Prevalence, Characterization, and Pathogenicity of Salmonella enterica Subspecies enterica Serovar Derby from Yaks in the Aba Tibetan Autonomous Prefecture, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
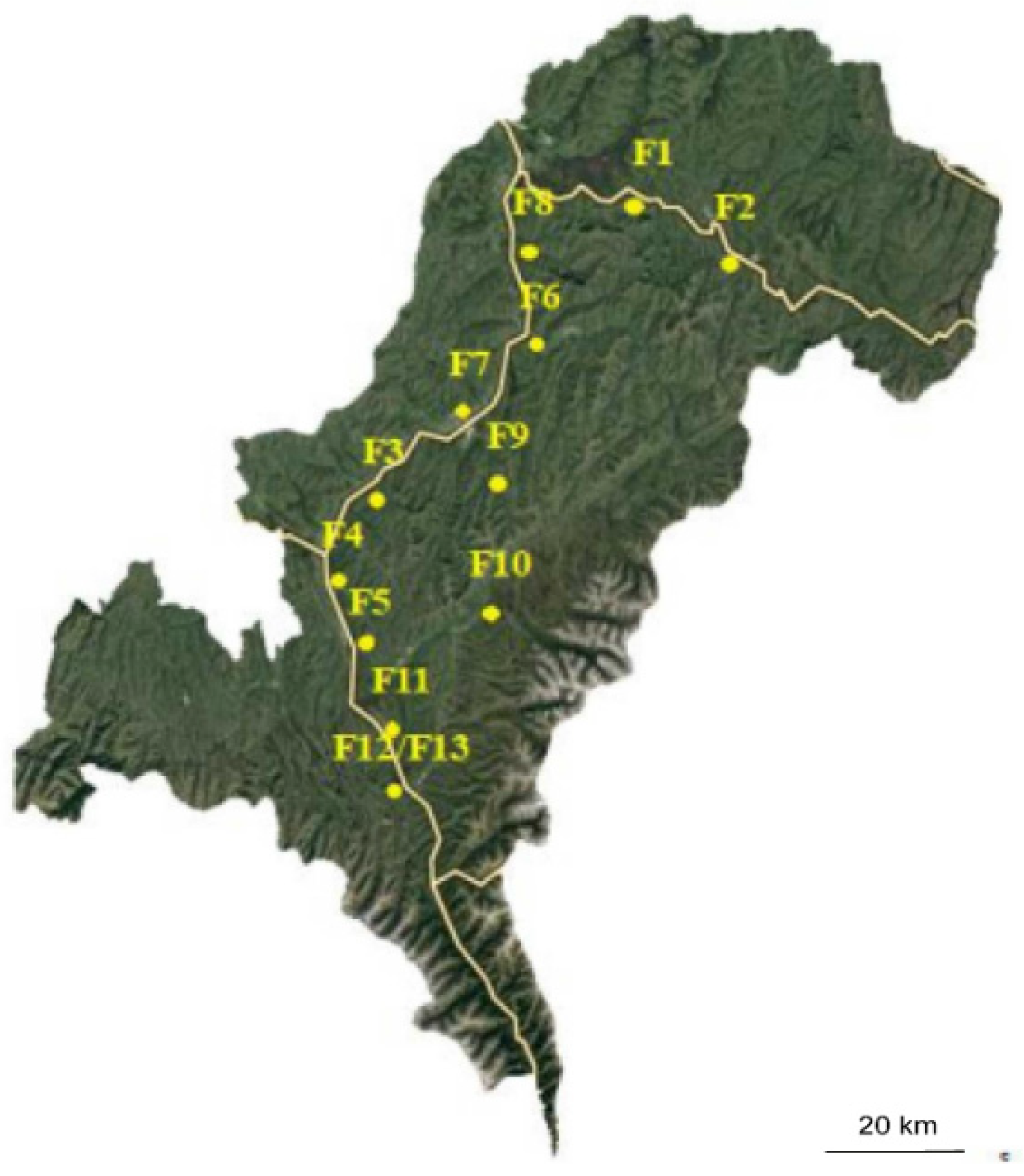
2.1. Sample Collection
2.2. Salmonella Isolation
2.3. Whole Genome Sequencing (WGS) and Bioinformatics Analyses
2.4. Animal Experiments
2.5. Statistical Analyses
3. Results
3.1. Prevalence and Serotyping of Salmonella
3.2. Molecular Typing of S. Derby and Whole Genome Sequencing (WGS) Anaylses
3.3. Virulence of S. Derby in Mice
3.3.1. LD50 Calculation
3.3.2. Fecal Shedding and Bacterial Loads in Organs
3.3.3. Histopathological Analyzation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoelzer, K.; Cummings, K.J.; Wright, E.M.; Rodriguez-Rivera, L.D.; Roof, S.E.; Switt, A.M.; Dumas, N.; Root, T.; Schoonmaker-Bopp, D.J.; Grohn, Y.T.; et al. Salmonella Cerro isolated over the past twenty years from various sources in the US represent a single predominant pulsed-field gel electrophoresis type. Vet. Microbiol. 2011, 150, 389–393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Navarro-Gonzalez, N.; Mentaberre, G.; Porrero, C.M.; Serrano, E.; Mateos, A.; Lopez-Martin, J.M.; Lavin, S.; Dominguez, L. Effect of cattle on Salmonella carriage, diversity and antimicrobial resistance in free-ranging wild boar (Sus scrofa) in Northeastern Spain. PLoS ONE 2012, 7, e51614. [Google Scholar] [CrossRef] [PubMed]
- Nova, M.V.; Durimel, K.; La, K.; Felten, A.; Bessières, P.; Mistou, M.Y.; Mariadassou, M.; Radomski, N. Genetic and metabolic signatures of Salmonella enterica subsp. enterica associated with animal sources at the pangenomic scale. BMC Genom. 2019, 20, 814. [Google Scholar]
- Jin, H.K.; Kim, H.J.; Jung, S.J.; Mizan, M.R.; Si, H.P.; Ha, S. Characterization of Salmonella spp.-specific bacteriophages and their biocontrol application in chicken breast meat. J. Food Sci. 2020, 85, 526–534. [Google Scholar]
- Albino, L.A.; Rostagno, M.H.; Húngaro, H.M.; Mendonça, R.C. Isolation, characterization, and application of bacteriophages for Salmonella spp. biocontrol in pigs. Foodborne Pathog. Dis. 2014, 11, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.A.; Ibrahim, M.; Arafa, S.A. Effect of dietary pomegranate by-product extract supplementation on growth performance, digestibility, and antioxidant status of growing rabbit. Trop. Anim. Health Prod. 2020, 52, 1893–1901. [Google Scholar] [CrossRef]
- Cetin, E.; Temelli, S.; Eyigor, A. Nontyphoid Salmonella prevalence, serovar distribution and antimicrobial resistance in slaughter sheep. Food Sci. Anim. Resour. 2019, 40, 21–33. [Google Scholar] [CrossRef]
