Cytokines That Serve as Embryokines in Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Cytokines of Interest and Their Signaling Pathways
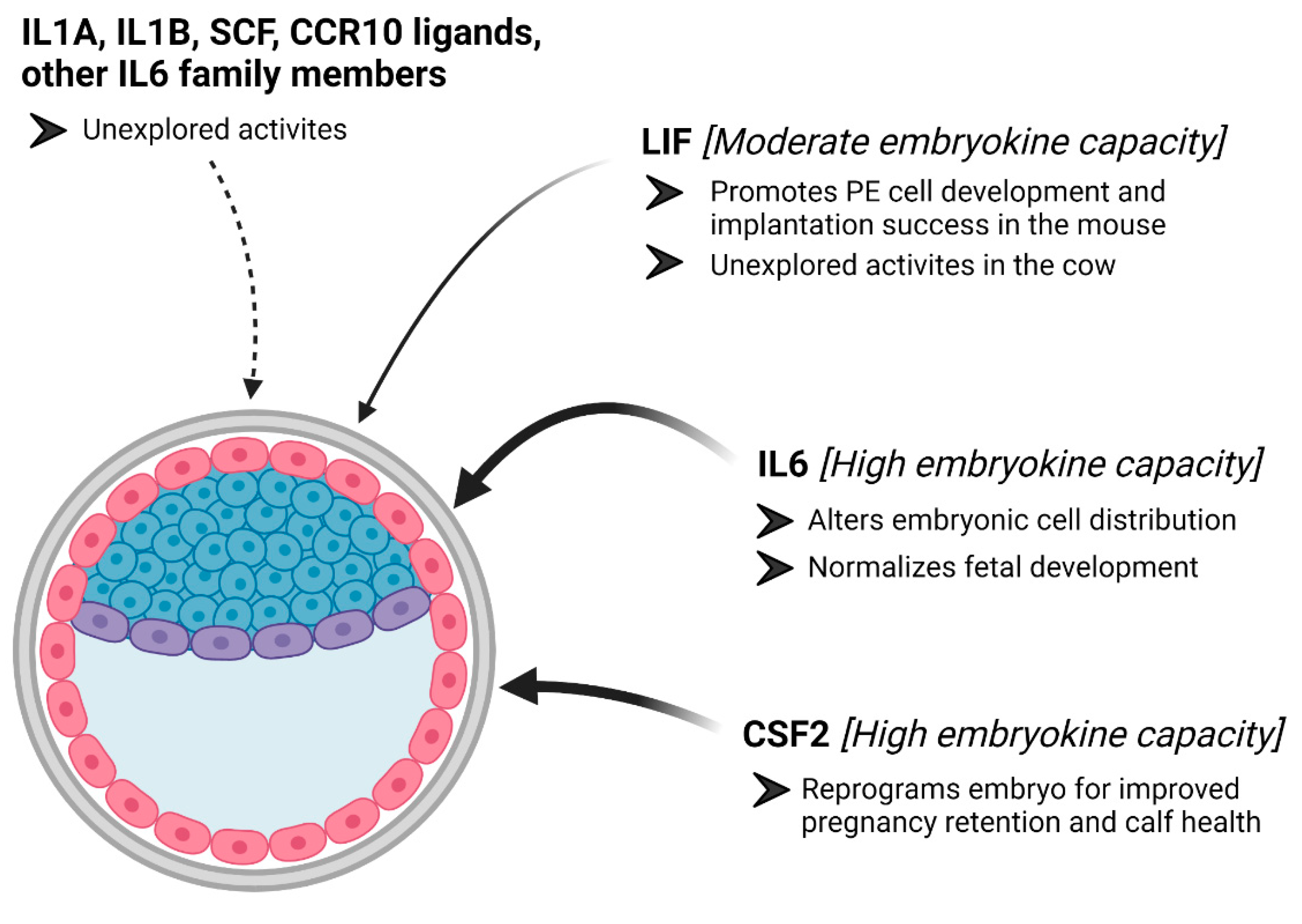
3. Colony Stimulating Factor 2
4. Interleukin 6
5. Leukemia Inhibitory Factor
6. Other Cytokines That May Serve as Embryokines
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viana, J.H. 2019 Statistics of embryo production and transfer in domestic farm animals. Embryo Technol. Newsl. 2019, 38, 1–15. [Google Scholar]
- Brackett, B.G.; Bousquet, D.; Boice, M.L.; Donawick, W.J.; Evans, J.F.; Dressel, M.A. Normal development following in vitro fertilization in the cow. Biol. Reprod. 1982, 27, 147–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonergan, P.; Rizos, D.; Gutierrez-Adan, A.; Fair, T.; Boland, M.P. Oocyte and embryo quality: Effect of origin, culture conditions and gene expression patterns. Reprod. Domest Anim. 2003, 38, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.; Fair, T. In vitro-produced bovine embryos: Dealing with the warts. Theriogenology 2008, 69, 17–22. [Google Scholar] [CrossRef]
- Ealy, A.D.; Wooldridge, L.K.; McCoski, S.R. BOARD INVITED REVIEW: Post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci. 2019, 97, 2555–2568. [Google Scholar] [CrossRef]
- Siqueira, L.G.B.; Dikmen, S.; Ortega, M.S.; Hansen, P.J. Postnatal phenotype of dairy cows is altered by in vitro embryo production using reverse X-sorted semen. J. Dairy Sci. 2017, 100, 5899–5908. [Google Scholar] [CrossRef] [Green Version]
- Hori, N.; Nagai, M.; Hirayama, M.; Hirai, T.; Matsuda, K.; Hayashi, M.; Tanaka, T.; Ozawa, T.; Horike, S. Aberrant CpG methylation of the imprinting control region KvDMR1 detected in assisted reproductive technology-produced calves and pathogenesis of large offspring syndrome. Anim. Reprod. Sci. 2010, 122, 303–312. [Google Scholar] [CrossRef]
- Suzuki, J., Jr.; Therrien, J.; Filion, F.; Lefebvre, R.; Goff, A.K.; Smith, L.C. In vitro culture and somatic cell nuclear transfer affect imprinting of SNRPN gene in pre- and post-implantation stages of development in cattle. BMC Dev. Biol. 2009, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Hagen, D.E.; Elsik, C.G.; Ji, T.; Morris, C.J.; Moon, L.E.; Rivera, R.M. Characterization of global loss of imprinting in fetal overgrowth syndrome induced by assisted reproduction. Proc. Natl. Acad. Sci. USA 2015, 112, 4618–4623. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, L.; Block, J.; Denicol, A.C.; Hansen, P.J. Consequences of transfer of an in vitro-produced embryo for the dam and resultant calf. J. Dairy Sci. 2014, 97, 229–239. [Google Scholar] [CrossRef] [Green Version]
- van Wagtendonk-de Leeuw, A.M.; Aerts, B.J.; den Daas, J.H. Abnormal offspring following in vitro production of bovine preimplantation embryos: A field study. Theriogenology 1998, 49, 883–894. [Google Scholar] [CrossRef]
- Hansen, P.J.; Dobbs, K.B.; Denicol, A.C. Programming of the preimplantation embryo by the embryokine colony stimulating factor 2. Anim. Reprod. Sci. 2014, 149, 59–66. [Google Scholar] [CrossRef]
- Block, J.; Drost, M.; Monson, R.L.; Rutledge, J.J.; Rivera, R.M.; Paula-Lopes, F.F.; Ocon, O.M.; Krininger, C.E., 3rd; Liu, J.; Hansen, P.J. Use of insulin-like growth factor-I during embryo culture and treatment of recipients with gonadotropin-releasing hormone to increase pregnancy rates following the transfer of in vitro-produced embryos to heat-stressed, lactating cows. J. Anim. Sci. 2003, 81, 1590–1602. [Google Scholar] [CrossRef]
