Annual Recurrences of Viral Hemorrhagic Septicemia Epizootics in Age 0 Pacific Herring Clupea pallasii Valenciennes, 1847
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
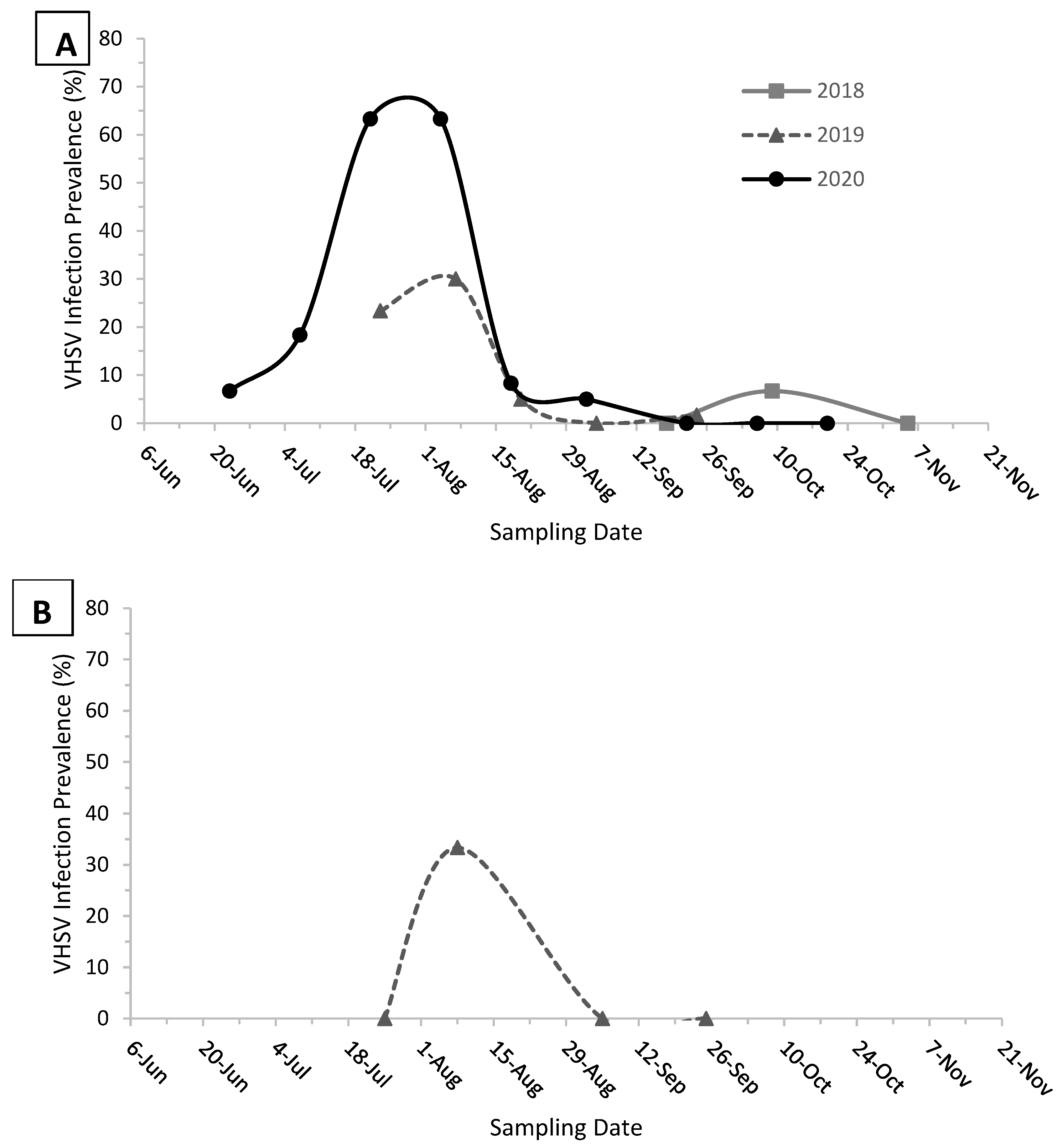
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isshiki, I.; Nishizawa, T.; Kobayashi, T.; Nagano, T.; Miyazaki, Y. An outbreak of VHSV (viral haemorrhagic septicemia virus) infection in farmed Japanese flounder Paralichthys olivaceus in Japan. Dis. Aquat. Org. 2001, 47, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Kim, S.R.; Kim, D.; Kim, J.O.; Park, M.A.; Kitamura, S.I.; Kim, H.Y.; Kim, D.H.; Han, H.J.; Jung, S.J.; et al. An outbreak of VHSV (viral hemorrhagic septicemia virus) infection in farmed olive flounder Paralichthys olivaceus in Korea. Aquaculture 2009, 296, 165–168. [Google Scholar] [CrossRef]
- Garver, K.A.; Traxler, G.S.; Hawley, L.M.; Richard, J.; Ross, J.; Lovy, J. Molecular epidemiology of viral haemorrhagic septicaemia virus (VHSV) in British Columbia, Canada, reveals transmission from wild to farmed fish. Dis. Aquat. Org. 2013, 104, 93–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyers, T.R.; Short, S.; Lipson, K. Isolation of the North American strain of viral hemorrhagic septicemia virus (VHSV) associated with epizootic mortality in two new host species of Alaskan marine fishes. Dis. Aquat. Org. 1999, 38, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hershberger, P.K.; MacKenzie, A.H.; Gregg, J.L.; Wilmot, M.D.; Powers, R.L.; Purcell, M.K. Long-term