Melatonin Promotes In Vitro Maturation of Vitrified-Warmed Mouse Germinal Vesicle Oocytes, Potentially by Reducing Oxidative Stress through the Nrf2 Pathway
Abstract
:Simple Summary
Abstract
1. Introduction
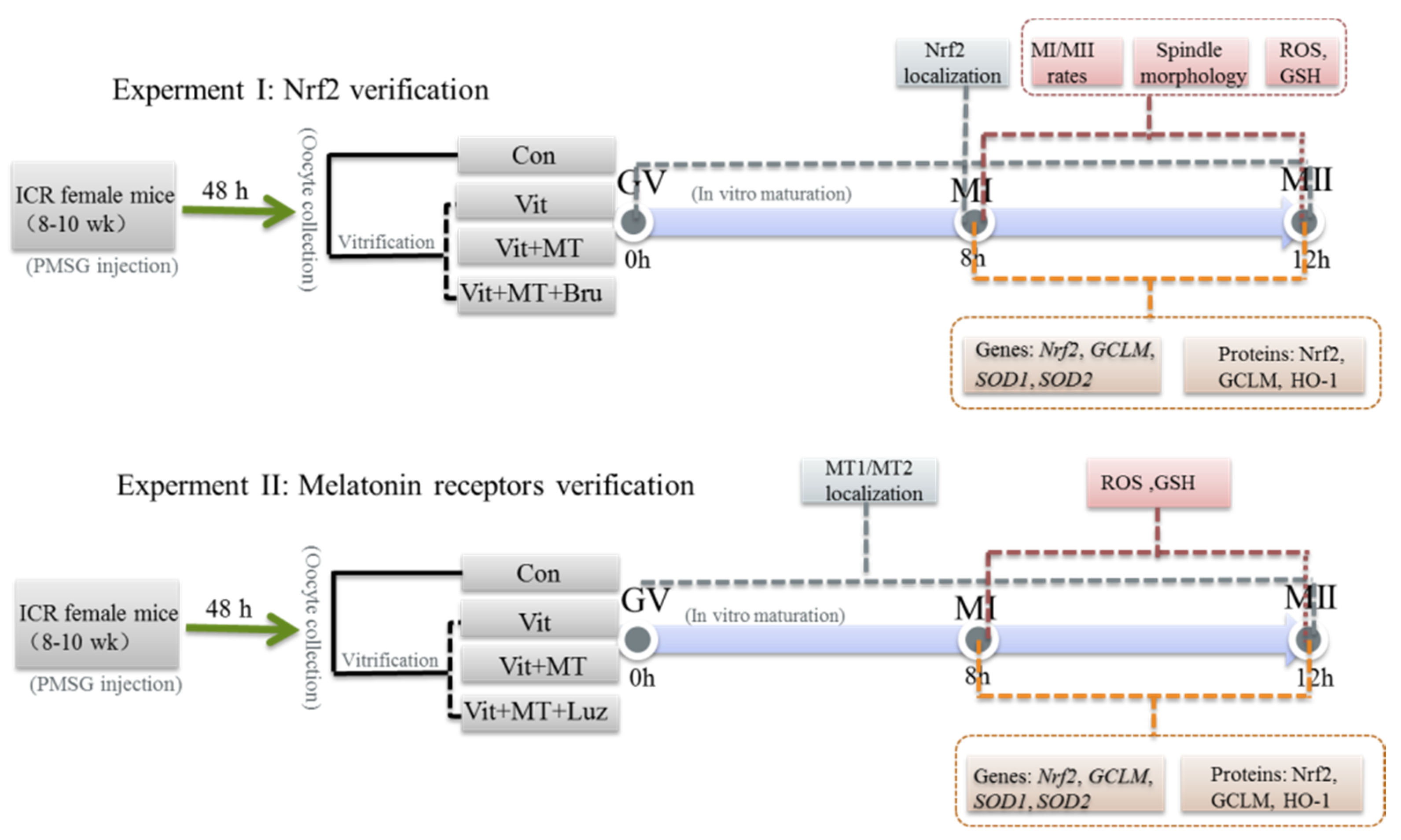
2. Materials and Methods
2.1. Oocyte Collection
2.2. Oocyte Vitrification and Warming
2.3. Oocyte Culture and In Vitro Maturation
2.4. Spindle Morphology and Classification
2.5. Measurement of Intracellular ROS and Glutathione (GSH) Levels
2.6. Quantitative Polymerase Chain Reaction (Q-PCR)
2.7. Immunofluorescent Staining
2.8. Statistical Analysis
3. Results
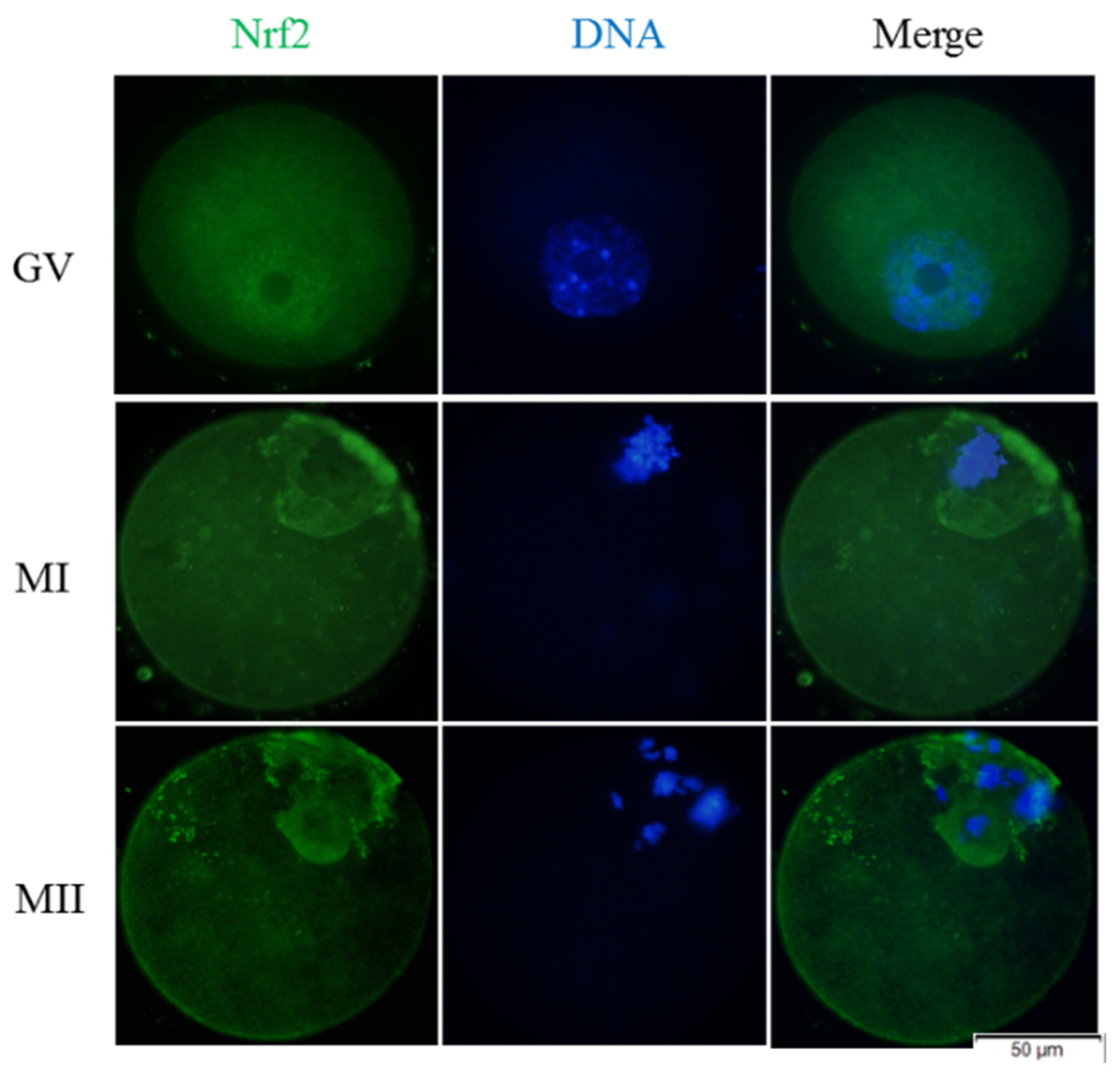
3.1. Melatonin Promotes In Vitro Development of Vitrified-Warmed Mouse GV Oocytes through Nrf2 Protein
3.2. Melatonin Improves the Spindle Morphology Grades of MI and MII Oocytes from the Vitrified-Warmed Mouse GV Oocytes through Nrf2 Protein
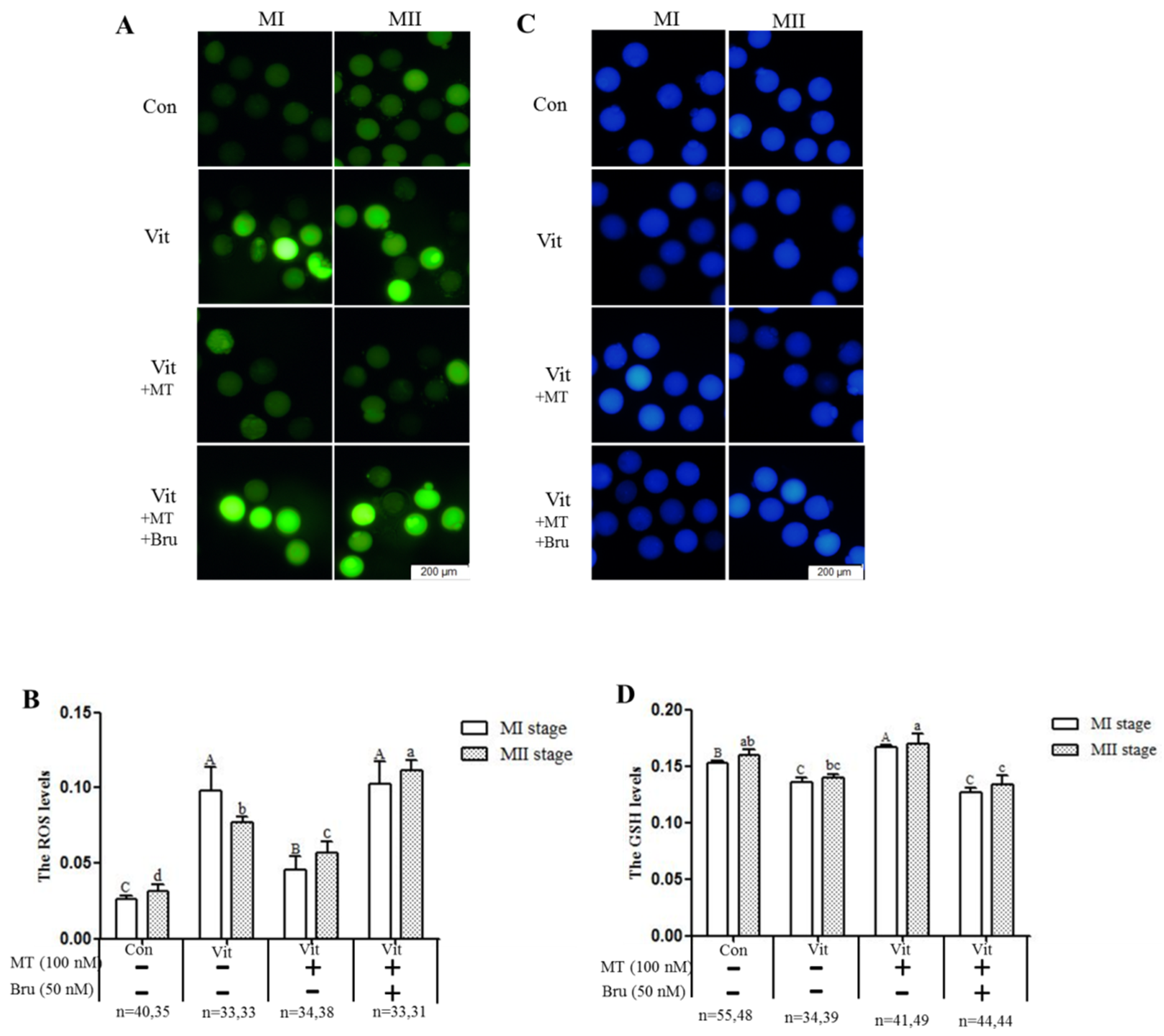
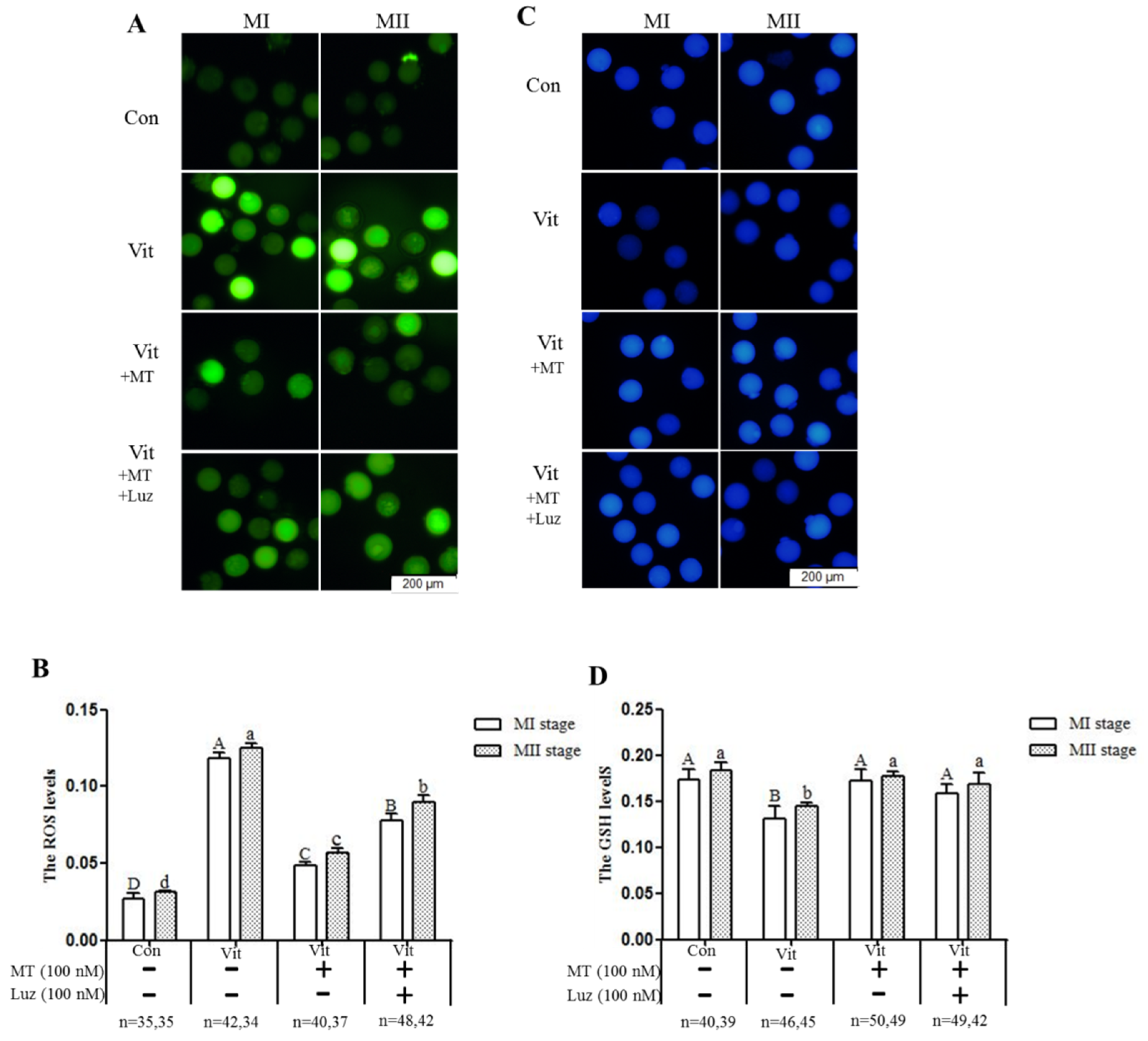
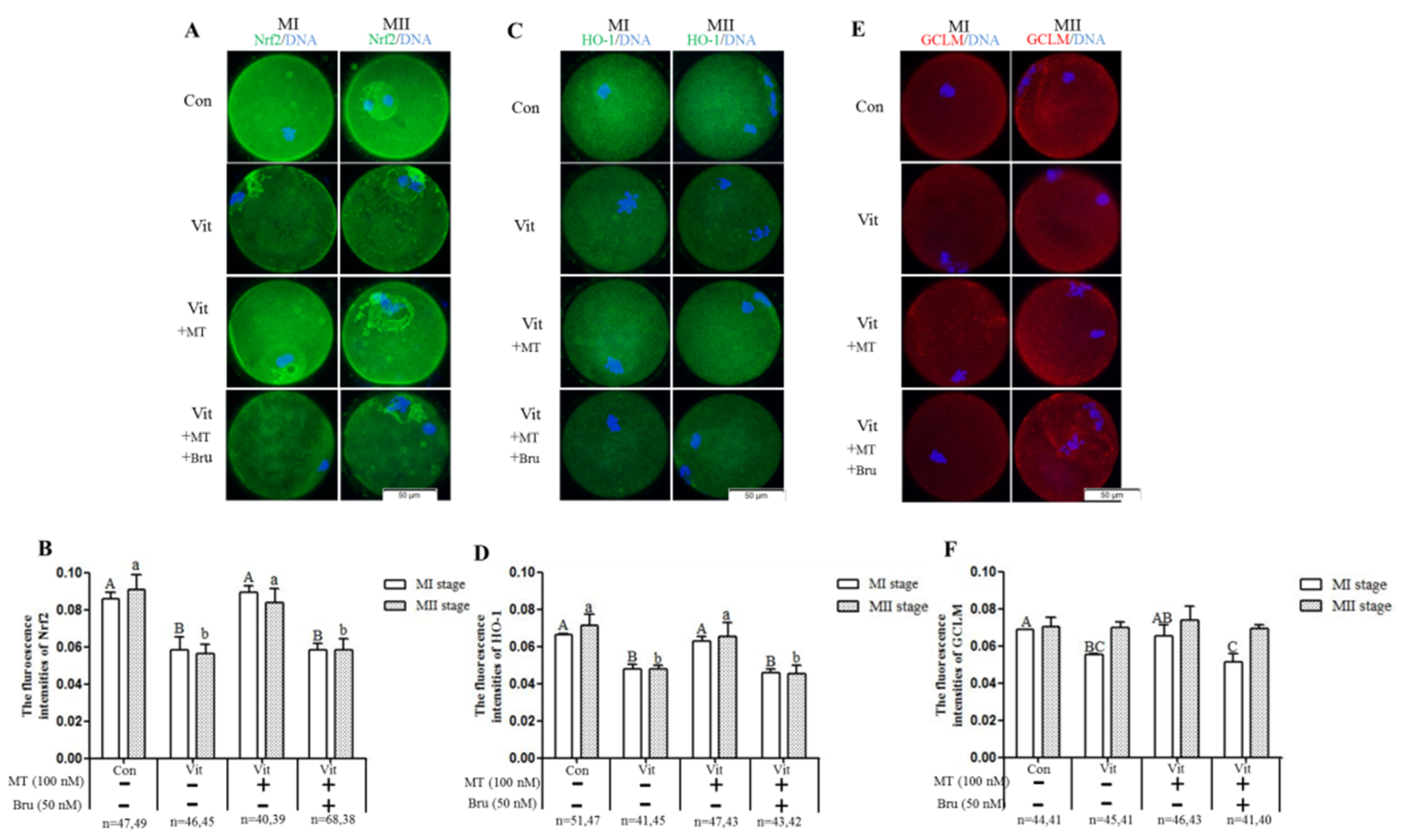
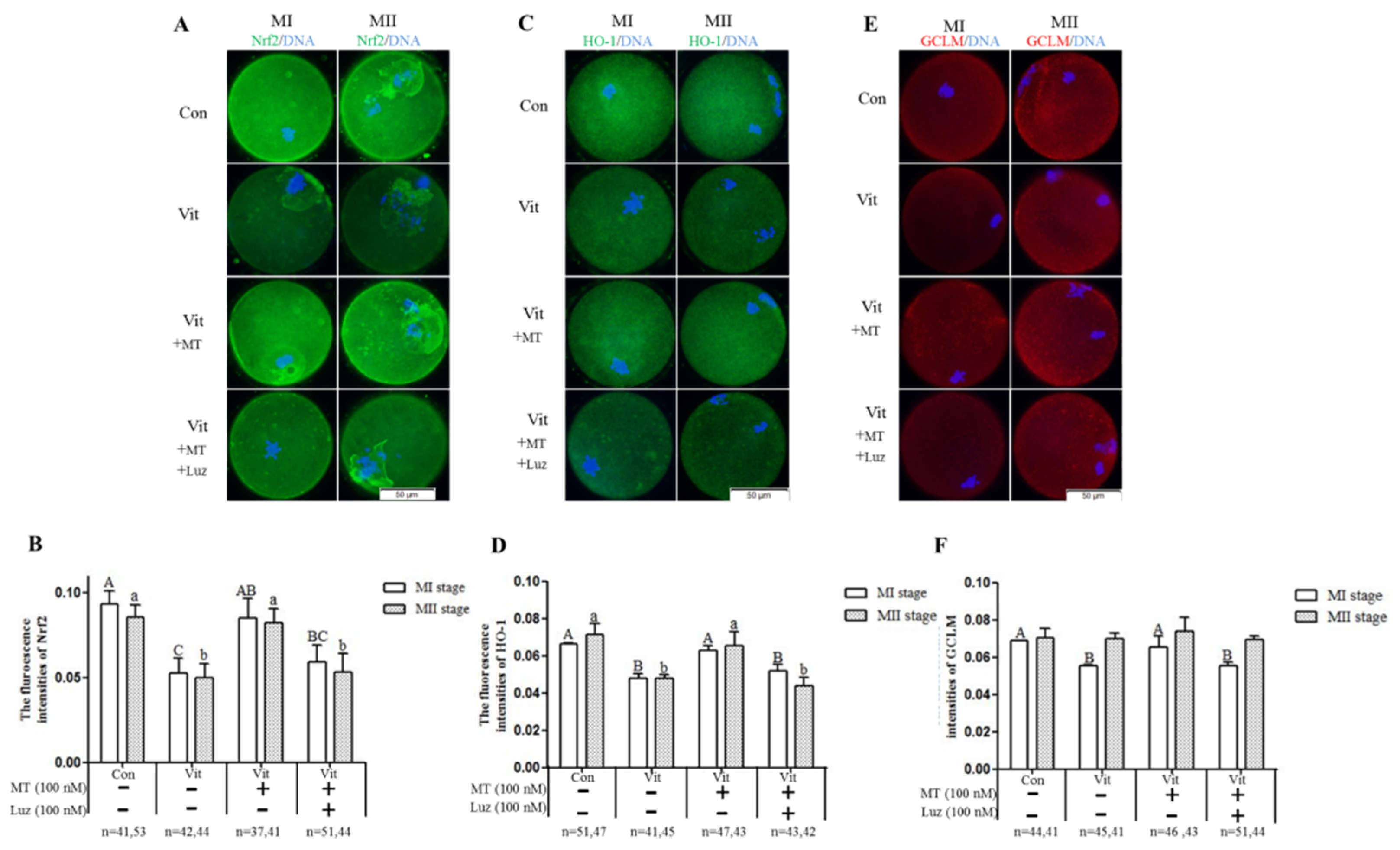
3.3. Melatonin Regulates Oxidative Stress of MI and MII Oocytes from the Vitrified-Warmed Mouse GV Oocytes through either Nrf2 Protein or Melatonin Receptors
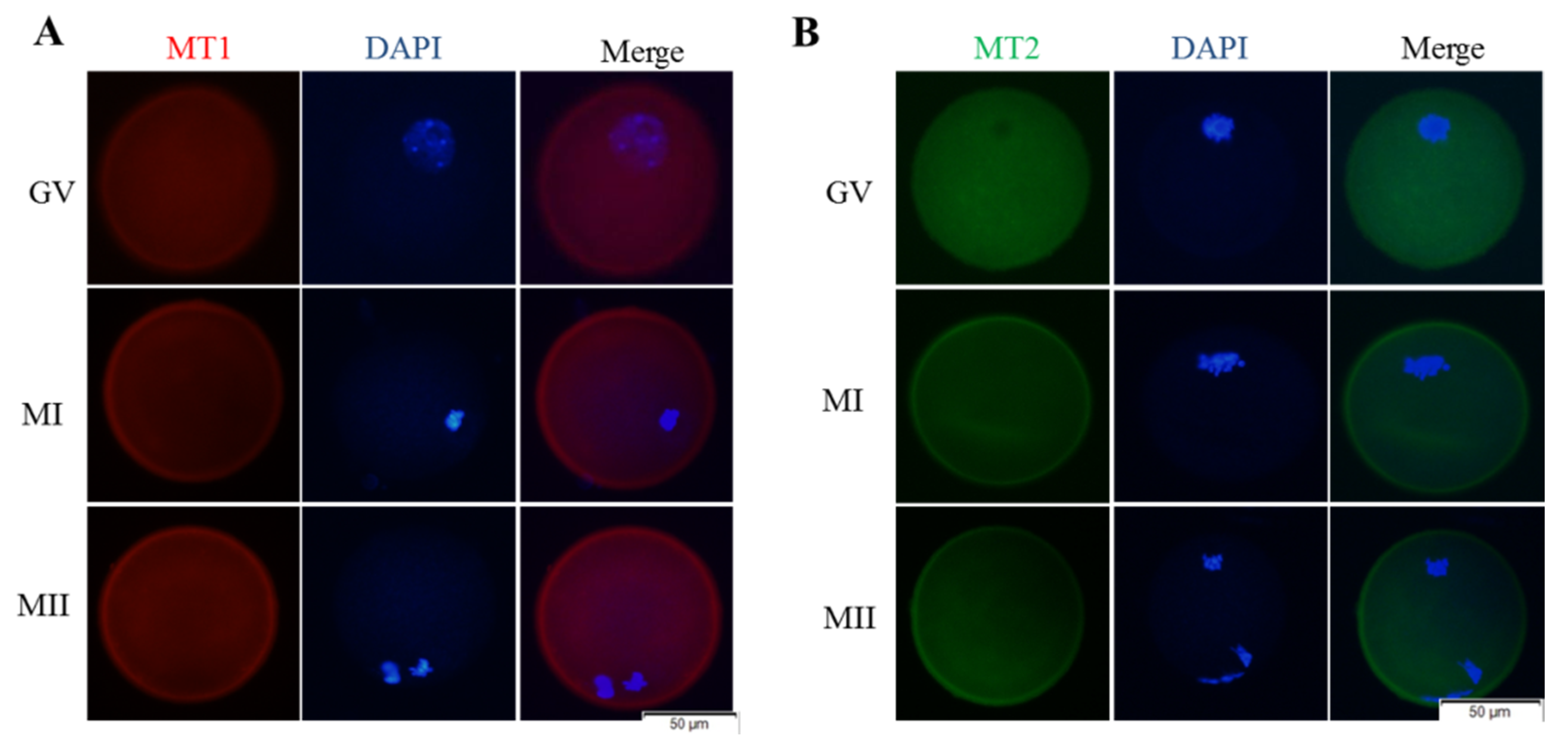
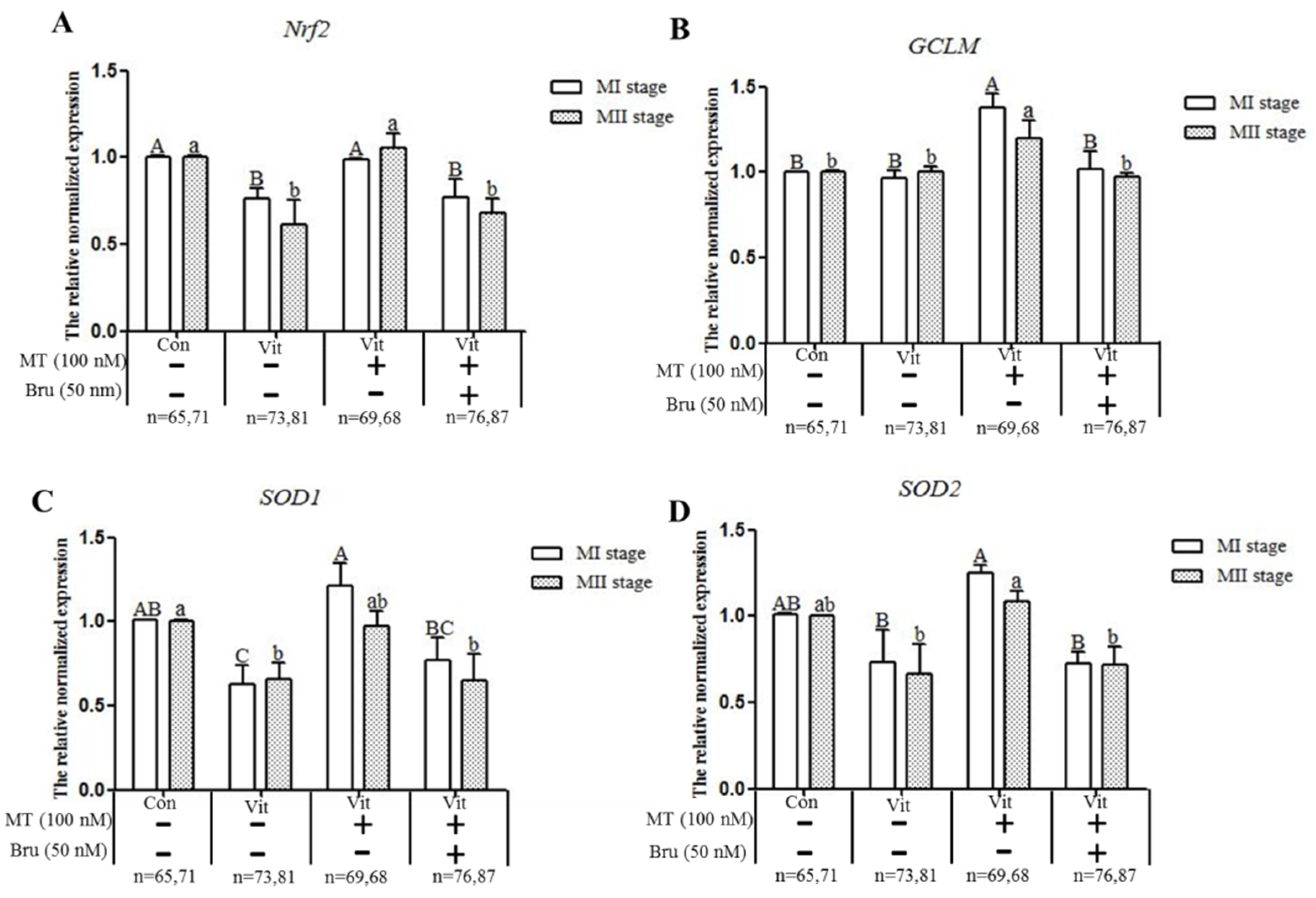
3.4. Melatonin Regulates Nrf2 Pathway-Related Genes and Proteins of MI and MII Oocytes from the Vitrified-Warmed Mouse GV Oocytes through Melatonin Receptors
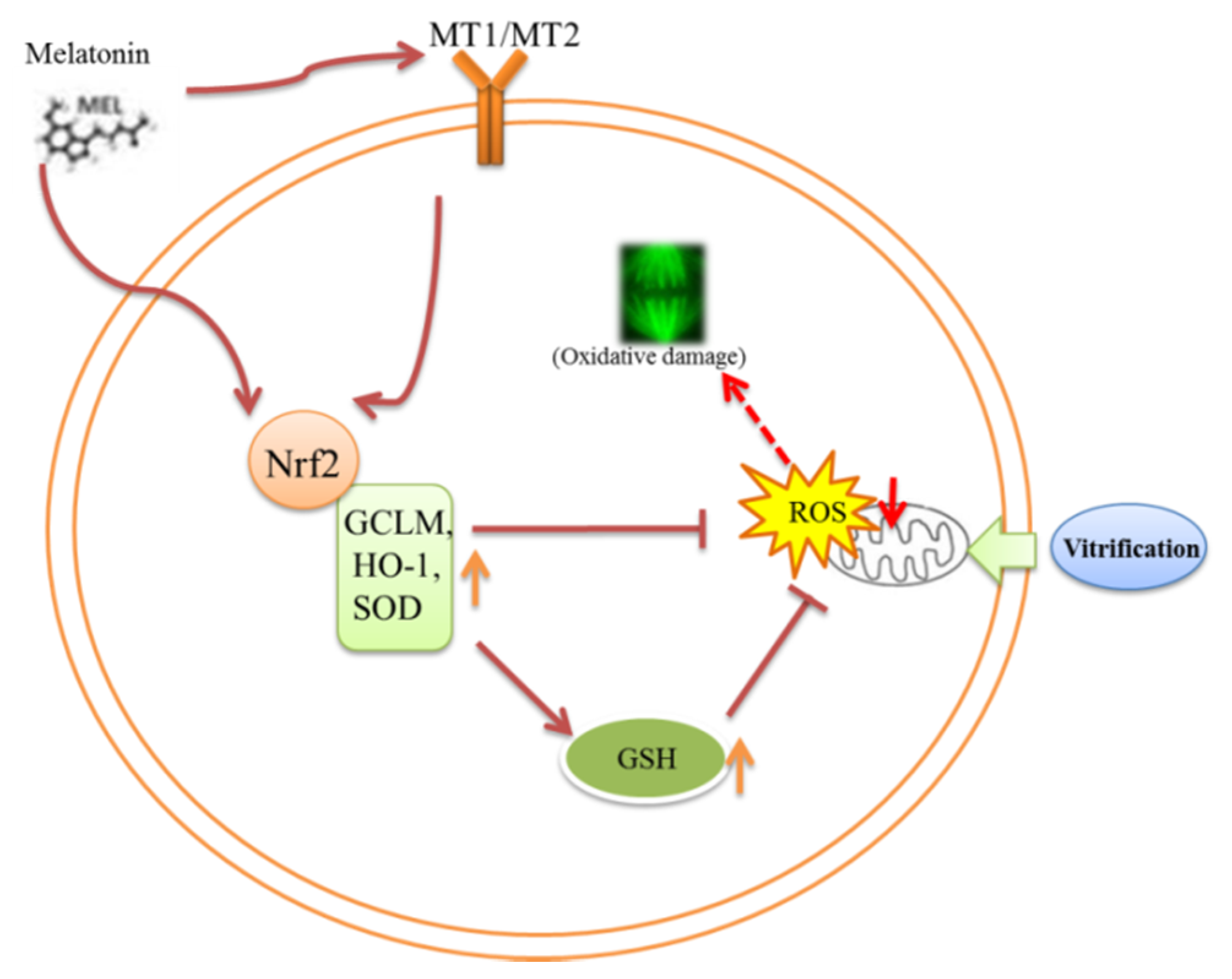
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Hwang, I.-S.; Shinichi, H. Recent progress in cryopreservation of bovine oocytes. Biomed. Res. Int. 2014, 2014, 570647. [Google Scholar] [CrossRef]
- Chian, R.C.; Wang, Y.; Li, Y.R. Oocyte vitrification: Advances, progress and future goals. J. Assist. Reprod. Genet. 2014, 31, 411–420. [Google Scholar] [CrossRef]
- János, K.; Katalin, K.; Rita, K.; Bence, S.; Sándor, C. Cryopreservation of Embryos and Oocytes in Human Assisted Reproduction. Biomed. Res. Int. 2014, 2014, 307268. [Google Scholar]
- Argyle, C.E.; Harper, J.C.; Davies, M.C. Oocyte cryopreservation: Where are we now? Hum. Reprod. Update 2016, 22, 440–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurchuk, T.; Petrushko, M.; Fuller, B. Science of cryopreservation in reproductive medicine Embryos and oocytes as exemplars. Early. Hum. Dev. 2018, 126, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Roser, M.; Mogas, T.; Maddox-Hyttel, P. Ultrastructure of bovine oocytes exposed to Taxol prior to OPS vitrification. Mol. Reprod. Dev. 2010, 75, 1318–1326. [Google Scholar]