- Jousan, F.D.; Hansen, P.J. Insulin-like growth factor-I promotes resistance of bovine preimplantation embryos to heat shock through actions independent of its anti-apoptotic actions requiring PI3K signaling. Mol. Reprod. Dev. 2007, 74, 189–196. [Google Scholar] [CrossRef]
- Xie, M.; McCoski, S.R.; Johnson, S.E.; Rhoads, M.L.; Ealy, A.D. Combinatorial effects of epidermal growth factor, fibroblast growth factor 2 and insulin-like growth factor 1 on trophoblast cell proliferation and embryogenesis in cattle. Reprod. Fertil. Dev. 2017, 29, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Kelso, A. Cytokines: Principles and prospects. Immunol. Cell. Biol. 1998, 76, 300–317. [Google Scholar] [CrossRef]
- Dougan, M.; Dranoff, G.; Dougan, S.K. GM-CSF, IL-3, and IL-5 Family of Cytokines: Regulators of Inflammation. Immunity 2019, 50, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Becher, B.; Tugues, S.; Greter, M. GM-CSF: From Growth Factor to Central Mediator of Tissue Inflammation. Immunity 2016, 45, 963–973. [Google Scholar] [CrossRef] [Green Version]
- Eulenfeld, R.; Dittrich, A.; Khouri, C.; Muller, P.J.; Mutze, B.; Wolf, A.; Schaper, F. Interleukin-6 signalling: More than Jaks and STATs. Eur J. Cell Biol. 2012, 91, 486–495. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicola, N.A.; Babon, J.J. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev. 2015, 26, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Sjoblom, C.; Roberts, C.T.; Wikland, M.; Robertson, S.A. Granulocyte-macrophage colony-stimulating factor alleviates adverse consequences of embryo culture on fetal growth trajectory and placental morphogenesis. Endocrinology 2005, 146, 2142–2153. [Google Scholar] [CrossRef]
- Lee, K.; Redel, B.K.; Spate, L.; Teson, J.; Brown, A.N.; Park, K.W.; Walters, E.; Samuel, M.; Murphy, C.N.; Prather, R.S. Piglets produced from cloned blastocysts cultured in vitro with GM-CSF. Mol. Reprod. Dev. 2012. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.S.; Lee, J.Y.; Choi, S.H.; Kwon, M.S.; Kim, T.; Kim, N.H. Mouse granulocyte-macrophage colony-stimulating factor enhances viability of porcine embryos in defined culture conditions. Anim. Reprod. Sci. 2004, 84, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Sjoblom, C.; Wikland, M.; Robertson, S.A. Granulocyte-macrophage colony-stimulating factor promotes human blastocyst development in vitro. Hum. Reprod. 1999, 14, 3069–3076. [Google Scholar] [CrossRef] [Green Version]
- Loureiro, B.; Bonilla, L.; Block, J.; Fear, J.M.; Bonilla, A.Q.; Hansen, P.J. Colony-stimulating factor 2 (CSF-2) improves development and posttransfer survival of bovine embryos produced in vitro. Endocrinology 2009, 150, 5046–5054. [Google Scholar] [CrossRef] [Green Version]
- Neira, J.A.; Tainturier, D.; Pena, M.A.; Martal, J. Effect of the association of IGF-I, IGF-II, bFGF, TGF-beta1, GM-CSF, and LIF on the development of bovine embryos produced in vitro. Theriogenology 2010, 73, 595–604. [Google Scholar] [CrossRef]
- de Moraes, A.A.; Davidson, J.A.; Fleming, J.G.; Bazer, F.W.; Edwards, J.L.; Betts, J.G.; Hansen, P.J. Lack of effect of granulocyte-macrophage colony-stimulating factor on secretion of interferon-tau, other proteins, and prostaglandin E2 by the bovine and ovine conceptus. Domest. Anim. Endocrinol. 1997, 14, 193–197. [Google Scholar] [CrossRef]
- Tribulo, P.; Bernal Ballesteros, B.H.; Ruiz, A.; Tribulo, A.; Tribulo, R.J.; Tribulo, H.E.; Bo, G.A.; Hansen, P.J. Consequences of exposure of embryos produced in vitro in a serum-containing medium to dickkopf-related protein 1 and colony stimulating factor 2 on blastocyst yield, pregnancy rate, and birth weight. J. Anim. Sci. 2017, 95, 4407–4412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobbs, K.B.; Khan, F.A.; Sakatani, M.; Moss, J.I.; Ozawa, M.; Ealy, A.D.; Hansen, P.J. Regulation of pluripotency of inner cell mass and growth and differentiation of trophectoderm of the bovine embryo by colony stimulating factor 2. Biol. Reprod. 2013, 89, 141. [Google Scholar] [CrossRef] [Green Version]
