The Combined Effect of Weaning Stress and Immune Activation during Pig Gestation on Serum Cytokine and Analyte Concentrations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry Analysis
2.2. Cytokine and Cortisol Analysis
2.3. Statistical Modeling and Testing
3. Results
3.1. Effect of Maternal Immune Activation, Weaning and Sex on Serum Chemistry and Cortisol Levels
3.2. Effect of Maternal Immune Activation, Weaning and Sex on Serum Immune Parameter Levels
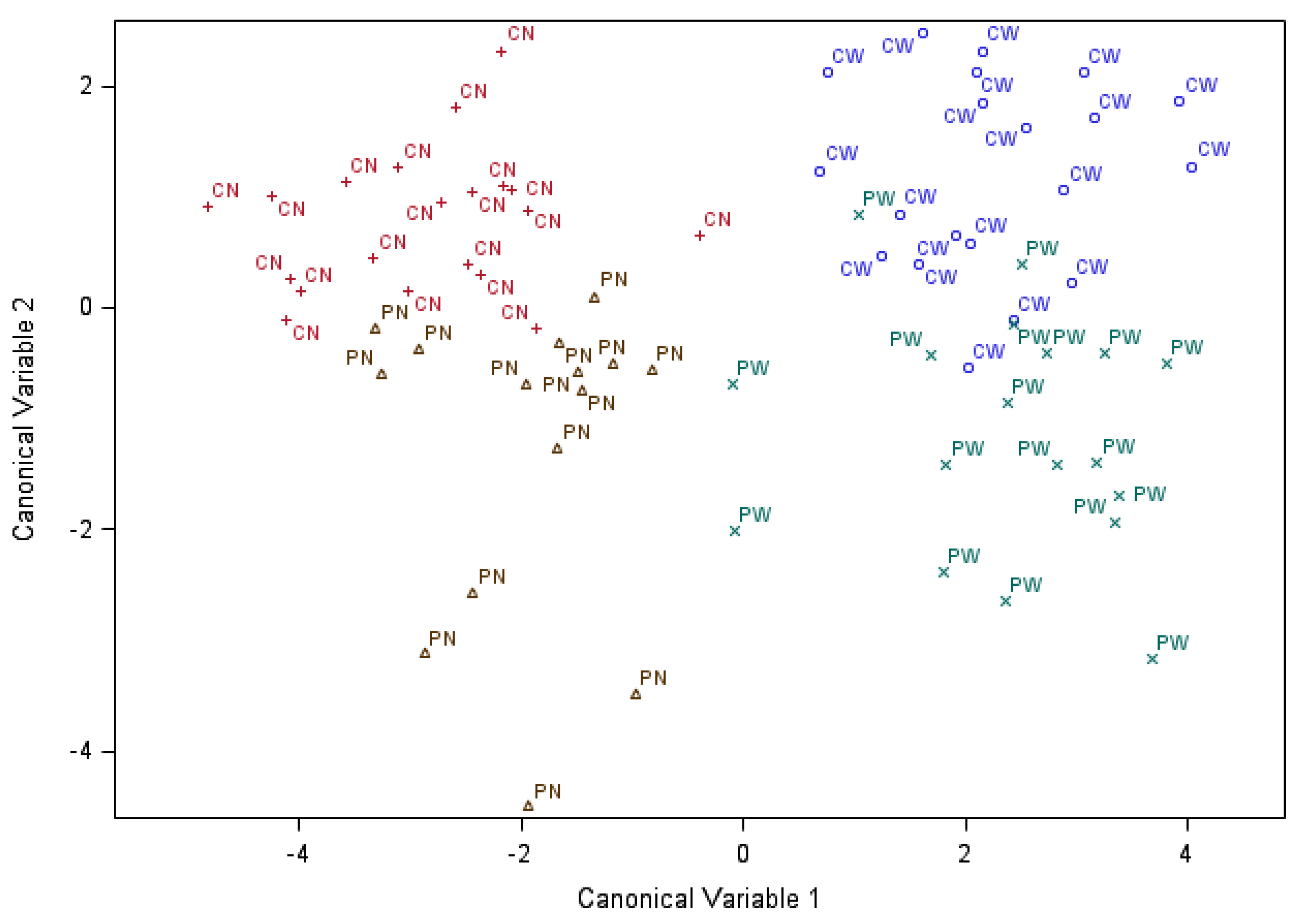
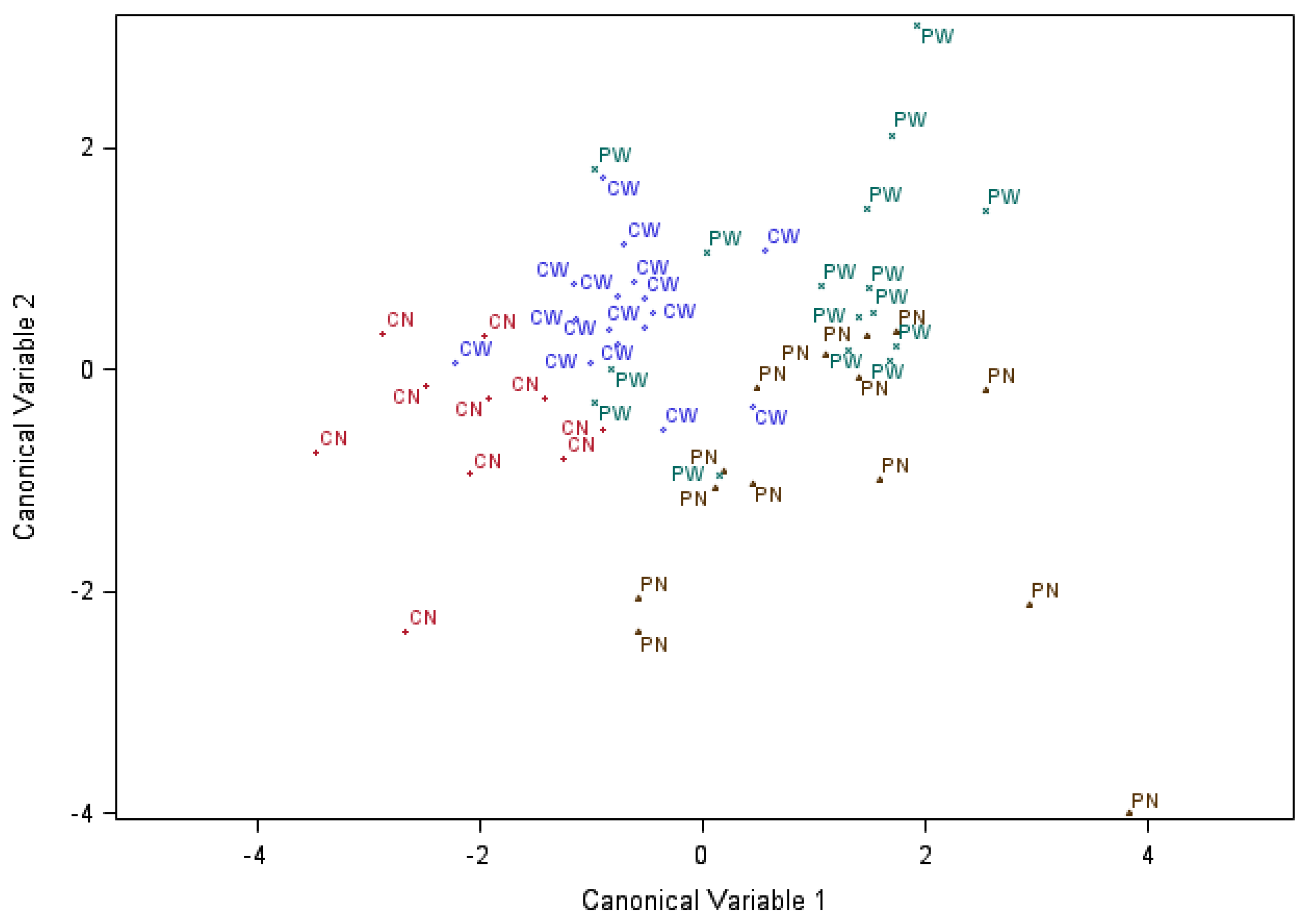
3.3. Discriminant Analysis of the Effects of Maternal Immune Activation, Weaning and Sex on Serum Chemistry and Immune Parameters
4. Discussion
4.1. Maternal Immune Activation, Weaning and Sex Effects on Serum Chemistry and Cortisol Levels
4.2. Maternal Immune Activation, Weaning and Sex Effects on Serum Immune Parameter Levels
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curley, J.P.; Jordan, E.R.; Swaney, W.T.; Izraelit, A.; Kammel, S.; Champagne, F.A. The Meaning of Weaning: Influence of the Weaning Period on Behavioral Development in Mice. Dev. Neurosci. 2009, 31, 318–331. [Google Scholar] [CrossRef]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef]
- Pié, S.; Lallès, J.P.; Blazy, F.; Laffitte, J.; Sève, B.; Oswald, I.P. Weaning is sssociated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [CrossRef]
- Gimsa, U.; Tuchscherer, M.; Kanitz, E. Psychosocial Stress and Immunity—What can we learn from pig studies? Front. Behav. Neurosci. 2018, 12, 64. [Google Scholar] [CrossRef]
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.F.; Blisklager, A.T.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 298, G352–G363. [Google Scholar] [CrossRef]
- Kick, A.R.; Tompkins, M.B.; Flowers, W.L.; Whisnant, C.S.; Almond, G.W. Effects of stress associated with weaning on the adaptive immune system in pigs1. J. Anim. Sci. 2012, 90, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Ribic, A.; de Roo, C.C.; Fuchs, E. Differential effects of maternal immune activation and juvenile stress on anxiety-like behaviour and physiology in adult rats: No evidence for the “double-hit hypothesis”. Behav. Brain Res. 2011, 224, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Nakamura, T.; Byrne, R.J.; Naicker, S.; Tynan, R.J.; Hunter, M.; Hodgson, D.M. Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: Implications for the double-hit hypothesis. Psychoneuroendocrinology 2009, 34, 1515–1525. [Google Scholar] [CrossRef]
- Maynard, T.M.; Sikich, L.; Lieberman, J.A.; LaMantia, A.-S. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr. Bull. 2001, 27, 457–476. [Google Scholar] [CrossRef]
- Markham, J.A.; Koenig, J.I. Prenatal stress: Role in psychotic and depressive diseases. Psychopharmacology 2011, 214, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Imanaka, A.; Morinobu, S.; Toki, S.; Yamawaki, S. Importance of early environment in the development of post-traumatic stress disorder-like behaviors. Behav. Brain Res. 2006, 173, 129–137. [Google Scholar] [CrossRef]
- Giovanoli, S.; Engler, H.; Engler, A.; Richetto, J.; Voget, M.; Willi, R.; Winter, C.; Riva, M.A.; Mortensen, P.B.; Feldon, J.; et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 2013, 339, 1095–1099. [Google Scholar] [CrossRef]
- Fair, T. The contribution of the maternal immune system to the establishment of pregnancy in cattle. Front. Immunol. 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Boulanger-Bertolus, J.; Pancaro, C.; Mashour, G.A. Increasing role of maternal immune activation in neurodevelopmental disorders. Front. Behav. Neurosci. 2018, 12, 230. [Google Scholar] [CrossRef]
