Effect of Sex and Reproductive Status on Inhibitory Control and Social Cognition in the Domestic Dog (Canis familiaris)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Cognitive Test Battery
2.3. Cylinder Test
2.4. Unsolvable Task
2.5. Data Analysis
3. Results
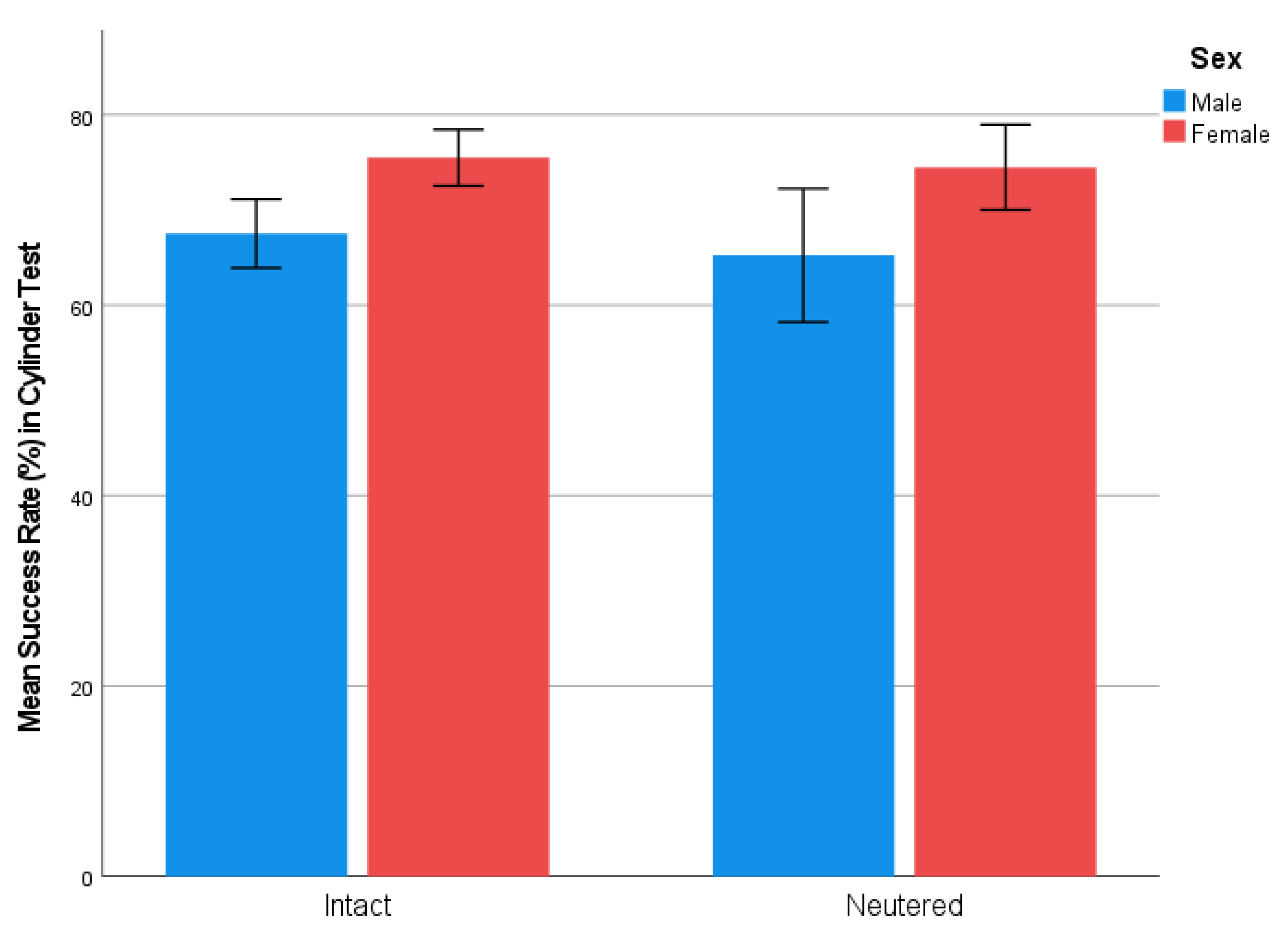
3.1. Cylinder Test
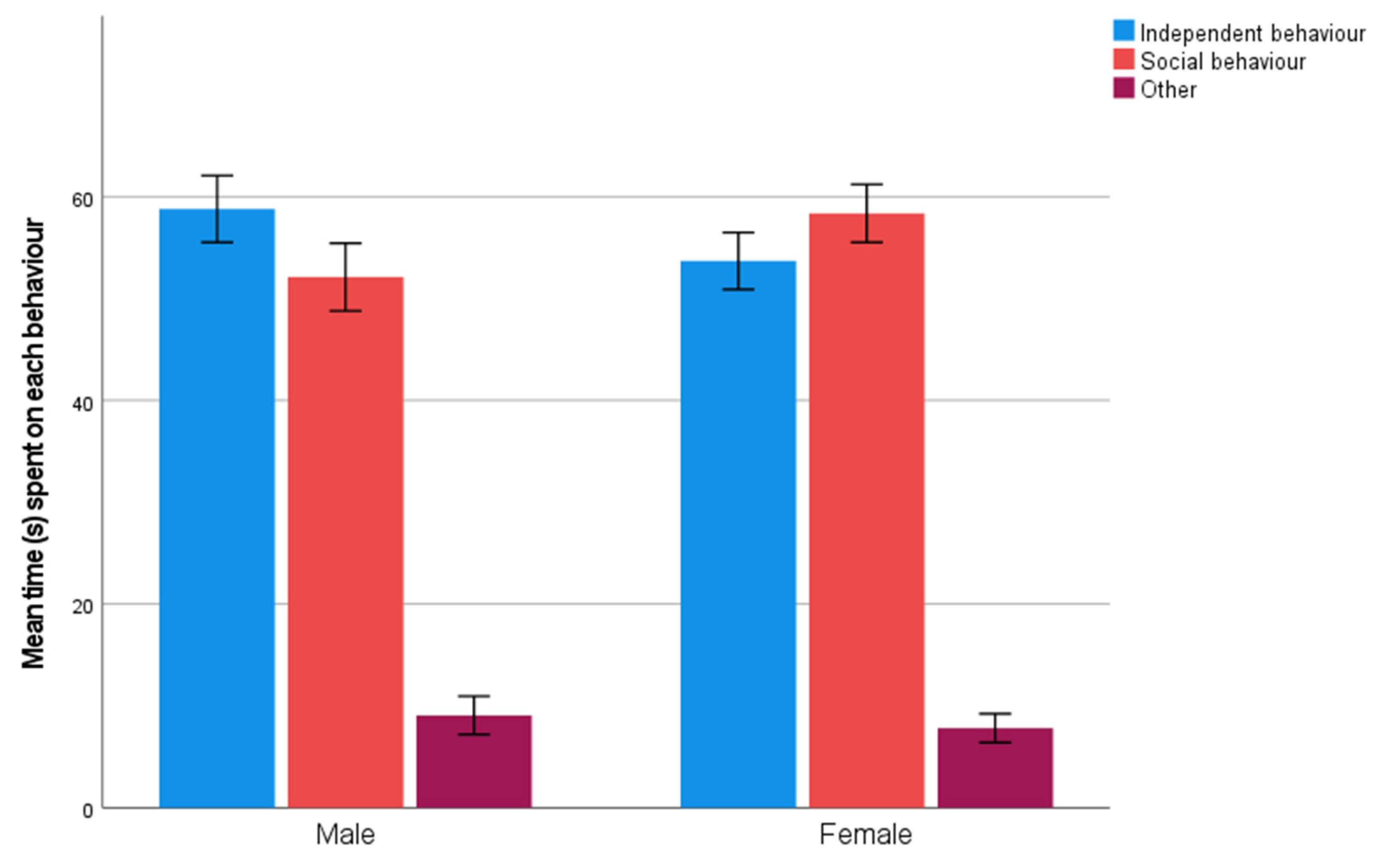
3.2. Unsolvable Task
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Breed | Total Number | Males | Females |
|---|---|---|---|
| Airedale Terrier | 2 | 2 | 0 |
| Akita | 1 | 0 | 1 |
| Alaskan Malamute | 3 | 1 | 2 |
| American Hairless Terrier | 13 | 3 | 10 |
| American Staffordshire Terrier | 3 | 1 | 2 |
| Appenzeller Sennenhund | 3 | 3 | 0 |
| Australian Cattle Dog | 2 | 1 | 1 |
| Australian Kelpie | 24 | 13 | 11 |
| Australian Shepherd | 23 | 12 | 11 |
| Australian Silky Terrier | 1 | 1 | 0 |
| Australian Terrier | 2 | 1 | 1 |
| Auvergne Pointer | 1 | 0 | 1 |
| Barbet | 2 | 2 | 0 |
| Basset Fauve de Bretagne | 1 | 0 | 1 |
| Beagle | 5 | 2 | 3 |
| Bearded Collie | 7 | 3 | 4 |
| Beauceron | 4 | 0 | 4 |
| Belgian Shepherd | 44 | 28 | 16 |
| Bernese Mountain Dog | 8 | 2 | 6 |
| Bichon Frisé | 1 | 0 | 1 |
| Black Russian Terrier | 3 | 2 | 1 |
| Bloodhound | 1 | 1 | 0 |
| Bohemian Shepherd | 4 | 1 | 3 |
| Bolognese | 3 | 1 | 2 |
| Border Collie | 60 | 24 | 36 |
| Border Terrier | 11 | 3 | 9 |
| Boston Terrier | 4 | 2 | 2 |
| Bouvier | 3 | 1 | 2 |
| Boxer | 2 | 0 | 2 |
| Braque du Bourbonnais | 2 | 1 | 1 |
| Broholmer | 2 | 2 | 0 |
| Bull Mastiff | 5 | 2 | 3 |
| Bull Terrier | 1 | 0 | 1 |
| Cairn Terrier | 2 | 0 | 2 |
| Cane Corso | 2 | 2 | 0 |
| Cavalier King Charles Spaniel | 3 | 2 | 1 |
| Ceskoslovenski Vlcak | 2 | 1 | 1 |
| Chesapeake Bay Retriever | 1 | 1 | 0 |
| Chihuahua | 1 | 1 | 0 |
| Chinese Crested Dog | 2 | 0 | 2 |
| Cocker Spaniel | 30 | 14 | 16 |
| Coton de Tulear | 1 | 1 | 0 |
| Croatian Sheepdog | 1 | 0 | 1 |
| Curly-Coated Retriever | 4 | 2 | 2 |
| Dalmatian | 3 | 2 | 1 |
| Danish-Swedish Farmdog | 25 | 17 | 8 |
| Dutch Shepherd | 7 | 2 | 5 |
| East European Shepherd | 2 | 1 | 1 |
| English Springer Spaniel | 6 | 5 | 1 |
| English Toy Terrier | 2 | 1 | 1 |
| Eurasier | 1 | 0 | 1 |
| Field Spaniel | 3 | 1 | 2 |
| Finnish Lapphund | 28 | 17 | 11 |
| Finnish Spitz | 5 | 1 | 4 |
| Flat-Coated Retriever | 28 | 14 | 14 |
| Fox Terrier | 1 | 0 | 1 |
| German Pointer | 4 | 1 | 3 |
| German Shepherd | 41 | 21 | 20 |
| Giant Schnauzer | 18 | 10 | 8 |
| Glen of Imaal Terrier | 1 | 1 | 0 |
| Golden Retriever | 21 | 6 | 15 |