- Hickman, C.F.; Ainslie, A.; Ealy, A.D.; Ashworth, C.J.; Rooke, J.A. Effect of ovine granulocyte-macrophage colony-stimulating factor on bovine in vitro embryo development and blastocyst interferon-tau secretion. Reprod. Domest. Anim. 2011, 46, 608–615. [Google Scholar] [CrossRef]
- Zolini, A.M.; Block, J.; Rabaglino, M.B.; Tribulo, P.; Hoelker, M.; Rincon, G.; Bromfield, J.J.; Hansen, P.J. Molecular fingerprint of female bovine embryos produced in vitro with high competence to establish and maintain pregnancydagger. Biol. Reprod. 2020, 102, 292–305. [Google Scholar] [CrossRef]
- Sosa, F.; Block, J.; Xiao, Y.; Hansen, P.J. Determinants of survival of the bovine blastocyst to cryopreservation stress: Treatment with colony stimulating factor 2 during the morula-to-blastocyst transition and embryo sex. CABI Agric. Biosci. 2020, 1. [Google Scholar] [CrossRef]
- Loureiro, B.; Oliveira, L.J.; Favoreto, M.G.; Hansen, P.J. Colony-stimulating Factor 2 Inhibits Induction of Apoptosis in the Bovine Preimplantation Embryo. Am. J. Reprod. Immunol. 2011. [Google Scholar] [CrossRef]
- Denicol, A.C.; Block, J.; Kelley, D.E.; Pohler, K.G.; Dobbs, K.B.; Mortensen, C.J.; Ortega, M.S.; Hansen, P.J. The WNT signaling antagonist Dickkopf-1 directs lineage commitment and promotes survival of the preimplantation embryo. FASEB J. 2014, 28, 3975–3986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozawa, M.; Sakatani, M.; Dobbs, K.B.; Kannampuzha-Francis, J.; Hansen, P.J. Regulation of gene expression in the bovine blastocyst by colony stimulating factor 2. BMC Res. Notes 2016, 9, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannampuzha-Francis, J.; Denicol, A.C.; Loureiro, B.; Kaniyamattam, K.; Ortega, M.S.; Hansen, P.J. Exposure to colony stimulating factor 2 during preimplantation development increases postnatal growth in cattle. Mol. Reprod. Dev. 2015, 82, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Ziebe, S.; Loft, A.; Povlsen, B.B.; Erb, K.; Agerholm, I.; Aasted, M.; Gabrielsen, A.; Hnida, C.; Zobel, D.P.; Munding, B.; et al. A randomized clinical trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) in embryo culture medium for in vitro fertilization. Fertil. Steril. 2013, 99, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Fu, L.; Zhou, W.; Li, Y. Relationship between granulocyte-macrophage colony-stimulating factor, embryo quality, and pregnancy outcomes in women of different ages in fresh transfer cycles: A retrospective study. J. Obstet. Gynaecol. 2020, 40, 626–632. [Google Scholar] [CrossRef]
- Loureiro, B.; Block, J.; Favoreto, M.G.; Carambula, S.; Pennington, K.A.; Ealy, A.D.; Hansen, P.J. Consequences of conceptus exposure to colony-stimulating factor 2 on survival, elongation, interferon-{tau} secretion, and gene expression. Reproduction 2011, 141, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Michael, D.D.; Wagner, S.K.; Ocon, O.M.; Talbot, N.C.; Rooke, J.A.; Ealy, A.D. Granulocyte-macrophage colony-stimulating-factor increases interferon-tau protein secretion in bovine trophectoderm cells. Am. J. Reprod. Immunol. 2006, 56, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, K.B.; Gagne, D.; Fournier, E.; Dufort, I.; Robert, C.; Block, J.; Sirard, M.A.; Bonilla, L.; Ealy, A.D.; Loureiro, B.; et al. Sexual dimorphism in developmental programming of the bovine preimplantation embryo caused by colony-stimulating factor 2. Biol. Reprod. 2014, 91, 80. [Google Scholar] [CrossRef]