- Antonson, A.M.; Radlowski, E.C.; Lawson, M.A.; Rytych, J.L.; Johnson, R.W. Maternal viral infection during pregnancy elicits anti-social behavior in neonatal piglet offspring independent of postnatal microglial cell activation. Brain Behav. Immun. 2017, 59, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Antonson, A.M.; Balakrishnan, B.; Radlowski, E.C.; Petr, G.; Johnson, R.W. Altered Hippocampal Gene Expression and Morphology in Fetal Piglets following Maternal Respiratory Viral Infection. Dev. Neurosci. 2018, 40, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Keever, M.R.; Zhang, P.; Bolt, C.R.; Antonson, A.M.; Rymut, H.E.; Caputo, M.P.; Houser, A.K.; Hernandez, A.G.; Southey, B.R.; Rund, L.A.; et al. Lasting and Sex-Dependent Impact of Maternal Immune Activation on Molecular Pathways of the Amygdala. Front. Neurosci. 2020, 14, 774. [Google Scholar] [CrossRef] [PubMed]
- Keever-Keigher, M.R.; Zhang, P.; Bolt, C.R.; Rymut, H.E.; Antonson, A.M.; Corbett, M.P.; Houser, A.K.; Hernandez, A.G.; Southey, B.R.; Rund, L.A.; et al. Interacting impact of maternal inflammatory response and stress on the amygdala transcriptome of pigs. G3 (Bethesda) 2021, jkab113. [Google Scholar] [CrossRef] [PubMed]
- Paysour, M.J.; Bolte, A.C.; Lukens, J.R. Crosstalk Between the Microbiome and Gestational Immunity in Autism-Related Disorders. DNA Cell Biol. 2019, 38, 405–409. [Google Scholar] [CrossRef]
- Madra, M.; Ringel, R.; Margolis, K.G. Gastrointestinal issues and autism spectrum disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2020, 29, 501–513. [Google Scholar] [CrossRef]
- Knutson, A.O.; Watters, J.J. All roads lead to inflammation: Is maternal immune activation a common culprit behind environmental factors impacting offspring neural control of breathing? Respir. Physiol. Neurobiol. 2020, 274, 103361. [Google Scholar] [CrossRef]
- Pileri, E.; Mateu, E. Review on the transmission porcine reproductive and respiratory syndrome virus between pigs and farms and impact on vaccination. Vet. Res. 2016, 47, 108. [Google Scholar] [CrossRef]
- Leibler, J.H.; Otte, J.; Roland-Holst, D.; Pfeiffer, D.U.; Soares Magalhaes, R.; Rushton, J.; Graham, J.P.; Silbergeld, E.K. Industrial food animal production and global health risks: Exploring the ecosystems and economics of avian influenza. EcoHealth 2009, 6, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Adda, J. Economic activity and the spread of viral diseases: Evidence from high frequency data. Q. J. Econ. 2016, 131, 891–941. [Google Scholar] [CrossRef]
- Li, Q.; Leung, Y.O.; Zhou, I.; Ho, L.C.; Kong, W.; Basil, P.; Wei, R.; Lam, S.; Zhang, X.; Law, A.C.K.; et al. Dietary supplementation with n-3 fatty acids from weaning limits brain biochemistry and behavioural changes elicited by prenatal exposure to maternal inflammation in the mouse model. Transl. Psychiat. 2015, 5, e641. [Google Scholar] [CrossRef] [PubMed]
- Rymut, H.E.; Bolt, C.R.; Caputo, M.P.; Houser, A.K.; Antonson, A.M.; Zimmerman, J.D.; Villamil, M.B.; Southey, B.R.; Rund, L.A.; Johnson, R.W.; et al. Long-lasting impact of maternal immune activation and interaction with a second immune challenge on pig behavior. Front. Vet. Sci. 2020, 9, 561151. [Google Scholar] [CrossRef] [PubMed]
- Rymut, H.E.; Rund, L.A.; Bolt, C.R.; Villamil, M.B.; Bender, D.E.; Southey, B.R.; Johnson, R.W.; Rodriguez-Zas, S.L. Biochemistry and Immune Biomarkers Indicate Interacting Effects of Pre- and Postnatal Stressors in Pigs across Sexes. Animals 2021, 11, 987. [Google Scholar] [CrossRef]
- Garay, P.A.; Hsiao, E.Y.; Patterson, P.H.; McAllister, A.K. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav. Immun. 2013, 31, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Bayer, T.A.; Falkai, P.; Maier, W. Genetic and non-genetic vulnerability factors in schizophrenia: The basis of the "two hit hypothesis". J. Psychiatr. Res. 1999, 33, 543–548. [Google Scholar] [CrossRef]
- Clark, S.M.; Notarangelo, F.M.; Li, X.; Chen, S.; Schwarcz, R.; Tonelli, L.H. Maternal immune activation in rats blunts brain cytokine and kynurenine pathway responses to a second immune challenge in early adulthood. Prog. Neuro-Psychopharmacol. Brain. Behav. Immun. 2019, 89, 286–294. [Google Scholar] [CrossRef]