| Great Dane | 2 | 1 | 1 |
| Hovawart | 23 | 11 | 12 |
| Hungarian Vizsla | 5 | 1 | 4 |
| Icelandic Sheepdog | 2 | 1 | 1 |
| Irish Setter | 4 | 4 | 0 |
| Irish Terrier | 3 | 0 | 3 |
| Irish Wolfhound | 2 | 1 | 1 |
| Italian Greyhound | 2 | 0 | 2 |
| Jack Russell Terrier | 9 | 4 | 5 |
| Karelian Bear Dog | 1 | 1 | 0 |
| Keeshond | 1 | 0 | 1 |
| Kerry Blue Terrier | 4 | 2 | 2 |
| Kleinspitz | 3 | 0 | 3 |
| Kooikerhondje | 6 | 4 | 2 |
| Labrador Retriever | 59 | 26 | 33 |
| Lagotto Romagnolo | 8 | 5 | 3 |
| Lancashire Heeler | 5 | 3 | 2 |
| Landseer | 1 | 1 | 0 |
| Lapponian Herder | 17 | 10 | 7 |
| Leonberger | 5 | 3 | 2 |
| Manchester Terrier | 1 | 0 | 1 |
| Medium Poodle | 6 | 3 | 3 |
| Miniature Daschund | 12 | 3 | 9 |
| Miniature Pinscher | 3 | 2 | 1 |
| Miniature Schnauzer | 5 | 2 | 3 |
| Mittelspitz | 9 | 7 | 2 |
| Mixed Breed | 90 | 27 | 63 |
| Mudi | 12 | 8 | 4 |
| Nova Scotia Duck Tolling Retriever | 15 | 4 | 11 |
| Parson Russell Terrier | 7 | 4 | 3 |
| Petit Brabacon | 2 | 2 | 0 |
| Pinscher | 3 | 3 | 0 |
| Portuguese Podengo | 5 | 1 | 4 |
| Portuguese Sheepdog | 1 | 1 | 0 |
| Portuguese Water Dog | 2 | 1 | 1 |
| Pumi | 2 | 2 | 0 |
| Pyrenean Sheepdog | 5 | 2 | 3 |
| Rhodesian Ridgeback | 10 | 2 | 8 |
| Rottweiler | 10 | 3 | 7 |
| Rough Collie | 10 | 5 | 5 |
| Russian Hound | 1 | 0 | 1 |
| Russian Tsvetnaya Bolonka | 1 | 1 | 0 |
| Saluki | 14 | 6 | 8 |
| Samoyed | 1 | 1 | 0 |
| Schapendoes | 3 | 3 | 0 |
| Schipperke | 2 | 0 | 2 |
| Schnauzer | 2 | 1 | 1 |
| Seiskarinkoira | 1 | 0 | 1 |
| Shetland Sheepdog | 16 | 8 | 8 |
| Shikoku | 4 | 2 | 2 |
| Siberian Husky | 1 | 0 | 1 |
| Silken Windhound | 1 | 0 | 1 |
| Small Munsterlander | 1 | 1 | 0 |
| Smooth Collie | 6 | 2 | 4 |
| Spanish Galgo | 2 | 1 | 1 |
| Spanish Water Dog | 29 | 9 | 20 |
| Stabyhoun | 1 | 1 | 0 |
| Staffordshire Bull Terrier | 4 | 2 | 2 |
| Standard Poodle | 7 | 1 | 6 |
| Swedish Vallhund | 19 | 13 | 6 |
| Tibetan Mastiff | 3 | 1 | 2 |
| Tibetan Spaniel | 3 | 0 | 3 |
| Weimaraner | 2 | 1 | 1 |
| Welsh Corgi | 4 | 2 | 2 |
| Welsh Springer Spaniel | 7 | 4 | 3 |
| West Highland White Terrier | 7 | 4 | 3 |
| Wheaten Terrier | 3 | 1 | 2 |
| Whippet | 2 | 1 | 1 |
| White Shepherd | 7 | 4 | 3 |
| Yorkshire Terrier | 1 | 1 | 0 |
| Not reported | 9 | 6 | 3 |
References
- Jacobs, L.F.; Gaulin, S.J.; Sherry, D.F.; Hoffman, G.E. Evolution of spatial cognition: Sex-specific patterns of spatial behavior predict hippocampal size. Proc. Natl. Acad. Sci. USA 1990, 87, 6349–6352. [Google Scholar] [CrossRef]