shedding and asymptomatic carriers indicate that Pacific herring are a marine reservoir for viral hemorrhagic septicemia virus. Dis. Aquat. Org. 2021, 144, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, P.K.; Kocan, R.M.; Elder, N.E.; Meyers, T.R.; Winton, J.R. Epizootiology of viral hemorrhagic septicemia virus in herring from the closed pound spawn-on-kelp fishery. Dis. Aquat. Org. 1999, 37, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocan, R.M.; Hershberger, P.K.; Elder, N.E.; Winton, J.R. Epidemiology of viral hemorrhagic septicemia (VHS) among juvenile Pacific herring and Pacific sand lances in Puget Sound, Washington. J. Aquat. Anim. Health 2001, 13, 77–85. [Google Scholar] [CrossRef]
- Hershberger, P.K.; Gregg, J.; Pacheco, C.; Winton, J.; Richard, J.; Traxler, G. Larval Pacific herring, Clupea pallasii (Valenciennes), are highly susceptible to viral hemorrhagic septicemia and survivors are partially protected after their metamorphosis to juveniles. J. Fish Dis. 2007, 30, 445–458. [Google Scholar] [CrossRef]
- Batts, W.N.; Lovy, J.; Getchell, R.; Faisal, M.; Standish, I.; Warg, J.V.; Phelps, N.B.D.; Glenney, G.; Winton, J.R. 2.2.7 Viral Hemorrhagic Septicemia. In Fish Health Section Blue Book—Suggested Procedures for the Detection and Identification of Certain Finfish and Shellfish Pathogens; American Fisheries Society: Bethesda, MD, USA, 2020; Available online: https://units.fisheries.org/fhs/fish-health-section-blue-book-2020/section-1-diagnostic/ (accessed on 21 January 2021).