- Lei, T.; Guo, N.; Liu, J.Q.; Tan, M.H.; Li, Y.F. Vitrification of in vitro matured oocytes: Effects on meiotic spindle configuration and mitochondrial function. Int. J. Clin. Exp. Pathol. 2014, 7, 1159–1165. [Google Scholar]
- Moussa, M.; Shu, J.; Zhang, X.H.; Zeng, F.Y. Cryopreservation of mammalian oocytes and embryos: Current problems and future perspectives. Sci. China. Life. Sci. 2014, 57, 903–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghaz, F.; Vaisi-Raygani, A.; Khazaei, M.; Arkan, E. Enhanced Cryoprotective Effect of Melatonin and Resveratrol by Coencapsulation: Improved In Vitro Development of Vitrified-Warmed Mouse Germinal Vesicle Oocytes. Biopreserv. Biobank. 2020, 19, 184–193. [Google Scholar] [CrossRef]
- Lu, M.C.; Ji, J.A.; Jiang, Z.Y.; You, Q.D. The Keap1–Nrf2–ARE Pathway As a Potential Preventive and Therapeutic Target: An Update. Med. Res. Rev. 2016, 36, 924–963. [Google Scholar] [CrossRef]
- Shaw, P.; Chattopadhyay, A. Nrf2–ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef] [PubMed]
- Adelusi, T.I.; Du, L.; Hao, M.; Zhou, X.; Yin, X. Keap1/Nrf2/ARE signaling unfolds therapeutic targets for redox imbalanced-mediated diseases and diabetic nephropathy. Biomed. Pharmacother. 2020, 123, 109732. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta. Mol. Basis. Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.; Cheng, L.; Wang, L.; Li, H. Protective role of Nrf2 against mechanical-stretch-induced apoptosis in mouse fibroblasts: A potential therapeutic target of mechanical-trauma-induced stress urinary incontinence. Int. Urogynecol. J. 2018, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Nie, X.; Chen, X.; Yin, L.; Luo, J.; Sun, L.; Wan, C. Nrf2 Signaling Elicits a Neuroprotective Role Against PFOS-mediated Oxidative Damage and Apoptosis. Neurochem. Res. 2018, 43, 2446–2459. [Google Scholar] [CrossRef]
- Ito, J.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Resveratrol treatment increases mitochondrial biogenesis and improves viability of porcine germinal-vesicle stage vitrified-warmed oocytes. Cryobiology 2020, 93, 37–43. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Ashrafizadeh, M. Melatonin as a potential modulator of Nrf2. Fundam. Clin. Pharmacol. 2020, 34, 11–19. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, J.; Mi, C.; Xu, B.; Jiao, C.; Li, Y.; Xu, D.; Liu, W.; Xu, Z. Melatonin Antagonizes Mn-Induced Oxidative Injury Through the Activation of Keap1–Nrf2–ARE Signaling Pathway in the Striatum of Mice. Neurotox. Res. 2015, 27, 156–171. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, C.; Meng, C.J.; Zhu, G.Q.; Sun, X.B.; Huo, L.; Zhang, J.; Liu, H.X.; He, W.C.; Shen, X.M.; et al. Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J. Pineal. Res. 2012, 53, 129–137. [Google Scholar] [CrossRef]
- Long, T.; Yang, Y.; Peng, L.; Li, Z. Neuroprotective Effects of Melatonin on Experimental Allergic Encephalomyelitis Mice Via Anti-Oxidative Stress Activity. J. Mol. Neurosci. 2018, 64, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Lei, S.; Tang, C.; Wang, K.; Xia, Z. Melatonin attenuates acute kidney ischemia/reperfusion injury in diabetic rats by activation of the SIRT1/Nrf2/HO-1 signaling pathway. Biosci. Rep. 2019, 39, BSR20181614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.; Wu, X.; Wang, Y.; Ji, S.; Zhang, W.; Kang, J.; Li, J.; Fei, G. Melatonin prevents LPS-induced epithelial-mesenchymal transition in human alveolar epithelial cells via the GSK-3β/Nrf2 pathway. Biomed. Pharmacother. 2020, 132, 110827. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, G.A.; Taweechaipaisankul, A.; Lee, S.H.; Lee, B.C. Melatonin enhances porcine embryo development via the Nrf2/ARE signaling pathway. J. Mol. Endocrinol. 2019, 63, 175–185. [Google Scholar] [CrossRef]
- Sun, T.C.; Liu, X.C.; Yang, S.H.; Song, L.L.; Zhou, S.J.; Deng, S.L.; Tian, L.; Cheng, L.Y. Melatonin Inhibits Oxidative Stress and Apoptosis in Cryopreserved Ovarian Tissues via Nrf2/HO-1 Signaling Pathway. Front. Mol. Biosci. 2020, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pan, B.; Qazi, I.H.; Yang, H.; Guo, S.; Yang, J.; Zhang, Y.; Zeng, C.; Zhang, M.; Han, H.; et al. Melatonin Improves In Vitro Development of Vitrified-Warmed Mouse Germinal Vesicle Oocytes Potentially via Modulation of Spindle Assembly Checkpoint-Related Genes. Cells 2019, 8, 1009. [Google Scholar] [CrossRef] [Green Version]