- Locklear, M.N.; Kritzer, M.F. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm. Behav. 2014, 66, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Bachevalier, J.; Hagger, C. Sex differences in the development of learning abilities in primates. Psychoneuroendocrinology 1991, 16, 177–188. [Google Scholar] [CrossRef]
- Müller, C.A.; Mayer, C.; Dörrenberg, S.; Huber, L.; Range, F. Female but not male dogs respond to a size constancy violation. Biol. Lett. 2011, 7, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Fugazza, C.; Mongillo, P.; Marinelli, L. Sex differences in dogs’ social learning of spatial information. Anim. Cogn. 2017, 20, 789–794. [Google Scholar] [CrossRef]
- Mongillo, P.; Scandurra, A.; D’Aniello, B.; Marinelli, L. Effect of sex and gonadectomy on dogs’ spatial performance. Appl. Anim. Behav. Sci. 2017, 191, 84–89. [Google Scholar] [CrossRef]
- Scandurra, A.; Pinelli, C.; Fierro, B.; Di Cosmo, A.; D’Aniello, B. Multimodal signaling in the visuo-acoustic mismatch paradigm: Similarities between dogs and children in the communicative approach. Anim. Cogn. 2020, 23, 833–841. [Google Scholar] [CrossRef]
- Arnold, A.P. The organizational–activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 2009, 55, 570–578. [Google Scholar] [CrossRef]
- Gnanadesikan, G.E.; Hare, B.; Snyder-Mackler, N.; MacLean, E.L. Estimating the heritability of cognitive traits across dog breeds reveals highly heritable inhibitory control and communication factors. Anim. Cogn. 2020, 23, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Fatjo, J.; Amat, M.; Mariotti, V.M.; de la Torre, J.L.R.; Manteca, X. Analysis of 1040 cases of canine aggression in a referral practice in Spain. J. Vet. Behav. 2007, 2, 158–165. [Google Scholar] [CrossRef]
- Vas, J.; Topál, J.; Péch, É.; Miklósi, Á. Measuring attention deficit and activity in dogs: A new application and validation of a human ADHD questionnaire. Appl. Anim. Behav. Sci. 2007, 103, 105–117. [Google Scholar] [CrossRef]
- Amat, M.; Manteca, X.; Mariotti, V.M.; de la Torre, J.L.R.; Fatjó, J. Aggressive behavior in the English cocker spaniel. J. Vet. Behav. 2009, 4, 111–117. [Google Scholar] [CrossRef]
- Wright, H.F.; Mills, D.S.; Pollux, P.M.J. Behavioural and physiological correlates of impulsivity in the domestic dog (Canis familiaris). Physiol. Behav. 2012, 105, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Piotti, P.; Satchell, L.P.; Lockhart, T.S. Impulsivity and behaviour problems in dogs: A Reinforcement Sensitivity Theory perspective. Behav. Process. 2018, 151, 104–110. [Google Scholar] [CrossRef]
