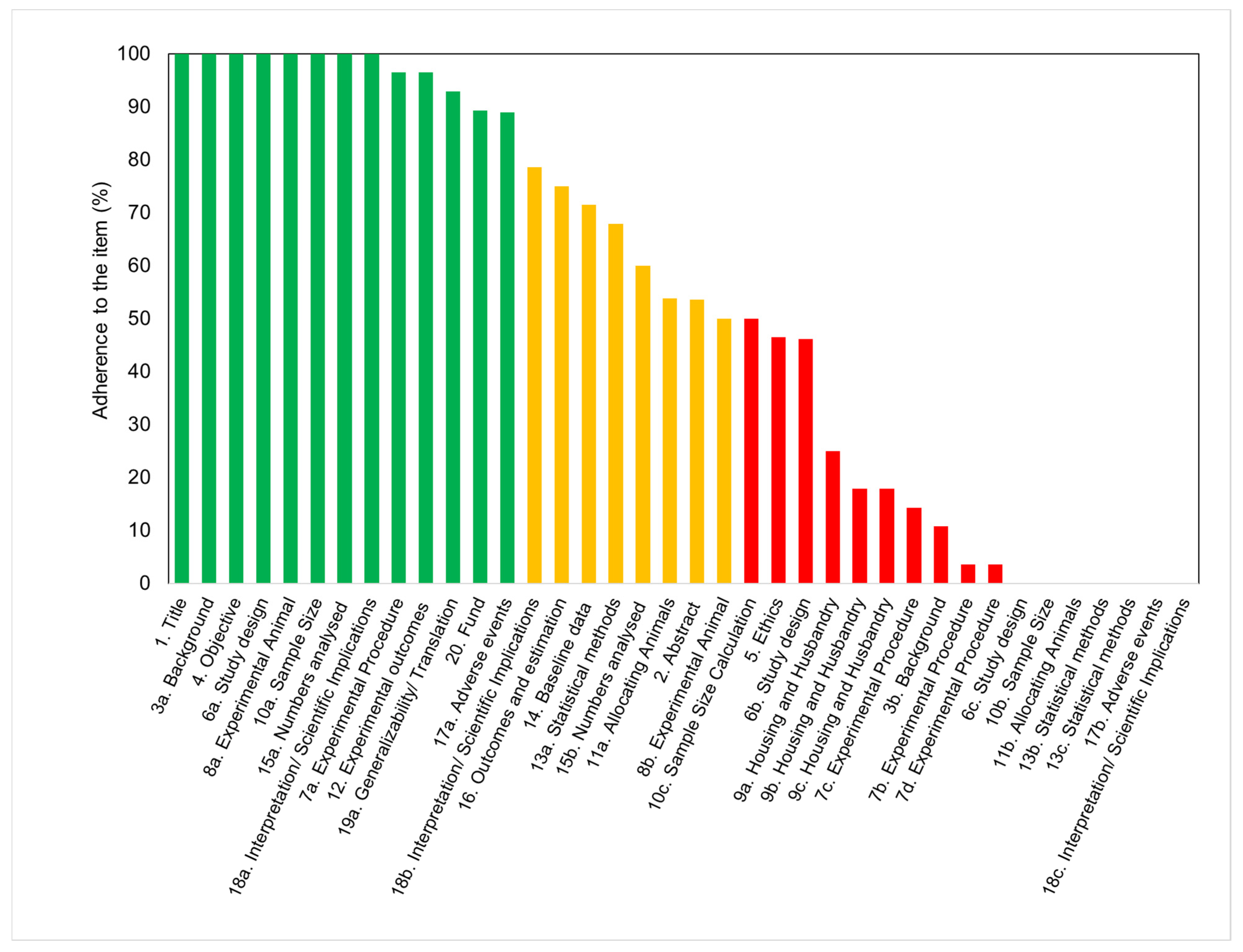
| (a) Item 5: Ethical statements | Ratio | % |
|---|---|---|
| Refers to guidelines | 25/28 | 89 |
| Approved protocol | 26/28 | 92 |
| (b) Item 6b: Steps to minimize subjective bias in the study design | ||
| Random allocation | 13/28 | 46 |
| Blind assessment | 0/28 | 0 |
| (c) Item 7a: Information about experimental procedures | ||
| Surgical procedure | 20/28 | 71 |
| Anesthesia | 22/28 | 79 |
| Post-operative analgesia | 4/28 | 14 |
| Method of euthanasia | 4/28 | 14 |
| (d) Item 8a: Details about animals in the experimental design | ||
| Sex | 28/28 | 100 |
| Weight and age | 4/28 | 14 |
| Weight but not age | 18/28 | 64 |
| Age but not weight | 0/28 | 0 |
| (e) Item 9a: Information about housing conditions | ||
| Type of facility | 5/28 | 18 |
| Type of cage | 3/28 | 11 |
| Bedding material | 1/28 | 4 |
| Number of cage companions | 1/28 | 4 |
| (f) Item 9b: Information about nutritional aspects and environment | ||
| Access to water / food | 3/28 | 11 |
| Breeding program | 2/28 | 7 |
| Temperature/humidity | 2/28 | 7 |
| Light/dark cycles | 2/28 | 7 |
| Environmental enrichment | 0/28 | 0 |
| (g) Item 18b: Disclosure of limitations in the results´ interpretation | ||
| General limitations, including potential sources of bias | 21/28 | 75 |
| Limitations of the animal model | 10/28 | 36 |
| Imprecision | 8/28 | 29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, T.O.; Elawad, A.; Pullattayil S., A.K.; Pennisi, C.P. Quality of Reporting in Preclinical Urethral Tissue Engineering Studies: A Systematic Review to Assess Adherence to the ARRIVE Guidelines. Animals 2021, 11, 2456. https://doi.org/10.3390/ani11082456
Abbas TO, Elawad A, Pullattayil S. AK, Pennisi CP. Quality of Reporting in Preclinical Urethral Tissue Engineering Studies: A Systematic Review to Assess Adherence to the ARRIVE Guidelines. Animals. 2021; 11(8):2456. https://doi.org/10.3390/ani11082456
Chicago/Turabian StyleAbbas, Tariq O., Abubakr Elawad, Abdul Kareem Pullattayil S., and Cristian Pablo Pennisi. 2021. "Quality of Reporting in Preclinical Urethral Tissue Engineering Studies: A Systematic Review to Assess Adherence to the ARRIVE Guidelines" Animals 11, no. 8: 2456. https://doi.org/10.3390/ani11082456
APA StyleAbbas, T. O., Elawad, A., Pullattayil S., A. K., & Pennisi, C. P. (2021). Quality of Reporting in Preclinical Urethral Tissue Engineering Studies: A Systematic Review to Assess Adherence to the ARRIVE Guidelines. Animals, 11(8), 2456. https://doi.org/10.3390/ani11082456