- Pan, B.; Yang, H.; Wu, Z.; Qazi, I.H.; Liu, G.; Han, H.; Meng, Q.; Zhou, G. Melatonin Improves Parthenogenetic Development of Vitrified(-)Warmed Mouse Oocytes Potentially by Promoting G1/S Cell Cycle Progression. Int. J. Mol. Sci. 2018, 19, 4029. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, W.; Ma, Y.; Wang, D.; Zhao, X.; Zeng, C.; Zhang, M.; Zeng, X.; Meng, Q.; Zhou, G. Improved development by melatonin treatment after vitrification of mouse metaphase II oocytes. Cryobiology 2016, 73, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Du, M.; Zhuan, Q.; Luo, Y.; Li, J.; Hou, Y.; Zeng, S.; Zhu, S.; Fu, X. Melatonin rescues the aneuploidy in mice vitrified oocytes by regulating mitochondrial heat product. Cryobiology 2012, 89, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal. Res. 2016, 60, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mu, Y.; Ding, D.; Zou, W.; Li, X.; Chen, B.; Leung, P.C.; Chang, H.; Zhu, Q.; Wang, K.; et al. Melatonin improves the effect of cryopreservation on human oocytes by suppressing oxidative stress and maintaining the permeability of the oolemma. J. Pineal. Res. 2021, 70, e12707. [Google Scholar] [CrossRef] [PubMed]
- Cecon, E.; Liu, L.; Jockers, R. Melatonin receptor structures shed new light on melatonin research. J. Pineal. Res. 2019, 67, e12606. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Wang, F.; Zhang, L.; He, C.; Ji, P.; Wang, J.; Zhang, Z.; Lv, D.; Abulizi, W.; Wang, X.; et al. Beneficial Effects of Melatonin on the In Vitro Maturation of Sheep Oocytes and Its Relation to Melatonin Receptors. Int. J. Mol. Sci. 2017, 18, 834. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.H.; Ridlo, M.R.; Lee, B.C.; Kim, G.A. Melatonin-Nrf2 Signaling Activates Peroxisomal Activities in Porcine Cumulus Cell-Oocyte Complexes. Antioxidants 2020, 9, 1080. [Google Scholar] [CrossRef]
- Barberino, R.S.; Menezes, V.G.; Ribeiro, A.E.A.S.; Palheta, R.C.J.; Jiang, X.; Smitz, J.E.J.; Matos, M.H.T. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biol. Reprod. 2017, 96, 1244–1255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, Z.; Wang, J.; Lv, D.; Zhu, T.; Wang, F.; Tian, X.; Yao, Y.; Ji, P.; Liu, G. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J. Pineal. Res. 2019, 66, e12550. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yan, Y.; Teng, X.; Wen, X.; Li, N.; Peng, S.; Liu, W.; Donadeu, F.X.; Zhao, S.; Hua, J. Melatonin prevents senescence of canine adipose-derived mesenchymal stem cells through activating NRF2 and inhibiting ER stress. Aging 2018, 10, 2954–2972. [Google Scholar] [CrossRef]