- Winstanley, C.A.; Eagle, D.M.; Robbins, T.W. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clin. Psychol. Rev. 2006, 26, 379–395. [Google Scholar] [CrossRef]
- MacLean, E.L.; Hare, B.; Nunn, C.L.; Addessi, E.; Amici, F.; Anderson, R.C.; Aureli, F.; Baker, J.M.; Bania, A.E.; Barnard, A.M.; et al. The evolution of self-control. Proc. Natl. Acad. Sci. USA 2014, 111, E2140–E2148. [Google Scholar] [CrossRef] [PubMed]
- Bray, E.E.; Bray, E.E.; MacLean, E.L.; MacLean, E.L.; Hare, B.A.; Hare, B.A. Context specificity of inhibitory control in dogs. Anim. Cogn. 2014, 17, 15–31. [Google Scholar] [CrossRef]
- Marshall-Pescini, S.; Virányi, Z.; Range, F. The Effect of Domestication on Inhibitory Control: Wolves and Dogs Compared. PLoS ONE 2015, 10, e0118469. [Google Scholar] [CrossRef]
- Fagnani, J.; Barrera, G.; Carballo, F.; Bentosela, M. Is previous experience important for inhibitory control? A comparison between shelter and pet dogs in A-not-B and cylinder tasks. Anim. Cogn. 2016, 19, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Vernouillet, A.A.A.; Stiles, L.R.; Andrew McCausland, J.; Kelly, D.M. Individual performance across motoric self-regulation tasks are not correlated for pet dogs. Learn. Behav. 2018, 46, 522–536. [Google Scholar] [CrossRef]
- Kabadayi, C.; Bobrowicz, K.; Osvath, M. The detour paradigm in animal cognition. Anim. Cogn. 2018, 21, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Krichbaum, S.; Lazarowski, L.; Smith, J.G.; Cox, E.; Katz, J.S. Assessing Inhibitory Control in Dogs Is No Easy Task: Factors to Consider, Canine Science Forum, 6–9 July 2021; Mills, D.S., Matos, R., Jacinto, D., Eds.; PsiAnimal: Lisboa, Portugal, 2021; pp. 164–165. [Google Scholar]
- Tiira, K.; Tikkanen, A.; Vainio, O. Inhibitory control—Important trait for explosive detection performance in police dogs? Appl. Anim. Behav. Sci. 2020, 224, 104942. [Google Scholar] [CrossRef]
- Weafer, J.; de Wit, H. Sex differences in impulsive action and impulsive choice. Addict. Behav. 2014, 39, 1573–1579. [Google Scholar] [CrossRef]
- Gaillard, A.; Fehring, D.J.; Rossell, S.L. A systematic review and meta-analysis of behavioural sex differences in executive control. Eur. J. Neurosci. 2021, 53, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Mongillo, P.; Scandurra, A.; Eatherington, C.J.; D’Aniello, B.; Marinelli, L. Development of a Spatial Discount Task to Measure Impulsive Choices in Dogs. Animals 2019, 9, 469. [Google Scholar] [CrossRef]