- Batts, W.N.; Winton, J.R. Enhanced detection of infectious hematopoietic necrosis viris and other fish viruses by pretreatment of cell monolayers with polyethylene glycol. J. Aquat. Amim. Health 1989, 1, 284–290. [Google Scholar] [CrossRef]
- Fijan, N.; Sulimanović, D.; Bearzotti, M.; Muzinić, D.; Zwillenberg, L.; Chilmonczyk, S.; Vautherot, J.; de Kinkelin, P. Some properties of the epithelioma papulosum cyprini (EPC) cell line from carp Cyprinus carpio. Ann. Inst. Pasteur Virol. 1983, 134, 207–220. [Google Scholar] [CrossRef]
- Winton, J.; Batts, W.; de Kinkelin, P.; LeBerre, M.; Bremont, M.; Fijan, N. Current lineages of the epithelioma papulosum cyprini (EPC) cell line are contaminated with Fathead Minnow, Pimephales promelas, cells. J. Fish Dis. 2010, 33, 701–704. [Google Scholar] [CrossRef]
- Garver, K.A.; Hawley, L.M.; McLure, C.A.; Schroeder, Y.T.; Aldous, S.; Doig, F.; Snow, M.; Edes, S.; Baynes, C.; Richard, J. Development and validation of a reverse transcription quantitative PCR for universal detection of viral hemorrhagic septicemia virus. Dis. Aquat. Org. 2011, 95, 97–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Methot, P.O. Why do parasites harm their host? On the origin and legacy of Theobald Smith’s law of “declining virulence”—1900–1980. Hist. Philos. Life Sci. 2012, 34, 561–601. [Google Scholar] [PubMed]
- Einer-Jensen, K.; Pherns, P.; Forsberg, R.; Lorenzen, N. Evolution of the fish rhabdovirus viral haemorrhagic septicaemia virus. J. Gen. Virol. 2004, 85, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Coevolution of hosts and parasites. Parasitology 1982, 85, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Alizon, S.; Hurford, A.; Midea, N.; Van Baalen, M. Virulence evolution and the trade-off hypothesis: History, current state of affairs, and the future. J. Evol. Biol. 2009, 22, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, P.K.; Gregg, J.L.; Grady, C.A.; Hart, L.; Roon, S.E.; Winton, J.R. Factors controlling the early stages of viral hemorrhagic septicemia epizootics: Low exposure levels, virus amplification, and fish-to-fish transmission. J. Fish Dis. 2011, 34, 893–899. [Google Scholar] [CrossRef]
- Wargo, A.R.; Garver, K.A.; Kurath, G. Virulence correlates with fitness in vivo for two M group genotypes of infectious hematopoietic necrosis virus (IHNV). Virology 2010, 404, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.; Faisal, M. Emergence and resurgence of the viral hemorrhagic septicemia virus (Novirhabdovirus, Rhabdoviridae, Mononegavirales). J. Adv. Res. 2011, 2, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Hershberger, P.K.; Garver, K.A.; Winton, J.R. Principles underlying the epizootiology of viral hemorrhagic septicemia in Pacific herring and other fishes throughout the North Pacific Ocean. Can. J. Fish. Aquat. Sci. 2016, 73, 853–859. [Google Scholar] [CrossRef] [Green Version]