- Jumnongprakhon, P.; Govitrapong, P.; Tocharus, C.; Tocharus, J. Melatonin promotes blood-brain barrier integrity in methamphetamine-induced inflammation in primary rat brain microvascular endothelial cells. Brain. Res. 2016, 1646, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.J.; Liu, Y.; Han, S.; Yang, C. Brusatol, an NRF2 inhibitor for future cancer therapeutic. Cell. Biosci. 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Villeneuve, N.F.; Jiang, T.; Wu, T.; Lau, A.; Toppin, H.A.; Zhang, D.D. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. USA 2011, 108, 1433–1438. [Google Scholar] [CrossRef] [Green Version]
- Estaras, M.; Marchena, A.M.; Fernandez-Bermejo, M.; Mateos, J.M.; Vara, D.; Roncero, V.; Salido, G.M.; Gonzalez, A. The melatonin receptor antagonist luzindole induces the activation of cellular stress responses and decreases viability of rat pancreatic stellate cells. J. Appl. Toxicol. 2020, 40, 1554–1565. [Google Scholar] [CrossRef]
- Yao, K.; Zhao, Y.F.; Zu, H.B. Melatonin receptor stimulation by agomelatine prevents Aβ-induced tau phosphorylation and oxidative damage in PC12 cells. Drug. Des. Devel. Ther. 2019, 13, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Cheng, K.; Zhang, Y.; Meng, Q.; Zhu, S.; Zhou, G. No effect of exogenous melatonin on development of cryopreserved metaphase II oocytes in mouse. J. Anim. Sci. Biotechnol. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Li, H.; Zhang, Y.; Lin, Y.; Qiu, X.; Xie, M.; Yao, B. The toxic effects and possible mechanisms of Brusatol on mouse oocytes. PLoS ONE 2017, 12, e0177844. [Google Scholar] [CrossRef] [Green Version]
- Soto-Heras, S.; Catalá, M.G.; Roura, M.; Menéndez-Blanco, I.; Piras, A.R.; Izquierdo, D.; Paramio, M.T. Effects of melatonin on oocyte developmental competence and the role of melatonin receptor 1 in juvenile goats. Reprod. Domest. Anim. 2019, 54, 381–390. [Google Scholar] [CrossRef]
- Tamura, A.N.; Huang, T.T.F.; Marikawa, Y. Impact of vitrification on the meiotic spindle and components of the microtubule-organizing center in mouse mature oocytes. Biol. Reprod. 2013, 89, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Lan, M.; Zang, X.W.; Li, Y.L.; Cui, X.S.; Kim, N.H.; Sun, S.C. Melatonin protects oocytes from MEHP exposure-induced meiosis defects in porcine. Biol. Reprod. 2018, 286–298. [Google Scholar] [CrossRef]
- Ma, R.; Liang, W.; Sun, Q.; Qiu, X.; Lin, Y.; Ge, X.; Jueraitetibaike, K.; Xie, M.; Zhou, J.; Huang, X.; et al. Sirt1/Nrf2 pathway is involved in oocyte aging by regulating Cyclin B1. Aging 2018, 10, 2991–3004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Du, W.H.; Wang, D.; Hao, H.S.; Liu, Y.; Qin, T.; Zhu, H.B. Recovery of mitochondrial function and endogenous antioxidant systems in vitrified bovine oocytes during extended in vitro culture. Mol. Reprod. Dev. 2011, 78, 942–950. [Google Scholar] [CrossRef]