- Siqueira, L.G.; Hansen, P.J. Sex differences in response of the bovine embryo to colony-stimulating factor 2. Reproduction 2016, 152, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, L.G.; Tribulo, P.; Chen, Z.; Denicol, A.C.; Ortega, M.S.; Negron-Perez, V.M.; Kannampuzha-Francis, J.; Pohler, K.G.; Rivera, R.M.; Hansen, P.J. Colony-stimulating factor 2 acts from days 5 to 7 of development to modify programming of the bovine conceptus at day 86 of gestationdagger. Biol. Reprod. 2017, 96, 743–757. [Google Scholar] [CrossRef]
- Li, Y.; Tribulo, P.; Bakhtiarizadeh, M.R.; Siqueira, L.G.; Ji, T.; Rivera, R.M.; Hansen, P.J. Conditions of embryo culture from days 5 to 7 of development alter the DNA methylome of the bovine fetus at day 86 of gestation. J. Assist. Reprod. Genet. 2020, 37, 417–426. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, J.; Dong, H.; Luo, O.; Zheng, X.; Obergfell, C.; Tang, Y.; Bi, J.; O’Neill, R.; Ruan, Y.; et al. Transcriptional profiles of bovine in vivo pre-implantation development. BMC Genom. 2014, 15, 756. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Uh, K.; Negron-Perez, V.M.; Haines, H.; Lee, K.; Hansen, P.J. Regulation of gene expression in the bovine blastocyst by colony-stimulating factor 2 is disrupted by CRISPR/Cas9-mediated deletion of CSF2RA. Biol. Reprod. 2021, 104, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Tribulo, P.; Siqueira, L.G.B.; Oliveira, L.J.; Scheffler, T.; Hansen, P.J. Identification of potential embryokines in the bovine reproductive tract. J. Dairy Sci. 2018, 101, 690–704. [Google Scholar] [CrossRef] [Green Version]
- Wooldridge, L.K.; Ealy, A.D. Interleukin-6 increases inner cell mass numbers in bovine embryos. BMC Dev. Biol. 2019, 19, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wooldridge, L.K.; Johnson, S.E.; Cockrum, R.R.; Ealy, A.D. Interleukin-6 requires JAK to stimulate inner cell mass expansion in bovine embryos. Reproduction 2019. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Ortiz, W.; Xiao, Y.; Estrada-Cortes, E.; Jannaman, E.A.; Hansen, P.J. Actions of putative embryokines on development of the preimplantation bovine embryo to the blastocyst stage. J. Dairy Sci. 2020, 103, 11930–11944. [Google Scholar] [CrossRef]
- Seekford, Z.K.; Wooldridge, L.K.; Dias, N.W.; Timlin, C.L.; Sales, A.F.; Speckhart, S.L.; Pohler, K.G.; Cockrum, R.R.; Mercadante, V.R.G.; Ealy, A.D. Interleukin-6 supplementation improves post-transfer embryonic and fetal development of in vitro-produced bovine embryos. Theriogenology 2021, 170, 15–22. [Google Scholar] [CrossRef]
- Eckert, J.J.; Fleming, T.P. Tight junction biogenesis during early development. Biochim. Biophys. Acta 2008, 1778, 717–728. [Google Scholar] [CrossRef] [Green Version]
- Al-Sadi, R.; Ye, D.; Boivin, M.; Guo, S.; Hashimi, M.; Ereifej, L.; Ma, T.Y. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS ONE 2014, 9, e85345. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [Green Version]
- Wooldridge, L.K.; Ealy, A.D. Interleukin-6 promotes primitive endoderm development in bovine blastocysts. BMC Dev. Biol. 2021, 21, 3. [Google Scholar] [CrossRef]