- Garcia-Valtanen, P.; van Diermen, B.A.; Lakhan, N.; Lousberg, E.L.; Robertson, S.A.; Hayball, J.D.; Diener, K.R. Maternal host responses to poly(I:C) during pregnancy leads to both dysfunctional immune profiles and altered behaviour in the offspring. Am. J. Reprod. Immunol. 2020, 84, e13260. [Google Scholar] [CrossRef] [PubMed]
- Carlezon, W.A., Jr.; Kim, W.; Missig, G.; Finger, B.C.; Landino, S.M.; Alexander, A.J.; Mokler, E.L.; Robbins, J.O.; Li, Y.; Bolshakov, V.Y.; et al. Maternal and early postnatal immune activation produce sex-specific effects on autism-like behaviors and neuroimmune function in mice. Sci. Reports. 2019, 9, 16928. [Google Scholar] [CrossRef]
- Daş, G.; Vernunft, A.; Görs, S.; Kanitz, E.; Weitzel, J.M.; Brüssow, K.P.; Metges, C.C. Effects of general anesthesia with ketamine in combination with the neuroleptic sedatives xylazine or azaperone on plasma metabolites and hormones in pigs12. J. Anim. Sci. 2016, 94, 3229–3239. [Google Scholar] [CrossRef]
- Kaneko, J.; Harvey, J.; Bruss, M. Clinical Biochemistry of Domestic Animals; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Vap, L.M.; Weiser, M.G. Field chemistry analysis. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 427–442. [Google Scholar] [CrossRef]
- Radostits, O.; Gay, C.; Hinchcliff, K.; Constable, P. Veterinary Medicine, A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 10th ed.; Saunders Ltd., Elsevier: London, UK, 2006; p. 2065. [Google Scholar]
- Castillo, C.; Abuelo, A.; Hernández, J. Usefulness of metabolic profiling in the assessment of the flock’s health status and productive performance. Small Rumin. Res. 2016, 142, 28–30. [Google Scholar] [CrossRef]
- Harris, G.; Chen, W. Profiling of Cytokine and Chemokine Responses Using Multiplex Bead Array Technology. In Immunoproteomics: Methods and Protocols; Fulton, K.M., Twine, S.M., Eds.; Springer: New York, NY, USA, 2019; pp. 79–94. [Google Scholar]
- Staples, E.; Ingram, R.J.M.; Atherton, J.C.; Robinson, K. Optimising the quantification of cytokines present at low concentrations in small human mucosal tissue samples using Luminex assays. J. Immunol. Methods 2013, 394, 1–9. [Google Scholar] [CrossRef]
- Yu, K.; Canalias, F.; Solà-Oriol, D.; Arroyo, L.; Pato, R.; Saco, Y.; Terré, M.; Bassols, A. Age-Related Serum Biochemical Reference Intervals Established for Unweaned Calves and Piglets in the Post-weaning Period. Front. Vet. Sci. 2019, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Ventrella, D.; Dondi, F.; Barone, F.; Serafini, F.; Elmi, A.; Giunti, M.; Romagnoli, N.; Forni, M.; Bacci, M.L. The biomedical piglet: Establishing reference intervals for haematology and clinical chemistry parameters of two age groups with and without iron supplementation. BMC Vet. Res. 2017, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Greene, N.; Kimbro, K. Increased circulating cytokine levels in African American women with obesity and elevated HbA1c. Cytokine 2020, 128, 154989. [Google Scholar] [CrossRef]
- Wu, G.; Flynn, N.E.; Knabe, D.A.; Jaeger, L.A. A cortisol surge mediates the enhanced polyamine synthesis in porcine enterocytes during weaning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R554–R559. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Krebs, N.; Smith, J.S.; Dailey, J.W.; Carroll, J.A.; McGlone, J.J. The effect of three space allowances on the physiology and behavior of weaned pigs during transportation. Livest. Sci. 2009, 126, 183–188. [Google Scholar] [CrossRef]
- Koopmans, S.J.; Guzik, A.C.; van der Meulen, J.; Dekker, R.; Kogut, J.; Kerr, B.J.; Southern, L.L. Effects of supplemental L-tryptophan on serotonin, cortisol, intestinal integrity, and behavior in weanling piglets. J. Anim. Sci. 2006, 84, 963–971. [Google Scholar] [CrossRef]