- Mateo-Otero, Y.; Yeste, M.; Damato, A.; Giaretta, E. Cryopreservation and oxidative stress in porcine oocytes. Res. Vet. Sci. 2021, 135, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Nohales-Córcoles, M.; Sevillano-Almerich, G.; Emidio, G.D.; Tatone, C.; Cobo, A.C.; Dumollard, R.; De Los Santos Molina, M.J. Impact of vitrification on the mitochondrial activity and redox homeostasis of human oocyte. Hum. Reprod. 2016, 31, 1850–1858. [Google Scholar] [CrossRef]
- Clérico, G.; Taminelli, G.; Veronesi, J.C.; Polola, J.; Pagura, N.; Pinto, C.; Sansinena, M. Mitochondrial function, blastocyst development and live foals born after ICSI of immature vitrified/warmed equine oocytes matured with or without melatonin. Theriogenology 2021, 160, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys Acta. Mo.l Cell. Res. 2018, 1865, 721–723. [Google Scholar] [CrossRef]
- Yan, G.; Yu, L.; Jiang, S.; Zhu, J. Melatonin antagonizes oxidative stress-induced mitochondrial dysfunction in retinal pigmented epithelium cells via melatonin receptor 1 (MT1). J. Toxicol. Sci. 2018, 43, 659–669. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Zhao, J.; Fu, B.; Li, D.; Mao, T.; Peng, W.; Wu, H.; Zhang, Y. Melatonin-mediated upregulation of Sirt3 attenuates sodium fluoride-induced hepatotoxicity by activating the MT1-PI3K/AKT-PGC-1α signaling pathway. Free. Radic. Biol. Med. 2017, 112, 616–630. [Google Scholar] [CrossRef]
- Wang, G.F.; Dong, Q.; Bai, Y.; Yuan, J.; Xu, Q.; Cao, C.; Liu, X. Oxidative stress induces mitotic arrest by inhibiting Aurora A-involved mitotic spindle formation. Free. Radic. Biol. Med. 2017, 103, 177–187. [Google Scholar] [CrossRef]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front. Endocrinol. (Lausanne) 2019, 10, 811. [Google Scholar] [CrossRef] [Green Version]
- Succu, S.; Bebbere, D.; Bogliolo, L.; Ariu, F.; Fois, S.; Leoni, G.G.; Berlinguer, F.; Naitana, S.; Ledda, S. Vitrification of in vitro matured ovine oocytes affects in vitro pre-implantation development and mRNA abundance. Mol. Reprod. Dev. 2008, 75, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Quintero, R.; Chen, B.; Shu, Y.; Polan, M.L.; Behr, B. Expression of CD9 in frozen-thawed mouse oocytes: Preliminary experience. Fertil. Steril. 2007, 88, 526–529. [Google Scholar] [CrossRef]