- Van den Abbeel, E.; Balaban, B.; Ziebe, S.; Lundin, K.; Cuesta, M.J.; Klein, B.M.; Helmgaard, L.; Arce, J.C. Association between blastocyst morphology and outcome of single-blastocyst transfer. Reprod. Biomed. Online 2013, 27, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Maddox-Hyttell, P.; Gjorret, J.O.; Vajta, G.; Alexopoulos, N.I.; Lewis, I.; Trounson, A.; Viuff, D.; Laurincik, J.; Muller, M.; Tveden-Nyborg, P.; et al. Morphological assessment of preimplantation embryo quality in cattle. Reprod. Suppl. 2003, 61, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Yoshiba, N.; Ushijima, H.; Watanabe, S.; Nakahara, T. Morphology and proportion of inner cell mass of bovine blastocysts fertilized in vitro and in vivo. J. Reprod. Fertil 1990, 90, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Pomar, F.J.; Teerds, K.J.; Kidson, A.; Colenbrander, B.; Tharasanit, T.; Aguilar, B.; Roelen, B.A. Differences in the incidence of apoptosis between in vivo and in vitro produced blastocysts of farm animal species: A comparative study. Theriogenology 2005, 63, 2254–2268. [Google Scholar] [CrossRef] [PubMed]
- Gjorret, J.O.; Knijn, H.M.; Dieleman, S.J.; Avery, B.; Larsson, L.I.; Maddox-Hyttel, P. Chronology of apoptosis in bovine embryos produced in vivo and in vitro. Biol. Reprod. 2003, 69, 1193–1200. [Google Scholar] [CrossRef]
- Knijn, H.M.; Gjorret, J.O.; Vos, P.L.; Hendriksen, P.J.; van der Weijden, B.C.; Maddox-Hyttel, P.; Dieleman, S.J. Consequences of in vivo development and subsequent culture on apoptosis, cell number, and blastocyst formation in bovine embryos. Biol. Reprod. 2003, 69, 1371–1378. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, M.; Beam, S.W.; Shim, H.; Bertolini, L.R.; Moyer, A.L.; Famula, T.R.; Anderson, G.B. Growth, development, and gene expression by in vivo- and in vitro-produced day 7 and 16 bovine embryos. Mol. Reprod. Dev. 2002, 63, 318–328. [Google Scholar] [CrossRef]
- Fischer-Brown, A.E.; Lindsey, B.R.; Ireland, F.A.; Northey, D.L.; Monson, R.L.; Clark, S.G.; Wheeler, M.B.; Kesler, D.J.; Lane, S.J.; Weigel, K.A.; et al. Embryonic disc development and subsequent viability of cattle embryos following culture in two media under two oxygen concentrations. Reprod. Fertil Dev. 2004, 16, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Mason, J.B.; Beam, S.W.; Carneiro, G.F.; Sween, M.L.; Kominek, D.J.; Moyer, A.L.; Famula, T.R.; Sainz, R.D.; Anderson, G.B. Morphology and morphometry of in vivo- and in vitro-produced bovine concepti from early pregnancy to term and association with high birth weights. Theriogenology 2002, 58, 973–994. [Google Scholar] [CrossRef]
- Block, J.; Fischer-Brown, A.E.; Rodina, T.M.; Ealy, A.D.; Hansen, P.J. The effect of in vitro treatment of bovine embryos with IGF-1 on subsequent development in utero to Day 14 of gestation. Theriogenology 2007, 68, 153–161. [Google Scholar] [CrossRef]
- Fischer-Brown, A.; Monson, R.; Parrish, J.; Rutledge, J. Cell allocation in bovine embryos cultured in two media under two oxygen concentrations. Zygote 2002, 10, 341–348. [Google Scholar] [CrossRef]
- Arman, E.; Haffner-Krausz, R.; Chen, Y.; Heath, J.K.; Lonai, P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc. Natl. Acad. Sci. USA 1998, 95, 5082–5087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalaby, F.; Rossant, J.; Yamaguchi, T.P.; Gertsenstein, M.; Wu, X.F.; Breitman, M.L.; Schuh, A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995, 376, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Assis Neto, A.C.; Pereira, F.T.; Santos, T.C.; Ambrosio, C.E.; Leiser, R.; Miglino, M.A. Morpho-physical recording of bovine conceptus (Bos indicus) and placenta from days 20 to 70 of pregnancy. Reprod. Domest Anim. 2010, 45, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, J.S.; Murray, R.W.; Foley, R.C. Observations on the morphogenesis and histochemistry of the bovine preattachment placenta between 16 and 33 days of gestation. Anat. Rec. 1958, 132, 321–341. [Google Scholar] [CrossRef]