- Xiao, Y.-P.; Wu, T.-X.; Hong, Q.-H.; Sun, J.-M.; Chen, A.-G.; Yang, C.-M.; Li, X.-Y. Response to weaning and dietary L-glutamine supplementation: Metabolomic analysis in piglets by gas chromatography/mass spectrometry. J. Zhejiang Univ. Sci. B 2012, 13, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.T.; Edwards, D.G.; Farquhar, W.B. The influence of dietary salt beyond blood pressure. Curr. Hypertens. Rep. 2019, 21, 42. [Google Scholar] [CrossRef]
- Abeni, F.; Petrera, F.; Dal Prà, A.; Rapetti, L.; Crovetto, G.M.; Galassi, G. Blood parameters in fattening pigs from two genetic types fed diet with three different protein concentrations1. Transl. Anim. Sci. 2018, 2, 372–382. [Google Scholar] [CrossRef]
- Boles, J.A.; Patience, J.F.; Schaefer, A.L.; Aalhus, J.L. Effect of oral loading of acid or base on the incidence of pale soft exudative pork (PSE) in stress-susceptible pigs. Meat Sci. 1994, 37, 181–194. [Google Scholar] [CrossRef]
- Huang, S.-S.; Chan, W.-L.; Leu, H.-B.; Huang, P.-H.; Lin, S.-J.; Chen, J.-W. Serum bilirubin levels predict future development of metabolic syndrome in healthy middle-aged nonsmoking men. Am. J. Med. 2015, 128, 1138.e1135–1138.e1141. [Google Scholar] [CrossRef]
- Andretta, I.; Pomar, C.; Rivest, J.; Pomar, J.; Radünz, J. Precision feeding can significantly reduce lysine intake and nitrogen excretion without compromising the performance of growing pigs. Animal 2016, 10, 1137–1147. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Yuan, L.; Azevedo, M.S.P.; Jeong, K.-I.; Gonzalez, A.-M.; Saif, L.J. Transfer of maternal cytokines to suckling piglets: In vivo and in vitro models with implications for immunomodulation of neonatal immunity. Vet. Immunol. Immunopathol. 2007, 117, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Liu, J.; Li, C.; Zhang, H.; Ma, P.; Luo, X.; Zeng, Z.; Hong, N.; Liu, X.; Wang, B.; et al. Positive effects of porcine IL-2 and IL-4 on virus-specific immune responses induced by the porcine reproductive and respiratory syndrome virus (PRRSV) ORF5 DNA vaccine in swine. J. Vet. Sci. 2014, 15, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The role of interleukin 6 during viral infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fu, S.; Feng, W.; Huang, B.; Xu, S.; Wang, W.; Liu, J. AMP010014A09 in Sus scrofa encodes an analog of G protein-coupled receptor 109A, which mediates the anti-inflammatory effects of beta-hydroxybutyric acid. Cell. Physiol. Biochem. 2017, 42, 1420–1430. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, H.; Liu, Z.; Lei, L.; Feng, Z.; Zhang, D.; Ren, Y.; Zhao, S. Comparative study of yeast selenium vs. sodium selenite on growth performance, nutrient digestibility, anti-inflammatory and anti-oxidative activity in weaned piglets challenged by Salmonella typhimurium. Innate Immun. 2019, 26, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Humann-Ziehank, E.; Menzel, A.; Roehrig, P.; Schwert, B.; Ganter, M.; Hennig-Pauka, I. Acute and subacute response of iron, zinc, copper and selenium in pigs experimentally infected with Actinobacillus pleuropneumoniae. Metallomics 2014, 6, 1869–1879. [Google Scholar] [CrossRef]
- Sipos, W.; Duvigneau, J.C.; Pietschmann, P.; Schilcher, F.; Hofbauer, G.; Hartl, R.T.; Schmoll, F. Porcine dermatitis and nephropathy syndrome (PDNS) is associated with a systemic cytokine expression profile indicative of proinflammation and a Th1 bias. Vet. Immunol. Immunopathol. 2005, 107, 303–313. [Google Scholar] [CrossRef]
- Wilson, G.D.A.; Harvey, D.G.; Snook, C.R. A Review of Factors Affecting Blood Biochemistry in the Pig. Br. Vet. J. 1972, 128, 596–610. [Google Scholar] [CrossRef]
- Wittish, L.M.; McElroy, A.P.; Harper, A.F.; Estienne, M.J. Performance and physiology of pigs administered spray-dried plasma protein during the late suckling period and transported after weaning1. J. Anim. Sci. 2014, 92, 4390–4399. [Google Scholar] [CrossRef] [PubMed]