- Lazarowski, L.; Krichbaum, S.; Waggoner, L.P.; Katz, J.S. The development of problem-solving abilities in a population of candidate detection dogs (Canis familiaris). Anim. Cogn. 2020, 23, 755–768. [Google Scholar] [CrossRef]
- Fadel, F.R.; Driscoll, P.; Pilot, M.; Wright, H.; Zulch, H.; Mills, D. Differences in Trait Impulsivity Indicate Diversification of Dog Breeds into Working and Show Lines. Sci. Rep. 2016, 6, 22162. [Google Scholar] [CrossRef]
- Salonen, M.; Sulkama, S.; Mikkola, S.; Puurunen, J.; Hakanen, E.; Tiira, K.; Araujo, C.; Lohi, H. Prevalence, comorbidity, and breed differences in canine anxiety in 13,700 Finnish pet dogs. Sci. Rep. 2020, 10, 2962. [Google Scholar] [CrossRef] [PubMed]
- Nave, G.; Nadler, A.; Zava, D.; Camerer, C. Single-Dose Testosterone Administration Impairs Cognitive Reflection in Men. Psychol. Sci. 2017, 28, 1398–1407. [Google Scholar] [CrossRef]
- Jentsch, J.D.; Taylor, J.R. Sex-related differences in spatial divided attention and motor impulsivity in rats. Behav. Neurosci. 2003, 117, 76–83. [Google Scholar] [CrossRef]
- Swalve, N.; Smethells, J.R.; Carroll, M.E. Progesterone attenuates impulsive action in a Go/No-Go task for sucrose pellets in female and male rats. Horm. Behav. 2016, 85, 43–47. [Google Scholar] [CrossRef]
- Hernandez, C.M.; Orsini, C.; Wheeler, A.; Ten Eyck, T.W.; Betzhold, S.M.; Labiste, C.C.; Wright, N.G.; Setlow, B.; Bizon, J.L. Testicular hormones mediate robust sex differences in impulsive choice in rats. eLife 2020, 9, e58604. [Google Scholar] [CrossRef]
- Mendes, J.W.W.; Resende, B.; Savalli, C. A review of the unsolvable task in dog communication and cognition: Comparing different methodologies. Anim. Cogn. 2021, 24, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Miklósi, Á.; Kubinyi, E.; Topál, J.; Gácsi, M.; Virányi, Z.; Csányi, V. A Simple Reason for a Big Difference: Wolves Do Not Look Back at Humans, but Dogs Do. Curr. Biol. 2003, 13, 763–766. [Google Scholar] [CrossRef]
- Marshall-Pescini, S.; Rao, A.; Virányi, Z.; Range, F. The role of domestication and experience in ‘looking back’ towards humans in an unsolvable task. Sci. Rep. 2017, 7, 46636. [Google Scholar] [CrossRef] [PubMed]
- Carballo, F.; Cavalli, C.; Martínez, M.; Dzik, V.; Bentosela, M. Asking for help: Do dogs take into account prior experiences with people? Learn. Behav. 2020, 48, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, C.; Carballo, F.; Dzik, M.V.; Bentosela, M. Gazing as a help requesting behavior: A comparison of dogs participating in animal-assisted interventions and pet dogs. Anim. Cogn. 2020, 23, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Konno, A.; Romero, T.; Inoue-Murayama, M.; Saito, A.; Hasegawa, T. Dog Breed Differences in Visual Communication with Humans. PLoS ONE 2016, 11, e0164760. [Google Scholar] [CrossRef]