| Year | Herring Stock | Collection Site | Collection Date | Gear Type | Adult/ Juvenile (A/J) | Mean Fork Length mm (SD) | VHSV Detection by Cell Culture (No. Positive/n) |
|---|---|---|---|---|---|---|---|
| 2001 | Cook Inlet, AK, USA | Kachemak Bay | 18 June | Purse Seine | J | NA | 0% (0/32) |
| 2002 | Cook Inlet, AK, USA | Kamishak Bay | 20 May | Purse Seine | A | NA | 0% (0/20) |
| 2003 | Puget Sound, WA, USA | Port Orchard | 6 February | Trawl | A | 179 (19) | 0% (0/60) |
| Port Madison | 6 February | Trawl | A | 173 (19) | 0% (0/60) | ||
| Skagit Bay | 12 February | Trawl | A | 161 (10) | 0% (0/60) | ||
| Port Susan | 12 February | Trawl | A | 171 (14) | 0% (0/60) | ||
| Port Gamble Bay | 25 February | Trawl | J | 122 (24) | 0% (0/60) | ||
| 2004 | Puget Sound, WA, USA | Cherry Point | 4 May | Trawl | A | 221 (16) | 1.7% (1/60) |
| N. Hecate Strait, AK, USA | Behm Canal | 11 April | Purse Seine | A | NA | 0% (0/20) | |
| Frederick Sound, AK, USA | Hidden Falls | 9 April | Cast Net | J | NA | 0% (0/15) | |
| 2005 | Cook Inlet, AK, USA | Dry Bay | 16 May | Purse Seine | A | NA | 0% (0/53) |
| 2006 | Cook Inlet, AK, USA | Nordyke Bay | 18 May | Purse Seine | A | NA | 0% (0/60) |
| Oil Bay | 21 May | Purse Seine | A | NA | 0% (0/60) | ||
| Iniskin Bay | 22 May | Purse Seine | A | NA | 0% (0/60) | ||
| 2007 | Prince William Sound, AK, USA | St. Matthews Bay | 5 April | Cast Net | A | 224 (17) | 0% (0/60) |
| Simpson Bay | 19 April | Purse Seine | J | 86 (6.3) | 0% (0/60) | ||
| Sawmill Bay | 30 November | Purse Seine | A | 215 (21) | 0% (0/60) | ||
| Simpson Bay | 2 December | Purse Seine | A | 187 (13) | 0% (0/60) | ||
| Sitka Sound, AK, USA | Cannon Island | 9 April | Cast Net | A | 215 (18) | 0% (0/60) | |
| Kodiak, AK, USA | Uganik Bay | 18 February | Purse Seine | A | NA | 0% (0/30) | |
| Uyak Bay | 21 February | Purse Seine | A | NA | 0% (0/35) | ||
| Cook Inlet, AK, USA | Iniskin Bay | 16 May | Purse Seine | A | NA | 1.7% (1/60) | |
| Amakdedulia Cove | 27 May | Purse Seine | A | NA | 1.7% (1/60) | ||
| Ursus Cove | 27 May | Purse Seine | A | NA | 0% (0/60) | ||
| 2008 | Prince William Sound, AK, USA | Unknown | 17 March | Purse Seine | J | 141 (11) | 0% (0/60) |
| Fish Bay | 19 March | Purse Seine | A | 236 (27) | 0% (0/45) | ||
| Evans Point | 24 March | Purse Seine | A | 208 (18) | 0% (0/60) | ||
| Whale Bay | 24 March | Purse Seine | J | 149 (22) | 0% (0/60) | ||
| St. Matthews Bay | 8 April | Purse Seine | A | NA | 0% (0/32) | ||
| Port Gravina | 8–12 November | Purse Seine/Jig | A | 197 (23) | 0% (0/80) | ||
| Sitka Sound, AK, USA | N. Middle Island | 26 March | Purse Seine | A | 249 (14) | 0% (0/60) | |
| 2009 | Prince William Sound, AK, USA | Port Gravina | 20 March | Purse Seine | A | 199 (15) | 0% (0/60) |
| Port Gravina | 20 March | Purse Seine | A | 168 (11) | 0% (0/60) | ||
| Simpson Bay | 22 March | Purse Seine | J | 94 (8) | 0% (0/60) | ||