- Obaidat, M.M. Prevalence and antimicrobial resistance of Listeria monocytogenes, Salmonella enterica and Escherichia coli O157:H7 in imported beef cattle in Jordan. Comp. Immunol. Microbiol. Infect. Dis. 2020, 70, 101447. [Google Scholar] [CrossRef]
- Xia, S.; Hendriksen, R.S.; Xie, Z.; Huang, L.; Zhang, J.; Guo, W.; Xu, B.; Ran, L.; Aarestrup, F.M. Molecular characterization and antimicrobial susceptibility of Salmonella isolates from infections in humans in Henan Province, China. J. Clin. Microbiol. 2009, 47, 401–409. [Google Scholar] [CrossRef]
- Gu, D.; Wang, Z.; Tian, Y.; Kang, X.; Jiao, X. Prevalence of Salmonella isolates and their distribution based on whole-genome sequence in a chicken slaughterhouse in Jiangsu, China. Front. Vet. Sci. 2020, 7, 29. [Google Scholar] [CrossRef]
- Xu, Z.; Song, Q.; Li, C.; Zhan, Y. Characterization of ciprofloxacin-resistant and ESBL-producing Salmonella enteric serotype Derby in Eastern China. BMC Microbiol. 2019, 19, 61. [Google Scholar] [CrossRef]
- Ebuchi, S.; Ai, B.; Uryu, K.; Hiwaki, H. Two outbreaks caused by Salmonella Derby and S. Anatum at grilled-meat restaurants in Fukuoka city. Jpn. J. Infect. Dis. 2006, 59, 405–406. [Google Scholar]
- Hendriksen, R.S.; Vieira, A.R.; Karlsmose, S.; Lo, F.; Jensen, A.B.; Wegener, H.C.; Aarestrup, F.M. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: Results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 2011, 8, 887–900. [Google Scholar] [CrossRef]
- Jackson, B.R.; Griffin, P.M.; Cole, D.; Walsh, K.A.; Chai, S.J. Outbreak-associated Salmonella enterica serotypes and food Commodities, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 1239–1244. [Google Scholar] [CrossRef]
- Arnedo-Pena, A.; Sabater-Vidal, S.; Herrera-León, S.; Bellido-Blasco, J.B.; Silvestre-Silvestre, E.; Meseguer-Ferrer, N.; Yague-Munoz, A.; Gil-Fortuno, M.; Romeu-García, A.; Moreno-Munoz, R. An outbreak of monophasic and biphasic Salmonella Typhimurium, and Salmonella Derby associated with the consumption of dried pork sausage in Castellon (Spain). Enferm. Infecc. Y Microbiol. Clin. 2016, 34, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Sévellec, Y.; Felten, A.; Radomski, N.; Granier, S.A.; Hello, S.L.; Petrovska, L.; Mistou, M.Y.; Cadel-Six, S. Genetic diversity of Salmonella derby from the poultry sector in europe. Pathogens 2019, 8, 46. [Google Scholar] [CrossRef]