- Mess, A.M.; Carreira, A.C.O.; Marinovic de Oliveira, C.; Fratini, P.; Favaron, P.O.; Barreto, R.; Pfarrer, C.; Meirelles, F.V.; Miglino, M.A. Vascularization and VEGF expression altered in bovine yolk sacs from IVF and NT technologies. Theriogenology 2017, 87, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.L.; Meirelles, F.V.; Perecin, F.; Ambrosio, C.E.; Favaron, P.O.; Franciolli, A.L.; Mess, A.M.; Dos Santos, J.M.; Rici, R.E.; Bertolini, M.; et al. Development of bovine embryos derived from reproductive techniques. Reprod. Fertil Dev. 2013, 25, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, F.S.; Oliveira, V.C.; Mancanares, C.A.; Ambrosio, C.E.; Miglino, M.A. Characterization of yolk sac proteins of Bos indicus cattle embryos. Genet. Mol. Res. 2012, 11, 3942–3954. [Google Scholar] [CrossRef] [Green Version]
- Galdos-Riveros, A.C.; Favaron, P.O.; Will, S.E.; Miglino, M.A.; Maria, D.A. Bovine yolk sac: From morphology to metabolomic and proteomic profiles. Genet. Mol. Res. 2015, 14, 6223–6238. [Google Scholar] [CrossRef]
- Oliveira, V.C.; Mancanares, C.A.; Oliveira, L.J.; Goncalves, N.J.; Miglino, M.A.; Perecin, F.; Meirelles, F.V.; Piedrahita, J.; Ambrosio, C.E. Characterization of putative haematopoietic cells from bovine yolk sac. J. Tissue Eng. Regen. Med. 2017, 11, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Vailes, M.T.; McCoski, S.R.; Wooldridge, L.K.; Reese, S.T.; Pohler, K.G.; Roper, D.A.; Mercadante, V.R.; Ealy, A.D. Post-transfer outcomes in cultured bovine embryos supplemented with epidermal growth factor, fibroblast growth factor 2, and insulin-like growth factor 1. Theriogenology 2019, 124, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, F.; Forrester-Gauntlett, B.; Turner, P.; Henderson, H.; Oback, B. Signal Inhibition Reveals JAK/STAT3 Pathway as Critical for Bovine Inner Cell Mass Development. Biol. Reprod. 2015, 93, 132. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.H.; Cui, X.S.; Lee, S.H.; Kim, N.H. Interleukin-6 enhances porcine parthenote development in vitro, through the IL-6/Stat3 signaling pathway. J. Reprod. Dev. 2012, 58, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Mathialagan, N.; Bixby, J.A.; Roberts, R.M. Expression of interleukin-6 in porcine, ovine, and bovine preimplantation conceptuses. Mol. Reprod. Dev. 1992, 32, 324–330. [Google Scholar] [CrossRef]
- Cao, S.; Han, J.; Wu, J.; Li, Q.; Liu, S.; Zhang, W.; Pei, Y.; Ruan, X.; Liu, Z.; Wang, X.; et al. Specific gene-regulation networks during the pre-implantation development of the pig embryo as revealed by deep sequencing. BMC Genom. 2014, 15, 4. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.H.; Han, Y.J.; Zhang, D.X.; Cui, X.S.; Kim, N.H. A link between the interleukin-6/Stat3 anti-apoptotic pathway and microRNA-21 in preimplantation mouse embryos. Mol. Reprod. Dev. 2009, 76, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Do, D.V.; Ueda, J.; Messerschmidt, D.M.; Lorthongpanich, C.; Zhou, Y.; Feng, B.; Guo, G.; Lin, P.J.; Hossain, M.Z.; Zhang, W.; et al. A genetic and developmental pathway from STAT3 to the OCT4-NANOG circuit is essential for maintenance of ICM lineages in vivo. Genes Dev. 2013, 27, 1378–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Tian, X.C. JAK-STAT3 and somatic cell reprogramming. JAKSTAT 2013, 2, e24935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, J.J.; Li, M.; Suthram, S.; Jiang, H.; Wong, W.H.; Blau, H.M. Early role for IL-6 signalling during generation of induced pluripotent stem cells revealed by heterokaryon RNA-Seq. Nat. Cell Biol. 2013, 15, 1244–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Huang, J.; Chen, T.; Wang, Y.; Xin, S.; Li, J.; Pei, G.; Kang, J. Yamanaka factors critically regulate the developmental signaling network in mouse embryonic stem cells. Cell Res. 2008, 18, 1177–1189. [Google Scholar] [CrossRef]