| Eaglek Bay | 14 November | Gill Net | J | 98 (4) | 0% (0/29) | ||
| Port Gravina | 15 November | Purse Seine | A | 179 (17) | 0% (0/60) | ||
| Lwr. Herring Bay | 16 November | Gill Net and Trawl | J | 99 (4.0) | 0% (0/14) | ||
| Elrington Pass | 17 November | Purse Seine | A | 216 (19) | 0% (0/60) | ||
| Simpson Bay | 19 November | Purse Seine | J | 87 (14) | 0% (0/60) | ||
| Simpson Bay | 19 November | Cast Net | J | 70 (12) | 0% (0/33) | ||
| Sitka Sound, AK, USA | Unknown | 24 March | Purse Seine | A | 270 (19) | 0% (0/44) | |
| St. John Babtist Bay | 26 March | Purse Seine | A | 248 (23) | 0% (0/67) | ||
| Unknown | 27 March | Purse Seine | J | 175 (7) | 0% (0/68) | ||
| Cook Inlet, AK, USA | Chenik Bay | 8 May | Purse Seine | A | NA | 0% (0/60) | |
| Rocky Cove | 21 May | Purse Seine | A | NA | 0% (0/59) | ||
| 2010 | Prince William Sound, AK, USA | Port Gravina | 16 March | Purse Seine | A | 213 (14) | 0% (0/60) |
| Port Fidalgo | 19 March | Purse Seine | A | 200 (15) | 0% (0/60) | ||
| Simpson Bay | 20 March | Purse Seine | J | 109 (23) | 2–5% A | ||
| Cordova Harbor | 2–13 June | Cast Net | J | 85 (11) | 0% (0/49) | ||
| Cordova Harbor | 18 August | Cast Net | J | 35 (6.8) | 0% (0/54) | ||
| Cordova Harbor | 28 September– 7 October | Cast Net | J | 50 (5.7) | 0% (0/22) | ||
| Whale Bay | 10–11 November | Gill Net | J | 95 (33) | 1.7% (1/60) | ||
| Sitka Sound, AK, USA | Indian River | 22 March | Purse Seine | A | 242 (22) | 0% (0/60) | |
| Kruzof Island | 24 March | Purse Seine | A | 241 (25) | 0% (0/60) | ||
| 2011 | Prince William Sound, AK, USA | Lower Herring Bay | 11 March | Cast Net | J | 95 (3.9) | 3.3% (2/60) |
| Eaglek Bay | 15 March | Cast Net | J | 113 (22) | 0% (0/60) | ||
| Port Fidalgo | 16 March | Cast Net | J | 76 (5.7) | 0% (0/60) | ||
| St. Matthew’s Bay | 2 April | Jig | A | 246 (19) | 0% (0/60) | ||
| Port Gravina | 4 April | Cast Net | A | 219 (20) | 0% (0/60) | ||
| Port Fidalgo | 6 April | Purse Seine | A | 253 (12) | 0% (0/60) | ||
| Simpson Bay | 13 October | Cast Net | J | 52 (3.0) | 0% (0/43) | ||
| Simpson Bay | 15 November | Cast Net | J | 60 (6.1) | 0% (0/60) | ||
| Whale Bay | 20 November | Cast Net | J | 83 (6.4) | 0% (0/60) | ||
| Port Gravina | 21 November | Purse Seine | A | 205 (19) | 0% (0/30) | ||
| Port Gravina | 22 November | Purse Seine | A | 157 (11) | 0% (0/30) | ||
| Simpson Bay | 13 December | Cast Net | J | 60 (5.0) | 0% (0/60) | ||
| Sitka Sound, AK, USA | Long Island | 22 March | Purse Seine | A | 232 (16) | 0% (0/60) | |
| Cook Inlet, AK, USA | Bruin Bay | 4 May | Purse Seine | A | NA | 0% (0/60) | |
| Rocky Cove | 13 May | Purse Seine | A | 224 (60) B | 0% (0/60) | ||
| Bristol Bay, AK, USA | Togiak Bay | 9 May | Purse Seine | A | NA | 0% (0/60) | |
| 2012 | Prince William Sound, AK, USA | Simpson Bay | 11 January | Cast Net | J | 57 (2.8) | 0% (0/60) |
| Glacier Island Pass | 8 February | Dip Net | A | 240 (13) | 0% (0/15) | ||