- Schmidt, J.W.; Brichta-Harhay, D.M.; Kalchayanand, N.; Bosilevac, J.M.; Shackelford, S.D.; Wheeler, T.L.; Koohmaraie, M. Prevalence, enumeration, serotypes, and antimicrobial resistance phenotypes of Salmonella enterica isolates from carcasses at two large United States pork processing plants. Appl. Environ. Microbiol. 2012, 78, 2716. [Google Scholar] [CrossRef]
- Cai, Y.; Tao, J.; Jiao, Y.; Fei, X.; Zhou, L.; Wang, Y.; Zheng, H.; Pan, Z.; Jiao, X. Phenotypic characteristics and genotypic correlation between Salmonella isolates from a slaughterhouse and retail markets in Yangzhou, China. Int. J. Food Microbiol. 2016, 222, 56–64. [Google Scholar] [CrossRef]
- Xu, C.; Ren, X.; Feng, Z.; Fu, Y.; Hong, Y.; Shen, Z.; Zhang, L.; Liao, M.; Xu, X.; Zhang, J. Phenotypic characteristics and genetic Diversity of Salmonella enterica serotype derby isolated from human patients and foods of animal origin. Foodborne Pathog. Dis. 2017, 14, 593–599. [Google Scholar] [CrossRef]
- Authority, E. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, e05077. [Google Scholar]
- Gong, X.; Liu, L.; Zheng, F.; Chen, Q.; Li, Z.; Cao, X.; Yin, H.; Zhou, J.; Cai, X. Molecular investigation of bovine viral diarrhea virus infection in yaks (Bos gruniens) from Qinghai, China. Virol. J. 2014, 11, 1–7. [Google Scholar] [CrossRef]
- Cui, P.; Feng, L.; Zhang, L.; He, J.; Yang, X. Antimicrobial resistance, virulence genes, and biofilm formation capacity among Enterococcus species from Yaks in Aba Tibetan autonomous prefecture, China. Front. Microbiol. 2020, 11, 1250. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, L.; Zhang, H.; Lei, Z.; Luo, H.; Mehmood, K.; Shahzad, M.; Lan, Y.; Wang, M.; Li, J. Epidemiological investigation and risk factors of Echinococcus granulosus in yaks (Bos grunniens), Tibetan pigs and Tibetans on Qinghai Tibetan plateau. Acta Trop. 2017, 173, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; An, T.; Cui, P.; Zhao, X.; Li, H.; Yang, X. Isolation, identification, and pathogenicity experiment of Salmonella enterica serovar derby from yaks (Poephagus grunniens) with diarrhea in Tibetan Qiang Autonomous Prefecture of Ngawa, China. J. Sichuan Univ. 2020, 57, 1022–1030. [Google Scholar]
- Tsai, H.L.; Hsu, B.M.; Hsu, T.K.; Huang, K.H.; Shih, F.C.; Chen, J.S.; Wang, H.J.; Kao, P.M.; Su, H.C. Evaluation of immunomagnetic separation for the improvement of Salmonella detection in aquatic environment. Environ. Earth Sci. 2014, 73, 7909–7914. [Google Scholar] [CrossRef]
- Popoff, M.Y.; Minor, L.L. Antigenic Formulas of the Salmonella Serovars; 8th Revision; WHO Collaborating Center for Reference and Research on Salmonella, Institute Pasteur: Paris, France, 2001. [Google Scholar]
- Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 23 April 2021).