- Erume, J.; Berberov, E.M.; Kachman, S.D.; Scott, M.A.; Zhou, Y.; Francis, D.H.; Moxley, R.A. Comparison of the Contributions of Heat-Labile Enterotoxin and Heat-Stable Enterotoxin b to the Virulence of Enterotoxigenic Escherichia coli in F4ac Receptor-Positive Young Pigs. Infect. Immun. 2008, 76, 3141–3149. [Google Scholar] [CrossRef]
- Burdick Sanchez, N.C.; Carroll, J.A.; Arthingon, J.D.; Lancaster, P.A. Exposure to lipopolysaccharide in utero alters the postnatal metabolic response in heifers1,2,3. J. Anim. Sci. 2017, 95, 5176–5183. [Google Scholar] [CrossRef][Green Version]
- Bădic, E.L.; Avram, N.; Sallay, A. The influence of bacterial endotoxins and of air microbial flora on serum biochemical parameters in pigs intensively exploited. Rev. Română Med. Vet. 2010, 20, 87–94. [Google Scholar]
- Blecha, F.; Kelley, K.W. Effects of cold and weaning stressors on the antibody-mediated immune response of pigs. J. Anim. Sci. 1981, 53, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Yang, J.-Y.; Upadhaya, S.D.; Lee, H.-J.; Yun, C.-H.; Ha, J.K. The stress of weaning influences serum levels of acute-phase proteins, iron-binding proteins, inflammatory cytokines, cortisol, and leukocyte subsets in Holstein calves. J. Vet. Sci. 2011, 12, 151–157. [Google Scholar] [CrossRef]
- Williams, P.N.; Collier, C.T.; Carroll, J.A.; Welsh, T.H.; Laurenz, J.C. Temporal pattern and effect of sex on lipopolysaccharide-induced stress hormone and cytokine response in pigs. Domest. Anim. Endocrinol. 2009, 37, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Webel, D.M.; Finck, B.N.; Baker, D.H.; Johnson, R.W. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 1997, 75, 1514–1520. [Google Scholar] [CrossRef]
- Chamberlin, W.G.; Middleton, J.R.; Spain, J.N.; Johnson, G.; Ellersieck, M.; Pithua, P. Subclinical hypocalcemia, plasma biochemical parameters, lipid metabolism, postpartum disease, and fertility in postparturient dairy cows. J. Dairy Sci. 2013, 96, 7001–7013. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.A.; Veum, T.L.; Matteri, R.L. Endocrine responses to weaning and changes in post-weaning diet in the young pig. Domest. Anim. Endocrinol. 1998, 15, 183–194. [Google Scholar] [CrossRef]
- Romero, E.; Guaza, C.; Castellano, B.; Borrell, J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: Implications for the etiopathology of schizophrenia. Mol. Psychiatry 2010, 15, 372–383. [Google Scholar] [CrossRef]
- Kim, M.H.; Yun, C.H.; Lee, C.H.; Ha, J.K. The effects of fermented soybean meal on immunophysiological and stress-related parameters in Holstein calves after weaning. J. Dairy Sci. 2012, 95, 5203–5212. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C.; Niu, X.; Zhang, Z.; Li, F.; Li, F. An intensive milk replacer feeding program benefits immune response and intestinal microbiota of lambs during weaning. BMC Vet. Res. 2018, 14, 366. [Google Scholar] [CrossRef]
- Che, T.M.; Song, M.; Liu, Y.; Johnson, R.W.; Kelley, K.W.; Van Alstine, W.G.; Dawson, K.A.; Pettigrew, J.E. Mannan oligosaccharide increases serum concentrations of antibodies and inflammatory mediators in weanling pigs experimentally infected with porcine reproductive and respiratory syndrome virus1,2. J. Anim. Sci. 2012, 90, 2784–2793. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Lippi, L.L.; Bevilacqua, E.; Bernardi, M.M. LPS Exposure Increases Maternal Corticosterone Levels, Causes Placental Injury and Increases IL-1Β Levels in Adult Rat Offspring: Relevance to Autism. PLoS ONE 2013, 8, e82244. [Google Scholar] [CrossRef] [PubMed]

| Analyte 1 | Maternal Immune Activation | Sex | Weaning | Activation-Sex | Activation-Weaning | Weaning-Sex | Activation-Weaning-Sex |
|---|---|---|---|---|---|---|---|
| AG Ratio | 0.053 | 0.609 | 0.666 | 0.355 | 0.040 | 0.801 | 0.175 |