| Eaglek Bay | 21 March | Gill Net | J | 99 (3.2) | 0% (0/30) | ||
| Port Gravina | 28 March | Purse Seine | A | 218 (16) | 0% (0/60) | ||
| Port Gravina | 31 March | Purse Seine | A | 216 (16) | 0% (0/60) | ||
| Fidalgo Bay | 2 April | Purse Seine | A | 231 (20) | 0% (0/60) | ||
| Simpson Bay | 20 April | Cast Net | J | 78 (16) | 0% (0/30) | ||
| Port Gravina | 9 November | Cast Net | J | 63 (5.5) | 0% (0/30) | ||
| Simpson Bay | 9 November | Cast Net | J | 72 (9.0) | 0% (0/30) | ||
| Zaikoff Bay | 13 November | Cast Net | J | 72 (4.4) | 0% (0/60) | ||
| Lower Herring Bay | 15 November | Cast Net | J | 90 (4.3) | 0% (0/30) | ||
| Port Gravina | 15 November | Purse Seine | A | 159 (14) | 0% (0/60) | ||
| Sitka Sound, AK, USA | N. Kasiana Isl. | 3 April | Cast Net | A | 233 (22) | 0% (0/60) | |
| St. John Bay | 4 April | Purse Seine | A | 214 (24) | 0% (0/60) | ||
| Sitka Harbor | 4 April | Cast Net | A | 225 (22) | 0% (0/60) | ||
| Cook Inlet, AK, USA | Bruin Bay | 7 May | Purse Seine | A | 254 (10) B | 0% (0/60) | |
| 2013 | Prince William Sound, AK, USA | Port Gravina | 27 March | Purse Seine | J | 147 (16) | 0% (0/60) |
| Port Gravina | 31 March | Purse Seine | A | 232 (20) | 0% (0/60) | ||
| Port Gravina | 1 April | Purse Seine | A | 225 (23) | 0% (0/60) | ||
| Lower Herring Bay | 9 November | Trawl, Cast Net | J | 93 (9.7) | 0% (0/60) | ||
| Port Gravina | 13 November | Cast Net | J | 90 (6.0) | 0% (0/40) | ||
| Cordova Harbor | 20 November | Cast Net | J | 70 (7.5) | 0% (0/60) | ||
| Sitka Sound, AK, USA | Apple Islands | 29 March | Cast Net | A | 246 (28) | 0% (0/60) | |
| Silver Bay | 30 March | Cast Net | A | 251 (16) | 0% (0/60) | ||
| Unknown | 30 March | Cast Net | A | 226 (26) | 0% (0/60) | ||
| Kodiak, AK, USA | Kiliuda Bay | 24 April | Purse Seine | A | 282 (60) B | 9.1% (1/11) | |
| Cook Inlet, AK, USA | Akumwarvik Bay | 20 May | Purse Seine | A | 219 (60) B | 0% (0/60) | |
| 2014 | Prince William Sound, AK, USA | Sheep Bay | 26 March | Purse Seine | A | 216 (14) | 1.7% (1/60) |
| Port Fidalgo | 28 March | Purse Seine | A | 228 (15) | 0% (0/60) | ||
| Port Gravina | 29 March | Purse Seine | A | 242 (14) | 0% (0/60) | ||
| Simpson Bay | 15–23 November | Trawl | J | 78 (12) | 0% (0/60) | ||
| Port Gravina | 16 November | Trawl | J | 70 (5.1) | 0% (0/61) | ||
| Eaglek Bay | 19 November | Trawl | J | 96 (4.3) | 0% (0/61) | ||
| Cook Inlet, AK, USA | Kamishak Bay | 30 April | Unknown | A | NA | 0% (0/60) | |
| Kamishak Bay | 13 May | Unknown | A | NA | 0% (0/59) | ||
| Sitka Sound, AK, USA | Sitka Harbor | 26 March | Cast Net | A | 245 (25) | 0% (0/60) | |
| Middle Island | 27 March | Cast Net | A | 241 (30) | 0% (0/60) | ||
| Inner Point | 28 March | Purse Seine | A | 222 (20) | 0% (0/60) | ||
| 2015 | Prince William Sound, AK, USA | Port Gravina | 3 April | Cast Net | A | 228 (17) | 0% (0/60) |
| Simpson Bay | 6 November | Trawl | J | 82 (11) B | 0% (0/46) | ||