- Queipo-Ortuño, M.I.; De Dios Colmenero, J.; Macias, M.; Bravo, M.J.; Morata, P. Preparation of bacterial DNA template by boiling and effect of immunoglobulin G as an inhibitor in real-time PCR for serum samples from patients with brucellosis. Clin. Vaccine Immunol. 2008, 15, 293. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, C.; Ke, F.; Huang, S.; Li, Q. Characterization of a bacterial biocontrol strain 1404 and its efficacy in controlling postharvest citrus anthracnose. Acta Microbiol. Sin. 2010, 50, 1208–1217. [Google Scholar]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Stevens, R. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2013, 42, D206–D214. [Google Scholar] [CrossRef]
- Kidgell, C.; Reichard, U.; Wain, J.; Linz, B.; Achtman, M. Salmonella typhi, the causative agent of typhoid fever, is approximately 50,000 years old. Infect. Genet. Evol. 2002, 2, 39–45. [Google Scholar] [CrossRef]
- EnteroBase. Available online: http://enterobase.warwick.ac.uk/species/index/senterica (accessed on 23 April 2021).
- Nabil-Fareed, A.; Zhou, Z.; Sergeant, M.J.; Mark, A.; Josep, C. A genomic overview of the population structure of Salmonella. PLoS Genet. 2018, 14, e1007261. [Google Scholar]
- Jolley, K.A.; Bliss, C.M.; Bennett, J.S.; Bratcher, H.B.; Brehony, C.; Colles, F.M.; Wimalarathna, H.; Harrison, O.B.; Sheppard, S.K.; Cody, A.J.; et al. Ribosomal multilocus sequence typing: Universal characterization of bacteria from domain to strain. Microbiology 2012, 158, 1005–1015. [Google Scholar] [CrossRef]
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS ONE 2014, 9, e104984. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Interactive Tree of Life (iTOL) v6. Available online: https://itol.embl.de/itol.cgi (accessed on 20 April 2021).