- Lavranos, T.C.; Rathjen, P.D.; Seamark, R.F. Trophic effects of myeloid leukaemia inhibitory factor (LIF) on mouse embryos. J. Reprod. Fertil 1995, 105, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Fedorcsak, P.; Storeng, R. Effects of leptin and leukemia inhibitory factor on preimplantation development and STAT3 signaling of mouse embryos in vitro. Biol. Reprod. 2003, 69, 1531–1538. [Google Scholar] [CrossRef]
- Kimber, S.J. Leukaemia inhibitory factor in implantation and uterine biology. Reproduction 2005, 130, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.L. Leukaemia inhibitory factor and the regulation of pre-implantation development of the mammalian embryo. Mol. Reprod. Dev. 1994, 39, 233–238. [Google Scholar] [CrossRef]
- Vejlsted, M.; Avery, B.; Gjorret, J.O.; Maddox-Hyttel, P. Effect of leukemia inhibitory factor (LIF) on in vitro produced bovine embryos and their outgrowth colonies. Mol. Reprod. Dev. 2005, 70, 445–454. [Google Scholar] [CrossRef]
- Fukui, Y.; Matsuyama, K. Development of in vitro matured and fertilized bovine embryos cultured in media containing human leukemia inhibitory factor. Theriogenology 1994, 42, 663–673. [Google Scholar] [CrossRef]
- Rodriguez, A.; De Frutos, C.; Diez, C.; Caamano, J.N.; Facal, N.; Duque, P.; Garcia-Ochoa, C.; Gomez, E. Effects of human versus mouse leukemia inhibitory factor on the in vitro development of bovine embryos. Theriogenology 2007, 67, 1092–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirisathien, S.; Hernandez-Fonseca, H.J.; Bosch, P.; Hollet, B.R.; Lott, J.D.; Brackett, B.G. Effect of leukemia inhibitory factor on bovine embryos produced in vitro under chemically defined conditions. Theriogenology 2003, 59, 1751–1763. [Google Scholar] [CrossRef]
- Kocyigit, A.; Cevik, M. Leucemia inhibitory factor; investigating the time-dependent effect on viability of vitrified bovine embryos. Reprod. Domest. Anim. 2017, 52, 1113–1119. [Google Scholar] [CrossRef]
- Morgani, S.M.; Brickman, J.M. LIF supports primitive endoderm expansion during pre-implantation development. Development 2015, 142, 3488–3499. [Google Scholar] [CrossRef] [Green Version]
- Eckert, J.; Niemann, H. mRNA expression of leukaemia inhibitory factor (LIF) and its receptor subunits glycoprotein 130 and LIF-receptor-beta in bovine embryos derived in vitro or in vivo. Mol. Hum. Reprod. 1998, 4, 957–965. [Google Scholar] [CrossRef] [Green Version]
- Kocyigit, A.; Cevik, M. Effects of leukemia inhibitory factor and insulin-like growth factor-I on the cell allocation and cryotolerance of bovine blastocysts. Cryobiology 2015, 71, 64–69. [Google Scholar] [CrossRef]
- Ware, C.B.; Horowitz, M.C.; Renshaw, B.R.; Hunt, J.S.; Liggitt, D.; Koblar, S.A.; Gliniak, B.C.; McKenna, H.J.; Papayannopoulou, T.; Thoma, B.; et al. Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal, neural and metabolic defects and results in perinatal death. Development 1995, 121, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Telugu, B.P.; Ezashi, T.; Sinha, S.; Alexenko, A.P.; Spate, L.; Prather, R.S.; Roberts, R.M. Leukemia inhibitory factor (LIF)-dependent, pluripotent stem cells established from inner cell mass of porcine embryos. J. Biol. Chem. 2011, 286, 28948–28953. [Google Scholar] [CrossRef] [Green Version]