| Albumin a | 0.553 | 0.810 | 0.169 | 0.335 | 0.223 | 0.757 | 0.059 |
| AlkPhos b | 0.110 | 0.397 | 0.804 | 0.808 | 0.214 | 0.657 | 0.599 |
| AnionGap c | 0.075 | 0.573 | 0.011 | 0.151 | 0.962 | 0.393 | 0.027 |
| AST b | 0.094 | 0.542 | 0.282 | 0.492 | 0.112 | 0.707 | 0.651 |
| Bicarbonate c | 0.136 | 0.885 | 0.083 | 0.634 | 0.433 | 0.711 | 0.045 |
| Bilirubin d | 0.548 | 0.514 | 0.011 | 0.361 | 0.246 | 0.433 | 0.018 |
| BUN d | 0.216 | 0.566 | 0.072 | 0.377 | 0.022 | 0.431 | 0.082 |
| Calcium d | 0.033 | 0.195 | 0.016 | 0.425 | 0.514 | 0.562 | 0.060 |
| Chloride c | 0.020 | 0.820 | 0.084 | 0.899 | 0.936 | 0.533 | 0.028 |
| Cholesterol d | 0.040 | 0.159 | 0.602 | 0.307 | 0.102 | 0.937 | 0.020 |
| CPK b | 0.034 | 0.988 | 0.230 | 0.037 | 0.151 | 0.795 | 0.082 |
| Creatinine d | 0.019 | 0.827 | 0.102 | 0.215 | 0.726 | 0.791 | 0.018 |
| GGT b | 0.897 | 0.830 | 0.015 | 0.415 | 0.665 | 0.490 | 0.257 |
| GLDH b | 0.547 | 0.444 | 0.697 | 0.707 | 0.521 | 0.363 | 0.254 |
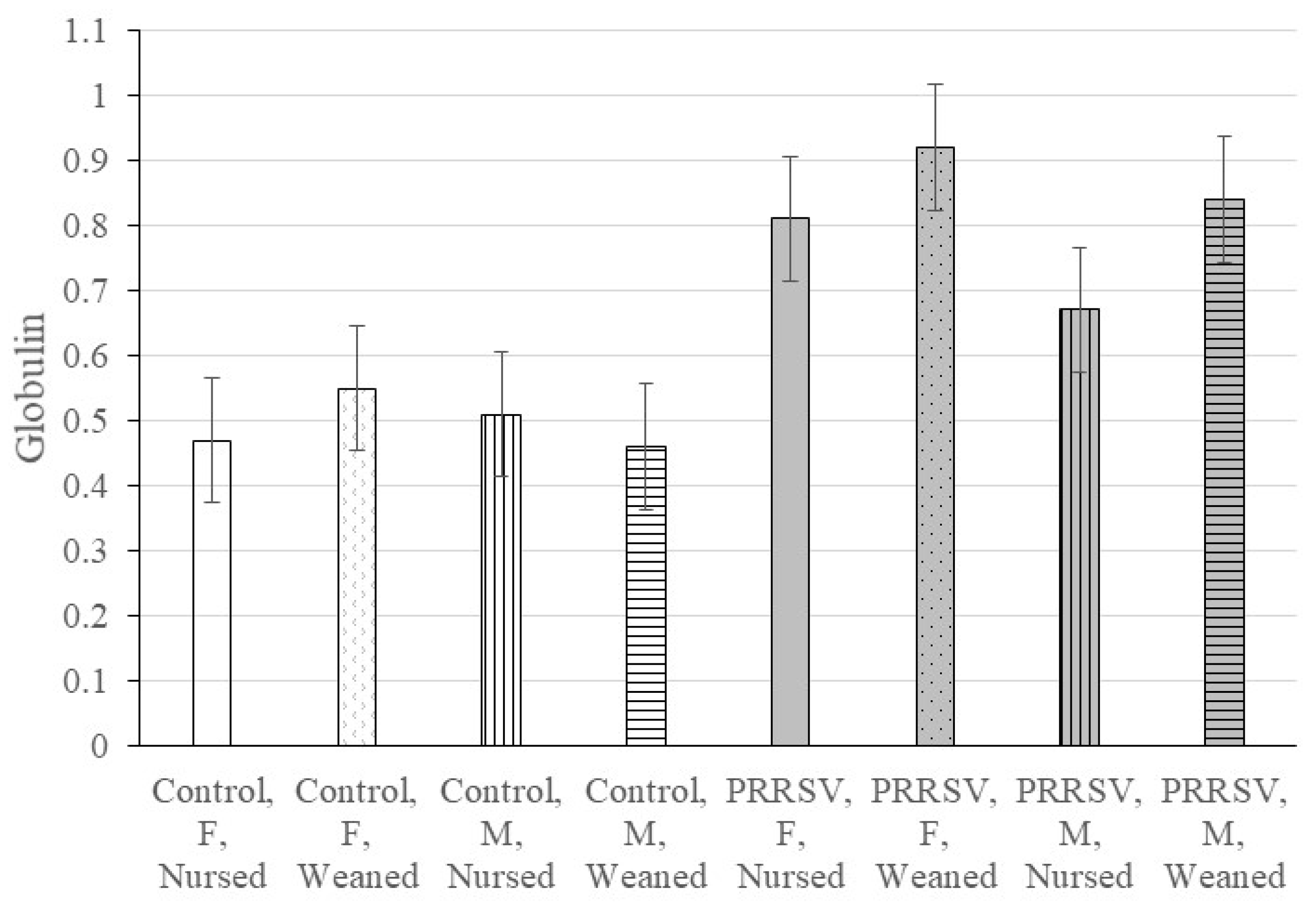
| Globulin e | 0.025 | 0.211 | 0.227 | 0.329 | 0.334 | 0.486 | 0.080 |
| Glucose d | 0.869 | 0.388 | 0.002 | 0.412 | 0.289 | 0.830 | 0.277 |
| NaK Ratio | 0.131 | 0.437 | 0.151 | 0.268 | 0.835 | 0.112 | 0.522 |
| Phosphorus d | 0.811 | 0.308 | 0.159 | 0.821 | 0.737 | 0.473 | 0.065 |
| Potassium c | 0.332 | 0.052 | 0.215 | 0.280 | 0.922 | 0.148 | 0.036 |
| Protein a | 0.045 | 0.556 | 0.170 | 0.289 | 0.804 | 0.948 | 0.028 |
| Sodium c | 0.028 | 0.626 | 0.416 | 0.723 | 0.948 | 0.483 | 0.061 |
| Triglycerides d | 0.197 | 0.705 | 0.004 | 0.707 | 0.777 | 0.283 | 0.085 |
| Cortisol e | 0.627 | 0.201 | 0.010 | 0.146 | 0.261 | 0.048 | 0.048 |
| BodyWeight f | 0.857 | 0.439 | 0.057 | 0.097 | 0.341 | 0.942 | 0.224 |
| Analyte 1 | Control Female | PRRSV Female | Control Male | PRRSV Male | Control | PRRSV | Female | Male |
|---|---|---|---|---|---|---|---|---|
| AG Ratio | 0.594 | 0.620 | 0.099 | 0.126 | 0.126 | 0.148 | 0.716 | 0.982 |
| Albumin | 0.013 | 0.720 | 0.065 | 0.847 | 0.008 | 0.530 | 0.071 | 0.178 |
| AlkPhos | 0.632 | 0.387 | 0.631 | 0.490 | 0.457 | 0.330 | 0.799 | 0.911 |
| Anion Gap | 0.026 | 0.107 | 0.214 | 0.080 | 0.058 | 0.034 | 0.023 | 0.090 |
| AST | 0.384 | 0.892 | 0.786 | 0.542 | 0.339 | 0.197 | 0.824 | 0.204 |
| Bicarbonate | 0.055 | 0.104 | 0.048 | 0.161 | 0.007 | 0.042 | 0.013 | 0.020 |
| Bilirubin | 0.053 | 0.284 | 0.027 | 0.870 | 0.897 | 0.039 | 0.077 | 0.673 |
| BUN | 0.008 | 0.473 | 0.176 | 0.570 | 0.009 | 0.407 | 0.202 | 0.871 |
| Calcium | 0.168 | 0.150 | 0.035 | 0.020 | 0.271 | 0.003 | 0.041 | 0.145 |
| Chloride | 0.372 | 0.092 | 0.031 | 0.107 | 0.011 | 0.008 | 0.015 | 0.005 |
| Cholesterol | 0.437 | 0.487 | 0.346 | 0.651 | 0.139 | 0.455 | 0.274 | 0.221 |
| CPK | 0.065 | 0.682 | 0.385 | 0.888 | 0.059 | 0.763 | 0.375 | 0.564 |
| Creatinine | 0.045 | 0.110 | 0.143 | 0.044 | 0.139 | 0.093 | 0.113 | 0.117 |
| GGT | 0.276 | 0.554 | 0.093 | 0.063 | 0.052 | 0.064 | 0.158 | 0.021 |
| GLDH | 0.610 | 0.141 | 0.709 | 0.759 | 0.662 | 0.222 | 0.169 | 0.839 |
| Globulin | 0.276 | 0.328 | 0.550 | 0.090 | 0.881 | 0.140 | 0.166 | 0.594 |
| Glucose | 0.753 | 0.109 | 0.835 | 0.235 | 0.950 | 0.337 | 0.361 | 0.299 |
| NaK Ratio | 0.453 | 0.745 | 0.258 | 0.348 | 0.798 | 0.974 | 0.408 | 0.069 |
| Phosphorus | 0.013 | 0.137 | 0.013 | 0.035 | 0.002 | 0.309 | 0.124 | 0.086 |
| Potassium | 0.515 | 0.969 | 0.064 | 0.191 | 0.502 | 0.292 | 0.822 | 0.027 |
| Protein | 0.075 | 0.034 | 0.459 | 0.084 | 0.264 | 0.433 | 0.316 | 0.369 |
| Sodium | 0.123 | 0.065 | 0.021 | 0.056 | 0.270 | 0.227 | 0.241 | 0.558 |
| Triglycerides | 0.012 | 0.144 | 0.300 | 0.359 | 0.011 | 0.086 | 0.006 | 0.168 |
| Cortisol | 0.006 | 0.097 | 0.174 | 0.958 | 0.002 | 0.149 | 0.003 | 0.321 |
| Body Weight | 0.273 | 0.093 | 0.237 | 0.137 | 0.619 | 0.069 | 0.201 | 0.251 |
| Analyte 1 | Female Nursed | Female Weaned | Male Nursed | Male Weaned | Nursed | Weaned | Male | Female |
|---|---|---|---|---|---|---|---|---|