- Dai, J.; Wu, C.; Muneri, C.W.; Niu, Y.; Zhang, S.; Rui, R.; Zhang, D. Changes in mitochondrial function in porcine vitrified MII-stage oocytes and their impacts on apoptosis and developmental ability. Cryobiology 2015, 71, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.L.; Wassmann, K. Multiple Duties for Spindle Assembly Checkpoint Kinases in Meiosis. Front. Cell. Dev. Biol. 2017, 5, 109. [Google Scholar] [CrossRef] [Green Version]
- Sadek, K.M.; Lebda, M.A.; Abouzed, T.K. The possible neuroprotective effects of melatonin in aluminum chloride-induced neurotoxicity via antioxidant pathway and Nrf2 signaling apart from metal chelation. Environ. Sci. Pollut. Res. Int. 2019, 26, 9174–9183. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Chung, Y.H.; Le, H.L.; Jeong, J.H.; Dang, D.K.; Nam, Y.; Wie, M.B.; Nah, S.Y.; Nabeshima, Y.; Nabeshima, T.; et al. Melatonin Attenuates Memory Impairment Induced by Klotho Gene Deficiency Via Interactive Signaling Between MT2 Receptor, ERK, and Nrf2-Related Antioxidant Potential. Int. J. Neuropsychopharmacol. 2015, 18, pyu105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasman, A.; Santoso, B.; Widjiati, W. The effect of vitrification after warming on the expressions of p38, CDK1, and cyclin B in immature goat oocytes followed by in vitro maturation. Vet. World 2020, 13, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Balboula, A.Z.; Schindler, K.; Kotani, T.; Kawahara, M.; Takahashi, M. Vitrification-induced activation of lysosomal cathepsin B perturbs spindle assembly checkpoint function in mouse oocytes. Mol. Hum. Reprod. 2020, 26, 689–701. [Google Scholar] [CrossRef]
- Jia, B.; Xiang, D.; Fu, X.; Shao, Q.; Hong, Q.; Quan, G.; Wu, G. Proteomic Changes of Porcine Oocytes After Vitrification and Subsequent in vitro Maturation: A Tandem Mass Tag-Based Quantitative Analysis. Front. Cell. Dev. Biol. 2020, 8, 614577. [Google Scholar] [CrossRef]
- Shah, S.A.; Khan, M.; Jo, M.H.; Jo, M.G.; Amin, F.U.; Kim, M.O. Melatonin Stimulates the SIRT1/Nrf2 Signaling Pathway Counteracting Lipopolysaccharide (LPS)-Induced Oxidative Stress to Rescue Postnatal Rat Brain. CNS. Neurosci. Ther. 2017, 23, 33–44. [Google Scholar] [CrossRef]
- Tripathi, D.N.; Jena, G.B. Effect of melatonin on the expression of Nrf2 and NF-κB during cyclophosphamide-induced urinary bladder injury in rat. J. Pineal. Res. 2010, 48, 324–331. [Google Scholar] [CrossRef]