- Dallman, T.J.; Lisa, B.; Ashton, P.M.; Cowley, L.A.; Perry, N.T.; Goutam, A.; Liljana, P.; Ellis, R.J.; Richard, E.; Anthony, U. Whole-genome sequencing for national surveillance of shiga toxin–producing Escherichia coli O157. Clin. Infect. Dis. 2015, 61, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kanagarajah, S.; Waldram, A.; Dolan, G.; Jenkins, C.; Ashton, P.M.; Martin, A.; Davies, R.; Frost, A.; Dallman, T.J.; Pinna, E. Whole genome sequencing reveals an outbreak of Salmonella Enteritidis associated with reptile feeder mice in the United Kingdom, 2012–2015. Food Microbiol. 2017, 71, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Valeria, B.; Kaas, R.S.; Etienne, R.; Roberts, M.C.; Stefan, S.; Vincent, C.; Alain, P.; Allesoe, R.L.; Rita, R.A.; Ferrer, F.A. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar]
- Roer, L.; Hendriksen, R.S.; Leekitcharoenphon, P.; Lukjancenko, O.; Kaas, R.S.; Hasman, H.; Aarestrup, F.M. Is the evolution of Salmonella enterica subsp. enterica linked to restriction-modification systems? Msystems 2016, 1, e00009–e00016. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2018, 47, 687–692. [Google Scholar] [CrossRef]
- Pizzi, M. Sampling variation of the fifty percent end-point, determined by the Reed-Muench (Behrens) method. Hum. Biol. 1950, 22, 151–190. [Google Scholar]
- Cevallos-Almeida, M.; Houdayer, C.; Rose, V.; Bailly, Y.; Paboeuf, F.; Fablet, C.; Denis, M.; Kerouanton, A. Colonization of pigs experimentally infected with a monophasic variant of Salmonella Typhimurium. Foodborne Pathog. Dis. 2018, 15, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Royston, P. A Remark on algorithm as 181: The W test for normality. J. R. Stat. Soc. Ser. C 1995, 44, 547–551. [Google Scholar]
- Cimolai, N.; Taylor, G.P.; Mah, D.; Morrison, B.J. Definition and application of a histopathological scoring scheme for an animal model of acute mycoplasma pneumoniae pulmonary infection. Microbiol. Immunol. 1992, 36, 465–478. [Google Scholar] [CrossRef]
- Oueslati, W.; Rjeibi, M.R.; Mhadhbi, M.; Jbeli, M.; Zrelli, S.; Ettriqui, A. Prevalence, virulence and antibiotic susceptibility of Salmonella spp. strains, isolated from beef in Greater Tunis (Tunisia). Meat Sci. 2016, 119, 154–159. [Google Scholar] [CrossRef]
- Mustefa, B.A.; Gebremedhin, E.Z. Carriage and antimicrobial resistance of non-typhoidal Salmonella in cattle slaughtered in Ambo municipality abattoir, West Shewa zone, Oromia, Ethiopia—A point prevalence survey. Ethiop. Vet. J. 2018, 22, 94. [Google Scholar] [CrossRef][Green Version]
- Sychanh, T.; Chaunchom, S.; Pulsrikarn, C.; Pornreongwong, S.; Boonmar, S. Salmonella prevalence in slaughtered buffaloes and cattle in Champasak province, Lao People’s Democratic Republic. Agric. Nat. Resour. 2013, 47, 561–570. [Google Scholar]
- Fedorka-Cray, P.J.; Dargatz, D.A.; Thomas, L.A.; Gray, J.T. Survey of Salmonella serotypes in feedlot cattle. J. Food Prot. 1998, 61, 525–530. [Google Scholar] [CrossRef]
- Guizelini, C.C.; Tutija, J.F.; Morais, D.R.; Bacha, F.B.; Ramos, C.A.N.; Leal, C.R.B.; Zaquetti, M.E.; Lemos, R.A.A. Outbreak investigation of septicemic salmonellosis in calves. J. Infect. Dev. Ctries. 2020, 14, 104–108. [Google Scholar] [CrossRef]
- Salaheen, S.; Sonnier, J.; Kim, S.W.; Haley, B.J.; Van Kessel, J.A.S. Interaction of Salmonella enterica with bovine epithelial cells demonstrates serovar-specific association and invasion patterns. Foodborne Pathog. Dis. 2020, 17, 608–610. [Google Scholar] [CrossRef]
- Hoelzer, K.; Switt, A.I.M.; Wiedmann, M. Animal contact as a source of human non-typhoidal salmonellosis. Vet. Res. 2011, 42, 1–28. [Google Scholar] [CrossRef]
- Guo, X.; Long, R.; Kreuzer, M.; Ding, L.; Shang, Z.; Zhang, Y.; Yang, Y.; Cui, G. Importance of functional ingredients in yak milk-derived food on health of Tibetan nomads living under high-altitude stress: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 292–302. [Google Scholar] [CrossRef]