- Hori, Y.; Kishi, H.; Inoue-Murayama, M.; Fujita, K. Dopamine receptor D4 gene (DRD4) is associated with gazing toward humans in domestic dogs (Canis familiaris). Open J. Anim. Sci. 2013, 2013. [Google Scholar] [CrossRef]
- Persson, M.E.; Roth, L.S.V.; Johnsson, M.; Wright, D.; Jensen, P. Human-directed social behaviour in dogs shows significant heritability. Genes Brain Behav. 2015, 14, 337–344. [Google Scholar] [CrossRef]
- Carballo, F.; Cavalli, C.M.; Gácsi, M.; Miklósi, Á.; Kubinyi, E. Assistance and Therapy Dogs Are Better Problem Solvers Than Both Trained and Untrained Family Dogs. Front. Vet. Sci. 2020, 7, 164. [Google Scholar] [CrossRef]
- Lore, R.K.; Eisenberg, F.B. Avoidance reactions of domestic dogs to unfamiliar male and female humans in a kennel setting. Appl. Anim. Behav. Sci. 1986, 15, 261–266. [Google Scholar] [CrossRef]
- Foyer, P.; Wilsson, E.; Wright, D.; Jensen, P. Early experiences modulate stress coping in a population of German shepherd dogs. Appl. Anim. Behav. Sci. 2013, 146, 79–87. [Google Scholar] [CrossRef]
- Nagasawa, M.; Mitsui, S.; En, S.; Ohtani, N.; Ohta, M.; Sakuma, Y.; Onaka, T.; Mogi, K.; Kikusui, T. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science 2015, 348, 333–336. [Google Scholar] [CrossRef]
- Duranton, C.; Bedossa, T.; Gaunet, F. When facing an unfamiliar person, pet dogs present social referencing based on their owners’ direction of movement alone. Anim. Behav. 2016, 113, 147–156. [Google Scholar] [CrossRef]
- Mongillo, P.; Pitteri, E.; Candaten, M.; Marinelli, L. Can attention be taught? Interspecific attention by dogs (Canis familiaris) performing obedience tasks. Appl. Anim. Behav. Sci. 2016, 182, 30–37. [Google Scholar] [CrossRef]
- D’Aniello, B.; Scandurra, A. Ontogenetic effects on gazing behaviour: A case study of kennel dogs (Labrador Retrievers) in the impossible task paradigm. Anim. Cogn. 2016, 19, 565–570. [Google Scholar] [CrossRef]
- Scandurra, A.; Alterisio, A.; Di Cosmo, A.; D’Ambrosio, A.; D’Aniello, B. Ovariectomy Impairs Socio-Cognitive Functions in Dogs. Animals 2019, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- SmartDOG Oy. Available online: https://www.smartdog.fi/english (accessed on 28 June 2021).