| AG Ratio | 0.105 | 0.031 | 0.640 | 0.022 | 0.246 | 0.017 | 0.119 | 0.039 |
| Albumin | 0.941 | 0.224 | 0.451 | 0.447 | 0.898 | 0.266 | 0.999 | 0.324 |
| AlkPhos | 0.180 | 0.407 | 0.221 | 0.144 | 0.136 | 0.336 | 0.073 | 0.127 |
| Anion Gap | 0.887 | 0.224 | 0.021 | 0.091 | 0.146 | 0.154 | 0.038 | 0.424 |
| AST | 0.457 | 0.717 | 0.486 | 0.163 | 0.362 | 0.095 | 0.502 | 0.693 |
| Bicarbonate | 0.442 | 0.097 | 0.125 | 0.075 | 0.186 | 0.063 | 0.073 | 0.160 |
| Bilirubin | 0.615 | 0.206 | 0.381 | 0.148 | 0.289 | 0.634 | 0.195 | 0.704 |
| BUN | 0.071 | 0.903 | 0.232 | 0.845 | 0.079 | 0.842 | 0.480 | 0.209 |
| Calcium | 0.484 | 0.435 | 0.162 | 0.250 | 0.109 | 0.187 | 0.233 | 0.123 |
| Chloride | 0.035 | 0.241 | 0.213 | 0.082 | 0.052 | 0.049 | 0.006 | 0.137 |
| Cholesterol | 0.300 | 0.675 | 0.086 | 0.716 | 0.044 | 0.323 | 0.624 | 0.196 |
| CPK | 0.088 | 0.005 | 0.930 | 0.539 | 0.312 | 0.012 | 0.578 | 0.005 |
| Creatinine | 0.521 | 0.706 | 0.028 | 0.221 | 0.062 | 0.180 | 0.012 | 0.450 |
| GGT | 0.781 | 0.466 | 0.754 | 0.708 | 0.407 | 0.706 | 0.308 | 0.861 |
| GLDH | 0.847 | 0.348 | 0.979 | 0.510 | 0.898 | 0.267 | 0.723 | 0.447 |
| Globulin | 0.028 | 0.022 | 0.262 | 0.017 | 0.076 | 0.016 | 0.057 | 0.017 |
| Glucose | 0.222 | 0.073 | 0.391 | 0.751 | 0.403 | 0.191 | 0.672 | 0.476 |
| NaK Ratio | 0.796 | 0.999 | 0.208 | 0.259 | 0.504 | 0.565 | 0.095 | 0.942 |
| Phosphorus | 0.505 | 0.555 | 0.285 | 0.840 | 0.648 | 0.322 | 0.525 | 0.586 |
| Potassium | 0.673 | 0.695 | 0.201 | 0.126 | 0.567 | 0.417 | 0.132 | 0.973 |
| Protein | 0.071 | 0.028 | 0.064 | 0.019 | 0.167 | 0.296 | 0.054 | 0.640 |
| Sodium | 0.153 | 0.229 | 0.281 | 0.090 | 0.145 | 0.098 | 0.029 | 0.063 |
| Triglycerides | 0.152 | 0.377 | 0.387 | 0.394 | 0.130 | 0.247 | 0.256 | 0.126 |
| Cortisol | 0.787 | 0.360 | 0.138 | 0.983 | 0.436 | 0.461 | 0.329 | 0.422 |
| Body Weight | 0.781 | 0.755 | 0.990 | 0.261 | 0.321 | 0.968 | 0.908 | 0.114 |
| Analyte 1 | Con Fem Nur | Con Fem Wea | Con Mal Nur | Con Mal Wea | PRR Fem Nur | PRR Fem Wea | PRR Mal Nur | PRR Mal Wea | SE 3 |
|---|---|---|---|---|---|---|---|---|---|
| AG Ratio 2 | 0.55 ab | 0.60 ab | 0.49 bc | 0.65 a | 0.26 d | 0.19 d | 0.41 c | 0.22 d | 0.07 |
| Albumin 4 | 1.05 | 1.16 | 1.04 | 1.13 | 1.06 | 1.08 | 1.09 | 1.08 | 0.04 |
| AlkPhos 5 | 6.73 | 6.78 | 6.84 | 6.79 | 6.51 | 6.64 | 6.65 | 6.56 | 0.07 |
| Anion Gap 6 | 2.81 abc | 2.96 ef | 2.89 def | 2.94 ef | 2.80 ab | 2.88 cde | 2.76 a | 2.84 bcd | 0.04 |
| AST 5 | 3.61 | 3.94 | 3.43 | 3.50 | 3.84 | 3.81 | 3.73 | 4.02 | 0.27 |
| Bicarbonate 6 | 3.17 bc | 3.08 a | 3.15 b | 3.09 a | 3.22 c | 3.17 bc | 3.22 c | 3.18 bc | 0.03 |
| Bilirubin 7 | −0.96 ab | 0.23 d | −1.02 ab | 0.07 d | −1.14 a | −0.51 c | −0.70 c | −0.80 abc | 0.22 |
| BUN 7 | 1.90 a | 2.19 bc | 1.92 ab | 2.07 abc | 2.33 c | 2.22 c | 2.20 c | 2.12 abc | 0.14 |
| Calcium 7 | 2.39 de | 2.34 bcd | 2.43 e | 2.33 abc | 2.37 cd | 2.31 ab | 2.37 cd | 2.28 a | 0.03 |
| Chloride 6 | 4.62 cd | 4.63 cd | 4.61 bc | 4.64 d | 4.59 a | 4.62 cd | 4.60 ab | 4.62 cd | 0.01 |
| Cholesterol 7 | 5.56 d | 5.44 cd | 5.30 bc | 5.14 ab | 5.39 cd | 5.51 cd | 5.01 a | 5.08 ab | 0.12 |
| CPK 5 | 6.42 c | 6.89 d | 6.27 bc | 6.50 c | 5.94 ab | 5.80 a | 6.25 bc | 6.29 bc | 0.18 |
| Creatinine 7 | −0.51 ab | −0.37 ab | −0.46 ab | −0.36 b | −0.56 ab | −0.40 ab | −0.65 a | −0.46 ab | 0.15 |
| GGT 5 | 3.65 | 3.79 | 3.63 | 3.80 | 3.61 | 3.69 | 3.66 | 3.84 | 0.08 |
| GLDH 5 | 0.70 | 0.57 | 0.45 | 0.49 | 0.65 | 0.38 | 0.46 | 0.38 | 0.18 |
| Globulin 7 | 0.47 a | 0.55 a | 0.51 a | 0.46 a | 0.81 c | 0.92 c | 0.67 b | 0.84 c | 0.06 |
| Glucose 6 | 4.75 | 4.82 | 4.87 | 4.83 | 5.13 | 4.67 | 5.12 | 4.80 | 0.12 |
| NaK Ratio | 3.49 | 3.54 | 3.56 | 3.51 | 3.51 | 3.54 | 3.50 | 3.47 | 0.04 |
| Phosphorus 6 | 2.26 | 2.33 | 2.22 | 2.29 | 2.23 | 2.31 | 2.17 | 2.29 | 0.02 |
| Potassium 5 | 1.42 cd | 1.37 ab | 1.35 a | 1.40 bc | 1.39 abc | 1.39 abc | 1.41 bc | 1.45 d | 0.02 |