- Hayward, M.R.; Petrovska, L.; Jansen, V.; Woodward, M.J. Population structure and associated phenotypes of Salmonella enterica serovars Derby and Mbandaka overlap with host range. BMC Microbiol. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- EFSA. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, e05926. [Google Scholar]
- Yann, S.; Marie-Léone, V.; Granier, S.A.; Renaud, L.; Carole, F.; Le, H.S.; Michel-Yves, M.; Sabrina, C.S. Polyphyletic Nature of Salmonella enterica Serotype Derby and Lineage-Specific Host-Association Revealed by Genome-Wide Analysis. Front. Microbiol. 2018, 9, 891. [Google Scholar]
- Hayward, M.R.; Jansen, V.A.; Woodward, M.J. Comparative genomics of Salmonella enterica serovars Derby and Mbandaka, two prevalent serovars associated with different livestock species in the UK. BMC Genom. 2013, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Hayward, M.R.; Abuoun, M.; Ragione, R.; Tchórzewska, M.; Woodward, M.J. SPI-23 of S. Derby: Role in adherence and invasion of porcine tissues. PLoS ONE 2014, 9, e107857. [Google Scholar]
- Olsen, J.E.; Brown, D.J.; Thomsen, L.E.; Platt, D.J.; Chadfield, M.S. Differences in the carriage and the ability to utilize the serotype associated virulence plasmid in strains of Salmonella enterica serotype Typhimurium investigated by use of a self-transferable virulence plasmid, pOG669. Microb. Pathog. 2004, 36, 337–347. [Google Scholar] [CrossRef]
- Swearingen, M.C.; Porwollik, S.; Desai, P.T.; Mcclelland, M.; Ahmer, B. Virulence of 32 Salmonella strains in mice. PLoS ONE 2012, 7, e36043. [Google Scholar] [CrossRef]
- Tennant, S.M.; Schmidlein, P.; Simon, R.; Pasetti, M.F.; Levine, M.M. Refined live attenuated Salmonella enterica Serovar typhimurium and enteritidis vaccines mediate homologous and heterologous serogroup protection in mice. Infect. Immun. 2015, 83, 4504–4512. [Google Scholar] [CrossRef] [PubMed]
- Plym, F.L.; Wierup, M. Salmonella contamination: A significant challenge to the global marketing of animal food products. Rev. Sci. Tech. 2006, 25, 541–554. [Google Scholar]
- Lamas, A.; Miranda, J.M.; Regal, P.; Vázquez, B.; Franco, C.M.; Cepeda, A. A comprehensive review of non-enterica subspecies of Salmonella enterica. Microbiol. Res. 2018, 206, 60–73. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Feng, L.; Kong, L.; Li, C.; Zhao, X.; Li, H.; Cui, P.; Yan, W.; Zhai, Y.; Zhang, L.; et al. Prevalence, Characterization, and Pathogenicity of Salmonella enterica Subspecies enterica Serovar Derby from Yaks in the Aba Tibetan Autonomous Prefecture, China. Animals 2021, 11, 2397. https://doi.org/10.3390/ani11082397
Fu X, Feng L, Kong L, Li C, Zhao X, Li H, Cui P, Yan W, Zhai Y, Zhang L, et al. Prevalence, Characterization, and Pathogenicity of Salmonella enterica Subspecies enterica Serovar Derby from Yaks in the Aba Tibetan Autonomous Prefecture, China. Animals. 2021; 11(8):2397. https://doi.org/10.3390/ani11082397
Chicago/Turabian StyleFu, Xue, Lan Feng, Linghan Kong, Chun Li, Xiaodong Zhao, Huade Li, Pengfei Cui, Wenjun Yan, Yaru Zhai, Lan Zhang, and et al. 2021. "Prevalence, Characterization, and Pathogenicity of Salmonella enterica Subspecies enterica Serovar Derby from Yaks in the Aba Tibetan Autonomous Prefecture, China" Animals 11, no. 8: 2397. https://doi.org/10.3390/ani11082397
APA StyleFu, X., Feng, L., Kong, L., Li, C., Zhao, X., Li, H., Cui, P., Yan, W., Zhai, Y., Zhang, L., Li, H., Wang, H., & Yang, X. (2021). Prevalence, Characterization, and Pathogenicity of Salmonella enterica Subspecies enterica Serovar Derby from Yaks in the Aba Tibetan Autonomous Prefecture, China. Animals, 11(8), 2397. https://doi.org/10.3390/ani11082397