- Passalacqua, C.; Marshall-Pescini, S.; Barnard, S.; Lakatos, G.; Valsecchi, P.; Prato Previde, E. Human-directed gazing behaviour in puppies and adult dogs, Canis lupus familiaris. Anim. Behav. 2011, 82, 1043–1050. [Google Scholar] [CrossRef]
- Marshall-Pescini, S.; Colombo, E.; Passalacqua, C.; Merola, I.; Prato-Previde, E. Gaze alternation in dogs and toddlers in an unsolvable task: Evidence of an audience effect. Anim. Cogn. 2013, 16, 933–943. [Google Scholar] [CrossRef]
- Orsini, C.A.; Setlow, B. Sex differences in animal models of decision making. J. Neurosci. Res. 2017, 95, 260–269. [Google Scholar] [CrossRef]
- Hosseini-Kamkar, N.; Morton, J.B. Sex differences in self-regulation: An evolutionary perspective. Front. Neurosci. 2014, 8, 233. [Google Scholar] [CrossRef]
- Duckworth, A.L.; Kern, M.L. A meta-analysis of the convergent validity of self-control measures. J. Res. Personal. 2011, 45, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Mayse, J.D.; Nelson, G.M.; Park, P.; Gallagher, M.; Lin, S. Proactive and reactive inhibitory control in rats. Front. Neurosci 2014, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Brucks, D.; Marshall-Pescini, S.; Wallis, L.J.; Huber, L.; Range, F. Measures of Dogs’ Inhibitory Control Abilities Do Not Correlate across Tasks. Front. Psychol. 2017, 8, 849. [Google Scholar] [CrossRef] [PubMed]
- Foyer, P.; Bjällerhag, N.; Wilsson, E.; Jensen, P. Behaviour and experiences of dogs during the first year of life predict the outcome in a later temperament test. Appl. Anim. Behav. Sci. 2014, 155, 93–100. [Google Scholar] [CrossRef]
- Proverbio, A.M. Sex differences in the social brain and in social cognition. J. Neurosci. Res. 2021, 1–9. [Google Scholar] [CrossRef]
- Paukner, A.; Anderson, J.R.; Fogassi, L.; Ferrari, P.F. Do facial gestures, visibility or speed of movement influence gaze following responses in pigtail macaques? Primates 2007, 48, 241–244. [Google Scholar] [CrossRef]
- Archer, J. Sex Differences in Social Behavior: Are the Social Role and Evolutionary Explanations Compatible? Am. Psychol. 1996, 51, 909–917. [Google Scholar] [CrossRef]
- Lazzaroni, M.; Marshall-Pescini, S.; Manzenreiter, H.; Gosch, S.; Přibilová, L.; Darc, L.; McGetrick, J.; Range, F. Why do dogs look back at the human in an impossible task? Looking back behaviour may be over-interpreted. Anim. Cogn. 2020, 23, 427–441. [Google Scholar] [CrossRef]
- Rao, A.; Bernasconi, L.; Lazzaroni, M.; Marshall-Pescini, S.; Range, F. Differences in persistence between dogs and wolves in an unsolvable task in the absence of humans. PeerJ 2018, 6, e5944. [Google Scholar] [CrossRef] [PubMed]
- MacLean, E.L.; Hare, B. Enhanced Selection of Assistance and Explosive Detection Dogs Using Cognitive Measures. Front. Vet. Sci. 2018, 5, 236. [Google Scholar] [CrossRef]
- Lazarowski, L.; Strassberg, L.R.; Waggoner, L.P.; Katz, J.S. Persistence and human-directed behavior in detection dogs: Ontogenetic development and relationships to working dog success. Appl. Anim. Behav. Sci. 2019, 220, 104860. [Google Scholar] [CrossRef]
- Sundman, A.; Persson, M.E.; Grozelier, A.; Halldén, L.; Jensen, P.; Roth, L.S.V. Understanding of human referential gestures is not correlated to human-directed social behaviour in Labrador retrievers and German shepherd dogs. Appl. Anim. Behav. Sci. 2018, 201, 46–53. [Google Scholar] [CrossRef]
- Kustritz, M.V.R. Early spay-neuter: Clinical considerations. Clin. Tech. Small Anim. Pract. 2002, 17, 124–128. [Google Scholar] [CrossRef]
- Farhoody, P.; Mallawaarachchi, I.; Tarwater, P.M.; Serpell, J.A.; Duffy, D.L.; Zink, C. Aggression toward Familiar People, Strangers, and Conspecifics in Gonadectomized and Intact Dogs. Front. Vet. Sci. 2018, 5, 18. [Google Scholar] [CrossRef]
- Starling, M.; Fawcett, A.; Wilson, B.; Serpell, J.; McGreevy, P. Behavioural risks in female dogs with minimal lifetime exposure to gonadal hormones. PLoS ONE 2019, 14, e0223709. [Google Scholar] [CrossRef]
- Wright, H.F.; Mills, D.S.; Pollux, P.M.J. Development and Validation of a Psychometric Tool for Assessing Impulsivity in the Domestic Dog (Canis familiaris). Int. J. Comp. Psychol. 2011, 24, 210–225. [Google Scholar] [CrossRef]
- Protopopova, A.; Hall, N.J.; Wynne, C.D.L. Association between increased behavioral persistence and stereotypy in the pet dog. Behav. Process. 2014, 106, 77–81. [Google Scholar] [CrossRef]