- Bourillot, P.Y.; Santamaria, C.; David, L.; Savatier, P. GP130 signaling and the control of naive pluripotency in humans, monkeys, and pigs. Exp. Cell Res. 2020, 386, 111712. [Google Scholar] [CrossRef]
- Xiao, Y.; Amaral, T.F.; Ross, P.J.; Soto, D.A.; Diffenderfer, K.E.; Pankonin, A.R.; Jeensuk, S.; Tribulo, P.; Hansen, P.J. Importance of WNT-dependent signaling for derivation and maintenance of primed pluripotent bovine embryonic stem cells. Biol. Reprod. 2021. [Google Scholar] [CrossRef]
- Bogliotti, Y.S.; Wu, J.; Vilarino, M.; Okamura, D.; Soto, D.A.; Zhong, C.; Sakurai, M.; Sampaio, R.V.; Suzuki, K.; Izpisua Belmonte, J.C.; et al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2090–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imakawa, K.; Tamura, K.; McGuire, W.J.; Khan, S.; Harbison, L.A.; Stanga, J.P.; Helmer, S.D.; Christenson, R.K. Effect of interleukin-3 on ovine trophoblast interferon during early conceptus development. Endocrine 1995, 3, 511–517. [Google Scholar] [CrossRef]
- Al Naib, A.; Mamo, S.; O’Gorman, G.M.; Lonergan, P.; Swales, A.; Fair, T. Regulation of non-classical major histocompatability complex class I mRNA expression in bovine embryos. J. Reprod. Immunol. 2011, 91, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Lupicka, M.; Bodek, G.; Shpigel, N.; Elnekave, E.; Korzekwa, A.J. Identification of pluripotent cells in bovine uterus: In situ and in vitro studies. Reproduction 2015, 149, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia-Alvarez, E.; Gomez, E.; Martin, D.; Carrocera, S.; Perez, S.; Otero, J.; Peynot, N.; Giraud-Delville, C.; Caamano, J.N.; Sandra, O.; et al. Expression and localization of interleukin 1 beta and interleukin 1 receptor (type I) in the bovine endometrium and embryo. J. Reprod. Immunol. 2015, 110, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Paula-Lopes, F.F.; de Moraes, A.A.; Edwards, J.L.; Justice, J.E.; Hansen, P.J. Regulation of preimplantation development of bovine embryos by interleukin-1beta. Biol. Reprod. 1998, 59, 1406–1412. [Google Scholar] [CrossRef] [Green Version]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef]
- Oliveira, L.J.; Mansouri-Attia, N.; Fahey, A.G.; Browne, J.; Forde, N.; Roche, J.F.; Lonergan, P.; Fair, T. Characterization of the Th profile of the bovine endometrium during the oestrous cycle and early pregnancy. PLoS ONE 2013, 8, e75571. [Google Scholar] [CrossRef] [PubMed]

| Cytokine Transcripts Endometrium 1 | Cytokine Transcripts Blastocyst 2 | Cytokine Receptor Transcripts Blastocyst 2 |
|---|---|---|
| CXCL3 | CCL17 | IL6R |
| IL8 | IFNT | IL6ST |
| CXCL12 | IL18 | IL2RB |
| CSF2 | IL1RN | IL10RB |
| IL1A | CXCL5 | IL13RA |
| CXCL10 | CCL24 | IFNGR |
| CX3CL1 | IL6 | IL36RN |
| CXCL16 | CXCL16 | IFNAR |
| CCL14 | IL27 | IL11RA |
| IL18 | CTF1 | IL17RA |
| IL33 | CLCF1 | IL20RB |
| IL6 | IL20RA | |
| IL16 | IL1R1 | |
| IL12A | IL17RC | |
| IL12B | CSF2RA | |
| IL34 | CCR10 | |
| IL1B | CNTFR | |
| CCL21 | LIFR | |
| IL15 | IL1RAP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ealy, A.D.; Speckhart, S.L.; Wooldridge, L.K. Cytokines That Serve as Embryokines in Cattle. Animals 2021, 11, 2313. https://doi.org/10.3390/ani11082313
Ealy AD, Speckhart SL, Wooldridge LK. Cytokines That Serve as Embryokines in Cattle. Animals. 2021; 11(8):2313. https://doi.org/10.3390/ani11082313
Chicago/Turabian StyleEaly, Alan D., Savannah L. Speckhart, and Lydia K. Wooldridge. 2021. "Cytokines That Serve as Embryokines in Cattle" Animals 11, no. 8: 2313. https://doi.org/10.3390/ani11082313
APA StyleEaly, A. D., Speckhart, S. L., & Wooldridge, L. K. (2021). Cytokines That Serve as Embryokines in Cattle. Animals, 11(8), 2313. https://doi.org/10.3390/ani11082313