| Protein 4 | 1.53 a | 1.58 ab | 1.52 a | 1.54 a | 1.64 bcd | 1.71 d | 1.63 bc | 1.69 cd | 0.04 |
| Sodium 5 | 4.92 | 4.94 | 4.92 | 4.94 | 4.90 | 4.93 | 4.90 | 4.93 | 0.01 |
| Triglycerides 6 | 3.92 a | 4.56 cd | 4.13 ab | 4.40 bc | 4.36 bc | 4.84 d | 4.39 bc | 4.66 cd | 0.20 |
| Cortisol 8 | 3.20 a | 3.90 d | 3.32 ab | 3.58 bc | 3.15 a | 3.61 bcd | 3.56 bc | 3.57 bc | 0.16 |
| Body Weight 9 | 6.35 | 5.89 | 5.86 | 6.13 | 5.69 | 6.31 | 5.45 | 5.92 | 0.25 |
| Cytokine | Maternal Immune Activation | Sex | Weaning | Activation-Sex | Activation-Weaning | Sex-Weaning | Activation-Weaning-Sex |
|---|---|---|---|---|---|---|---|
| GM-CSF 1 | 0.512 | 0.400 | 0.759 | 0.136 | 0.217 | 0.702 | 0.679 |
| IFN-γ | 0.407 | 0.025 | 0.164 | 0.551 | 0.055 | 0.533 | 0.434 |
| IL-1α | 0.463 | 0.021 | 0.513 | 0.096 | 0.119 | 0.132 | 0.419 |
| IL-1β | 0.490 | 0.050 | 0.440 | 0.330 | 0.019 | 0.071 | 0.290 |
| IL-1ra | 0.505 | 0.707 | 0.369 | 0.791 | 0.085 | 0.408 | 0.725 |
| IL-4 | 0.511 | 0.047 | 0.275 | 0.201 | 0.022 | 0.084 | 0.339 |
| IL-2 | 0.642 | 0.052 | 0.398 | 0.195 | 0.019 | 0.067 | 0.337 |
| IL-6 | 0.911 | 0.079 | 0.604 | 0.503 | 0.019 | 0.122 | 0.411 |
| IL-10 | 0.494 | 0.038 | 0.398 | 0.147 | 0.047 | 0.068 | 0.285 |
| IL-12 | 0.481 | 0.890 | 0.497 | 0.273 | 0.144 | 0.138 | 0.161 |
| IL-18 | 0.452 | 0.035 | 0.370 | 0.209 | 0.022 | 0.084 | 0.315 |
| TNF-α | 0.799 | 0.157 | 0.423 | 0.500 | 0.120 | 0.777 | 0.950 |
| Analyte 1 | Con Fem Nur 2 | Con Fem Wea | Con Mal Nur | Con Mal Wea | PRR Fem Nur | PRR Fem Wea | PRR Mal Nur | PRR Mal Wea | SE 3 |
|---|---|---|---|---|---|---|---|---|---|
| GM-CSF | 0.19 | 0.15 | 0.16 | 0.12 | 0.15 | 0.18 | 0.22 | 0.32 | 0.08 |
| IFN-γ | 57.12 | 38.29 | 47.31 | 30.25 | 60.00 | 70.78 | 52.67 | 47.80 | 10.33 |
| IL-1α | 0.25 | 0.20 | 0.25 | 0.19 | 0.29 | 0.44 | 0.24 | 0.23 | 0.06 |
| IL-1β | 1.05 a | 0.94 a | 1.02 a | 0.68 a | 0.99 a | 1.86 b | 1.00 a | 1.03 a | 0.25 |
| IL-1ra | 1.32 | 1.27 | 1.30 | 1.13 | 0.78 | 1.28 | 0.92 | 1.10 | 0.28 |
| IL-2 | 1.44 a | 1.34 a | 1.47 a | 0.97 a | 1.40 a | 2.90 b | 1.30 a | 1.45 a | 0.40 |
| IL-4 | 6.31 a | 5.55 a | 6.63 a | 3.66 a | 5.23 a | 12.57 b | 4.80 a | 5.29 a | 1.90 |
| IL-6 | 0.52 ab | 0.44 a | 0.5 ab | 0.33 a | 0.33 a | 0.67 b | 0.33 a | 0.36 a | 0.11 |
| IL-10 | 2.47 a | 2.32 a | 2.54 a | 1.74 a | 2.59 a | 4.96 b | 2.40 a | 2.34 a | 0.66 |
| IL-12 | 0.80 | 0.69 | 0.74 | 0.62 | 0.69 | 0.89 | 0.90 | 0.78 | 0.10 |
| IL18 | 4.65 a | 4.22 a | 4.59 a | 3.19 a | 4.70 a | 8.54 b | 4.40 a | 4.65 a | 1.07 |
| TNF-α | 0.65 | 0.53 | 0.55 | 0.39 | 0.47 | 0.53 | 0.44 | 0.47 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rymut, H.E.; Rund, L.A.; Bolt, C.R.; Villamil, M.B.; Southey, B.R.; Johnson, R.W.; Rodriguez-Zas, S.L. The Combined Effect of Weaning Stress and Immune Activation during Pig Gestation on Serum Cytokine and Analyte Concentrations. Animals 2021, 11, 2274. https://doi.org/10.3390/ani11082274
Rymut HE, Rund LA, Bolt CR, Villamil MB, Southey BR, Johnson RW, Rodriguez-Zas SL. The Combined Effect of Weaning Stress and Immune Activation during Pig Gestation on Serum Cytokine and Analyte Concentrations. Animals. 2021; 11(8):2274. https://doi.org/10.3390/ani11082274
Chicago/Turabian StyleRymut, Haley E., Laurie A. Rund, Courtni R. Bolt, Maria B. Villamil, Bruce R. Southey, Rodney W. Johnson, and Sandra L. Rodriguez-Zas. 2021. "The Combined Effect of Weaning Stress and Immune Activation during Pig Gestation on Serum Cytokine and Analyte Concentrations" Animals 11, no. 8: 2274. https://doi.org/10.3390/ani11082274
APA StyleRymut, H. E., Rund, L. A., Bolt, C. R., Villamil, M. B., Southey, B. R., Johnson, R. W., & Rodriguez-Zas, S. L. (2021). The Combined Effect of Weaning Stress and Immune Activation during Pig Gestation on Serum Cytokine and Analyte Concentrations. Animals, 11(8), 2274. https://doi.org/10.3390/ani11082274