- Vanderstichel, R.; Forzán, M.J.; Pérez, G.E.; Serpell, J.A.; Garde, E. Changes in blood testosterone concentrations after surgical and chemical sterilization of male free-roaming dogs in southern Chile. Theriogenology 2015, 83, 1021–1027. [Google Scholar] [CrossRef]
- Frank, L.A.; Mullins, R.; Rohrbach, B.W. Variability of estradiol concentration in normal dogs. Vet. Dermatol. 2010, 21, 490–493. [Google Scholar] [CrossRef]
- Thun, R.; Eggenberger, E.; Zerobin, K. 24-hour profiles of plasma cortisol and testosterone in the male dog: Absence of circadian rhythmicity, seasonal influence and hormonal inter-relationships. Reprod. Domest. Anim. 1990, 25, 68–77. [Google Scholar] [CrossRef]
- Svartberg, K. A comparison of behaviour in test and in everyday life: Evidence of three consistent boldness-related personality traits in dogs. Appl. Anim. Behav. Sci. 2005, 91, 103–128. [Google Scholar] [CrossRef]
- Starling, M.J.; Branson, N.; Thomson, P.C.; McGreevy, P.D. Age, sex and reproductive status affect boldness in dogs. Vet. J. 2013, 197, 868–872. [Google Scholar] [CrossRef]
- Scandurra, A.; Alterisio, A.; Di Cosmo, A.; D’Aniello, B. Behavioral and Perceptual Differences between Sexes in Dogs: An Overview. Animals 2018, 8, 151. [Google Scholar] [CrossRef]
- Svartberg, K. Shyness–boldness predicts performance in working dogs. Appl. Anim. Behav. Sci. 2002, 79, 157–174. [Google Scholar] [CrossRef]
- Guillette, L.M.; Hahn, A.H.; Hoeschele, M.; Przyslupski, A.; Sturdy, C.B. Individual differences in learning speed, performance accuracy and exploratory behaviour in black-capped chickadees. Anim. Cogn. 2015, 18, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Mazza, V.; Eccard, J.A.; Zaccaroni, M.; Jacob, J.; Dammhahn, M. The fast and the flexible: Cognitive style drives individual variation in cognition in a small mammal. Anim. Behav. 2018, 137, 119–132. [Google Scholar] [CrossRef]
- Daniel, D.K.; Bhat, A. Bolder and Brighter? Exploring Correlations Between Personality and Cognitive Abilities Among Individuals Within a Population of Wild Zebrafish, Danio rerio. Front. Behav. Neurosci. 2020, 14, 138. [Google Scholar] [CrossRef]
- Passalacqua, C.; Marshall-Pescini, S.; Merola, I.; Palestrini, C.; Previde, E.P. Different problem-solving strategies in dogs diagnosed with anxiety-related disorders and control dogs in an unsolvable task paradigm. Appl. Anim. Behav. Sci. 2013, 147, 139–148. [Google Scholar] [CrossRef]
- Overall, K.L.; Dunham, A.E.; Scheifele, P.; Sonstrom Malowski, K. Fear of noises affects canine problem solving behavior and locomotion in standardized cognitive tests. Appl. Anim. Behav. Sci. 2019, 221, 104863. [Google Scholar] [CrossRef]
- Wells, D.L.; Hepper, P.G. Male and female dogs respond differently to men and women. Appl. Anim. Behav. Sci. 1999, 61, 341–349. [Google Scholar] [CrossRef]



| Sex and Reproductive Status | No. of Dogs with Reproductive Status Known | No. of Dogs with Sex Known | Mean Age |
|---|---|---|---|
| Intact dogs | 392 | 3.7 | |
| Neutered dogs | 155 | 5.3 | |
| Males | 255 | 483 | 4.1 |
| Intact | 198 | ||
| Neutered | 61 | ||
| Females | 292 | 549 | 4.2 |
| Intact | 198 | ||
| Neutered | 94 | ||
| All dogs | 547 | 1032 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junttila, S.; Huohvanainen, S.; Tiira, K. Effect of Sex and Reproductive Status on Inhibitory Control and Social Cognition in the Domestic Dog (Canis familiaris). Animals 2021, 11, 2448. https://doi.org/10.3390/ani11082448
Junttila S, Huohvanainen S, Tiira K. Effect of Sex and Reproductive Status on Inhibitory Control and Social Cognition in the Domestic Dog (Canis familiaris). Animals. 2021; 11(8):2448. https://doi.org/10.3390/ani11082448
Chicago/Turabian StyleJunttila, Saara, Salla Huohvanainen, and Katriina Tiira. 2021. "Effect of Sex and Reproductive Status on Inhibitory Control and Social Cognition in the Domestic Dog (Canis familiaris)" Animals 11, no. 8: 2448. https://doi.org/10.3390/ani11082448
APA StyleJunttila, S., Huohvanainen, S., & Tiira, K. (2021). Effect of Sex and Reproductive Status on Inhibitory Control and Social Cognition in the Domestic Dog (Canis familiaris). Animals, 11(8), 2448. https://doi.org/10.3390/ani11082448