| Lower Herring Bay | 11 November | Gill Net | J | 85 (5.0) | 0% (054) | ||
| Whale Bay | 12 November | Trawl | J | 89 (7.2) | 0% (0/60) | ||
| Cook Inlet, AK, USA | Chenik Bay | 27 April | Purse Seine | A | 239 (10) B | 0% (0/60) | |
| Sitka Sound, AK, USA | Bieli Rock | 20 March | Purse Seine | A | 239 (26) | 0% (0/60) | |
| Bieli Rock | 22 March | Purse Seine | A | 250 (22) | 0% (0/60) | ||
| Bieli Rock | 22 March | Purse Seine | A | 231 (24) | 0% (0/60) | ||
| Ketchican, AK, USA | Craig | 17 December | Purse Seine | A | 193 (18) | 0% (0/76) | |
| 2016 | Prince William Sound, AK, USA | Red Head Point | 7 April | Jig | A | 205 (17) | 0% (0/60) |
| Knowles Head | 8 April | Cast Net | A | 223 (24) | 0% (0/120) | ||
| Simpson Bay | 29 October | Trawl | J | 82 (3.5) | 0% (0/60) | ||
| Eaglek Bay | 30 October | Trawl | J | 95 (4.6) | 0% (0/60) | ||
| Lower Herring Bay | 2 November | Trawl | J | 96 (4.4) | 0% (0/60) | ||
| Sitka Sound, AK, USA | S. Salsbury Sound | 21 March | Purse Seine | A | 218 (22) | 0% (0/60) | |
| North Crest | 22 March | Purse Seine | A | 215 (13) | 0% (0/60) | ||
| Point Brown | 22 March | Cast Net | A | 217 (24) | 0% (0/60) | ||
| 2017 | Prince William Sound, AK, USA | Port Gravina | 7 April | Purse Seine | A | 195 (14) | 0% (0/60) |
| Rocky Bay | 10 April | Purse Seine | J | 140 (46) | 0% (0/60) | ||
| Port Fidalgo | 10 April | Purse Seine | A | 191 (14) | 0% (0/60) | ||
| Sitka Sound, AK, USA | Unknown | 24 March | Cast Net | A | 221 (14) | 0% (0/60) | |
| S. Magoun Island | 25 March | Cast Net | A | 225 (15) | 0% (0/60) | ||
| Unknown | 25 March | Cast Net | A | 225 (16) | 0% (0/60) | ||
| 2018 | Prince William Sound, AK, USA | Port Fidalgo | 10–11 April | Purse Seine | J | 152 (36) | 0% (0/60) |
| Cedar Bay | 12 April | Purse Seine | A | 201 (15) | 0% (0/60) | ||
| Rocky Bay | 13 April | Purse Seine | A | 204 (16) | 0% (0/60) | ||
| Sitka Sound, AK, USA | Guide Island | 22 March | Cast Net | A | 221 (15) | 0% (0/56) | |
| Unknown | 23 March | Cast Net | A | 215 (16) | 0% (0/49) | ||
| Kruzof Island | 23 March | Cast Net | A | 224 (23) | 0% (0/72) | ||
| 2019 | Puget Sound, WA, USA | Oak Bay | 29 August | Dip Net | J | 103 (3.7) | 0% (0/60) |
| Prince William Sound, AK, USA | Double Bay | 5 April | Purse Seine | A | 176 (14) | 0% (0/60) | |
| Canoe Pass | 6 April | Purse Seine | A | 157 (29) | 0% (0/60) | ||
| Windy Bay | 6 April | Purse Seine | A | 179 (13) | 0% (0/59) | ||
| Sitka Sound, AK, USA | Krestof Isl. | 25 March | Cast Net | A | 205 (18) | 0% (0/60) | |
| Krestof Isl. | 26 March | Cast Net | A | 197 (17) | 0% (0/60) | ||
| Whitestone Narrows | 27 March | Cast Net | A | 204 (18) | 0% (0/60) | ||
| 2020 | Puget Sound | Eagle Harbor | 26 August | Cast Net | J | 69 (3.4) | 0% (0/60) |
| Prince William Sound, AK, USA | Canoe Pass | 8 April | Purse Seine | A | 213 (13) | 0% (0/130) | |
| Double Bay | 10 April | Purse Seine | A | 220 (26) | 0% (0/59) | ||