| Gene | Accession Number | Primer Sequence (5′–3′) | Tm (°C) |
|---|---|---|---|
| Nrf2 | NM_010902.4 | F:GTCTTCACTGCCCCTCATC R:TCGGGAATGGAAAATAGCTCC | 55 |
| Gclm | NM_008129.4 | F:CTTGGAGCATTTACAGCCTTAC R:GTGAGTCAGTAGCTGTATGTCA | 55 |
| Sod1 | NM_011434.2 | F:CACTCTAAGAAACATGGTGG R:GATCACACGATCTTCAATGG | 55 |
| Sod2 | NM_013671.3 | F:AAGGGAGATGTTACAACTCAGG R:GCTCAGGTTTGTCCAGAAAATG | 55 |
| Gapdh | NM_008084.3 | F:CATGGCCTTCCGTGTTCCTA R:GCCTGCTTACCACCTTCTT | 55 |
| Group | Melatonin (mol/L) | No. GV Oocytes Vitrified | No. GV Oocytes Recovered | No. GV Oocytes with Normal Morphology for (MI, MII) | No. GV Oocytes That Matured to | |
|---|---|---|---|---|---|---|
| MI Stage at 9 Hpi (%) | MII Stage at 13 Hpi (%) | |||||
| C | 0 | 60, 73 | 46(76.87 ± 1.82) a | 57(77.03 ± 1.97) a | ||
| V | 0 | 170 | 164 | 72, 73 | 43(59.42 ± 2.44) b | 41(56.92 ± 3.05) b |
| V + M | 10−7 | 151 | 145 | 65, 63 | 48(73.95 ± 0.53) a | 49(72.62 ± 2.03) a |
| V + M + B | 10−7 | 155 | 147 | 64, 63 | 39(59.85 ± 4.07) b | 35(56.01 ± 2.01) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Yang, J.; Qin, J.; Qazi, I.H.; Pan, B.; Zang, S.; Lv, T.; Deng, S.; Fang, Y.; Zhou, G. Melatonin Promotes In Vitro Maturation of Vitrified-Warmed Mouse Germinal Vesicle Oocytes, Potentially by Reducing Oxidative Stress through the Nrf2 Pathway. Animals 2021, 11, 2324. https://doi.org/10.3390/ani11082324
Guo S, Yang J, Qin J, Qazi IH, Pan B, Zang S, Lv T, Deng S, Fang Y, Zhou G. Melatonin Promotes In Vitro Maturation of Vitrified-Warmed Mouse Germinal Vesicle Oocytes, Potentially by Reducing Oxidative Stress through the Nrf2 Pathway. Animals. 2021; 11(8):2324. https://doi.org/10.3390/ani11082324
Chicago/Turabian StyleGuo, Shichao, Jinyu Yang, Jianpeng Qin, Izhar Hyder Qazi, Bo Pan, Shengqin Zang, Tianyi Lv, Shoulong Deng, Yi Fang, and Guangbin Zhou. 2021. "Melatonin Promotes In Vitro Maturation of Vitrified-Warmed Mouse Germinal Vesicle Oocytes, Potentially by Reducing Oxidative Stress through the Nrf2 Pathway" Animals 11, no. 8: 2324. https://doi.org/10.3390/ani11082324
APA StyleGuo, S., Yang, J., Qin, J., Qazi, I. H., Pan, B., Zang, S., Lv, T., Deng, S., Fang, Y., & Zhou, G. (2021). Melatonin Promotes In Vitro Maturation of Vitrified-Warmed Mouse Germinal Vesicle Oocytes, Potentially by Reducing Oxidative Stress through the Nrf2 Pathway. Animals, 11(8), 2324. https://doi.org/10.3390/ani11082324