| Sitka Sound, AK, USA | Kruzof | 31 March | Cast Net | A | 215 (14) | 0% (0/60) | |
| Low Island | 1 April | Cast Net | A | 215 (14) | 0% (0/60) | ||
| Silver Bay | 2 April | Cast Net | A | 204 (10) | 0% (0/60) | ||
| Kodiak, AK, USA | Uganik Bay | 21 April | Purse Seine | A | 199 (10) B | 0% (0/60) | |
| Region | Year | Stock | Collection Site | Collection Date | Gear Type | Adult/Juvenile (A/J) | Mean Fork Length mm (SD) | VHSV Detection by Cell Culture (No. Positive/n) |
|---|---|---|---|---|---|---|---|---|
| AK, USA | 2011 | Sitka Sound | Bear Cove Bay | 24 March | Cast Net | J | 108 (11) | 63% (38/60) |
| BC, Canada | 2018 | W. Vancouver Isl. | Hot Springs Cv. | 24 June | Dip Net | J | NA B | 85% (22/26) |
| 2019 | W. Vancouver Isl. | Hot Springs Cv. | 27 June | Beach Seine | J | NA | 96% (29/30) | |
| WA, USA | 2014 | Puget Sound | Lopez Isl. A | 11 September | Beach Seine | J | NA C | 27% (6/22) |
| Puget Sound | Waldron Isl. A | 12 September | Beach Seine | J | NA C | 13% (3/24) | ||
| 2018 | Puget Sound | Pt. Angeles Hbr. | 18 September–5 November | Cast Net | J | 67 (8.0)–78 (7.4) | 6.7% (2/30) D | |
| 2019 | Puget Sound | Pt. Ludlow Hbr. | 25 July–25 September | Cast Net | J | 52 (2.4)–78 (6.8) | 33% (20/60) D | |
| Puget Sound | Pt. Angeles Hbr. | 23 July–24 September | Cast Net | J | 64 (6.7)–76 (7.0) | 30% (18/60) D | ||
| 2020 | Puget Sound | Pt. Angeles Hbr. | 23 June–20 October | Cast Net | J | 37 (2.0)–63 (8.3) | 63% (38/60) D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hershberger, P.K.; Meyers, T.R.; Gregg, J.L.; Groner, M.L.; Hall, S.A.; Jayasekera, H.T.; MacKenzie, A.H.; Neat, A.S.; Piatt, E.N.; Garver, K.A. Annual Recurrences of Viral Hemorrhagic Septicemia Epizootics in Age 0 Pacific Herring Clupea pallasii Valenciennes, 1847. Animals 2021, 11, 2426. https://doi.org/10.3390/ani11082426
Hershberger PK, Meyers TR, Gregg JL, Groner ML, Hall SA, Jayasekera HT, MacKenzie AH, Neat AS, Piatt EN, Garver KA. Annual Recurrences of Viral Hemorrhagic Septicemia Epizootics in Age 0 Pacific Herring Clupea pallasii Valenciennes, 1847. Animals. 2021; 11(8):2426. https://doi.org/10.3390/ani11082426
Chicago/Turabian StyleHershberger, Paul K., Theodore R. Meyers, Jacob L. Gregg, Maya L. Groner, Sophie A. Hall, Hiruni T. Jayasekera, Ashley H. MacKenzie, Abigail S. Neat, Ella N. Piatt, and Kyle A. Garver. 2021. "Annual Recurrences of Viral Hemorrhagic Septicemia Epizootics in Age 0 Pacific Herring Clupea pallasii Valenciennes, 1847" Animals 11, no. 8: 2426. https://doi.org/10.3390/ani11082426
APA StyleHershberger, P. K., Meyers, T. R., Gregg, J. L., Groner, M. L., Hall, S. A., Jayasekera, H. T., MacKenzie, A. H., Neat, A. S., Piatt, E. N., & Garver, K. A. (2021). Annual Recurrences of Viral Hemorrhagic Septicemia Epizootics in Age 0 Pacific Herring Clupea pallasii Valenciennes, 1847. Animals, 11(8), 2426. https://doi.org/10.3390/ani11082426








