Effect of Wet Aging on Color Stability, Tenderness, and Sensory Attributes of Longissimus lumborum and Gluteus medius Muscles from Water Buffalo Bulls
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals Slaughtering
2.3. Muscle Sampling and Aging Processing
2.4. Study Parameters
2.4.1. Instrumental Color
2.4.2. pH Measurement
2.4.3. Metmyoglobin Content
2.4.4. Water Holding Capacity (WHC)
2.4.5. Cooking Loss
2.4.6. Warner–Bratzler Shear Force
2.4.7. Myofibrillar Fragmentation Index (MFI)
2.4.8. Sensory Analysis
2.5. Statistical Analysis
3. Results
3.1. Instrumental Color
3.2. pH
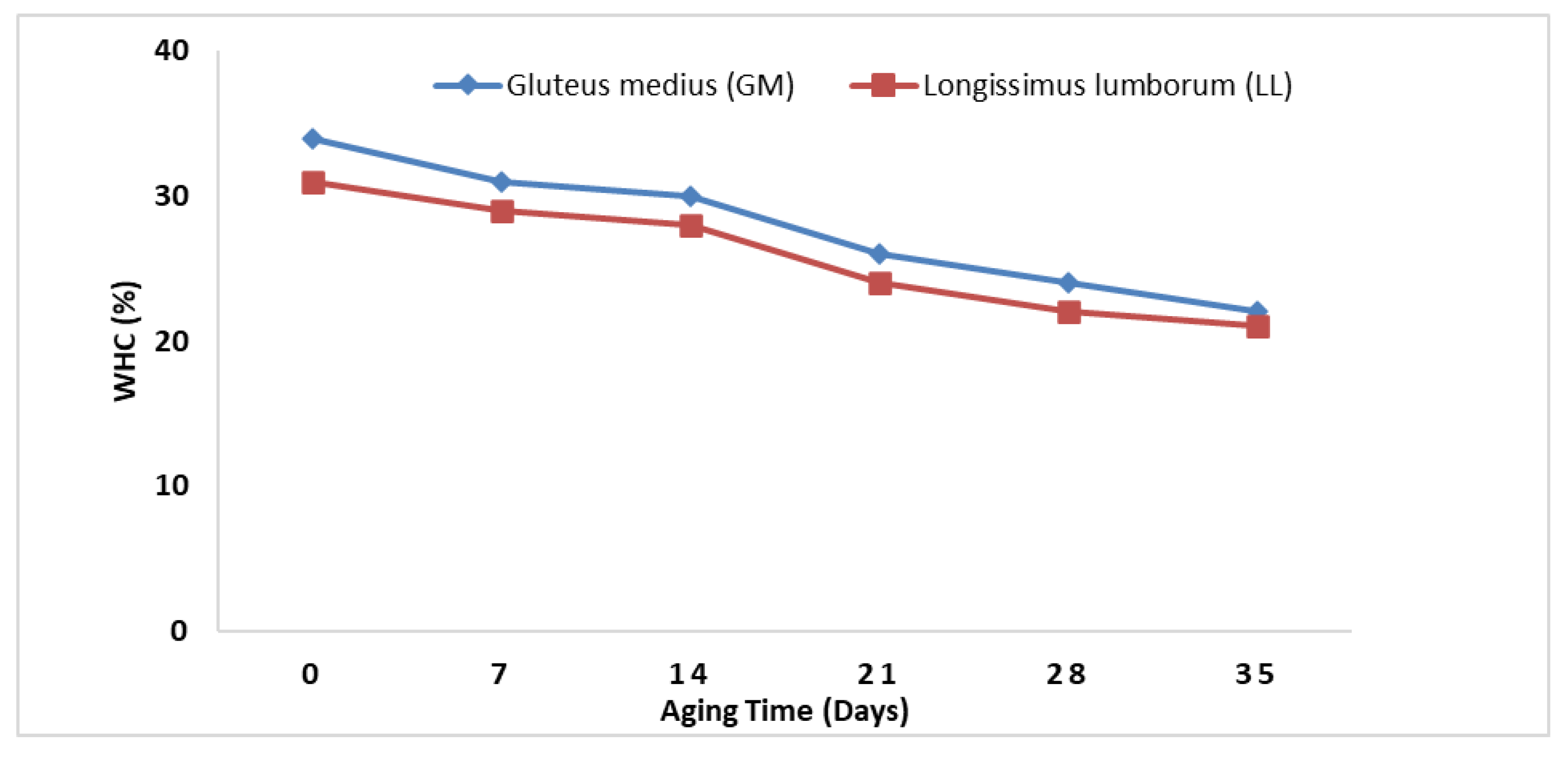
3.3. Water Holding Capacity (WHC)
3.4. Cooking Loss
3.5. Warner–Bratzler Shear Force
3.6. Myofibrillar Fragmentation Index (MFI)
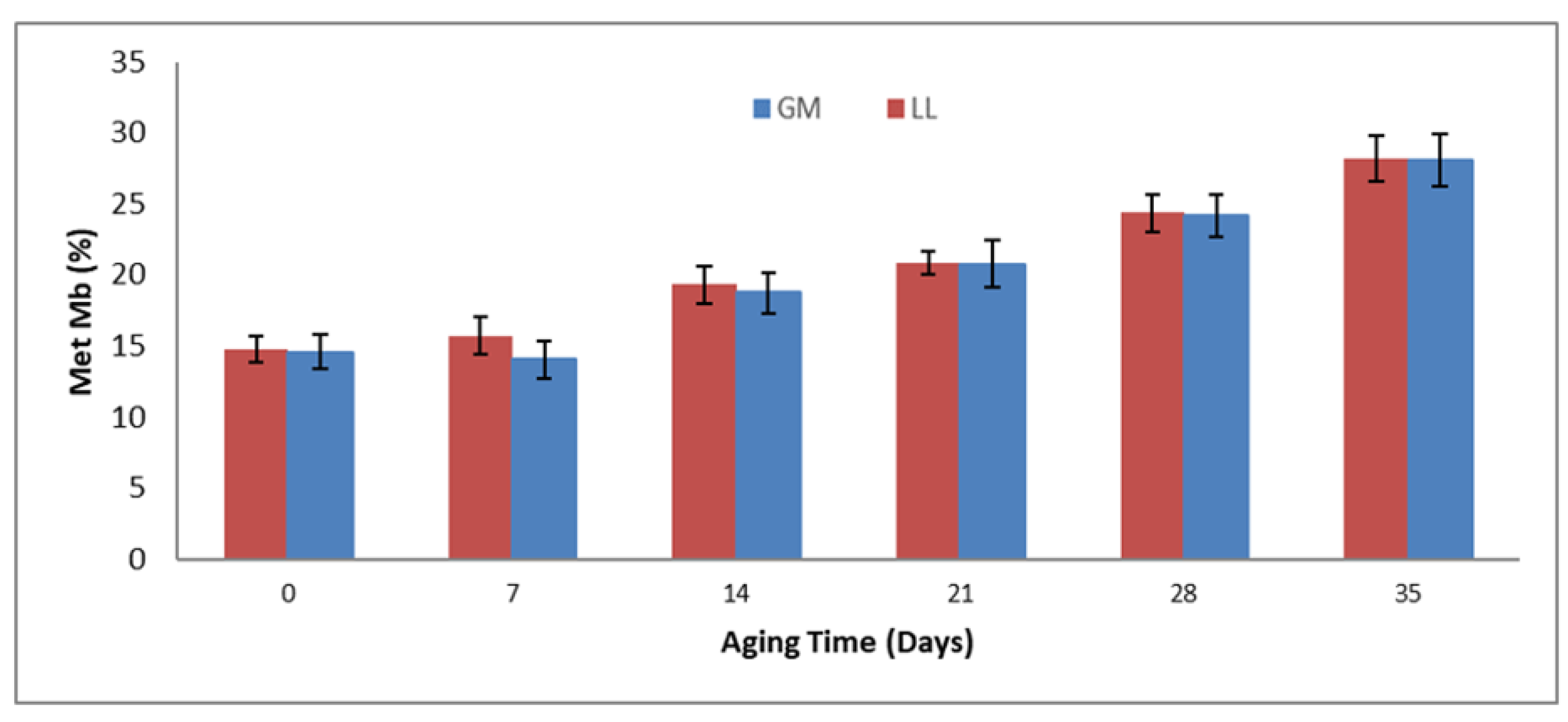
3.7. Metmyoglobin (%)
3.8. Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaspal, M.H.; Ijaz, M.; Akhtar, M.J.; Nasir, J.; Ullah, S.; Badar, I.H.; Yar, M.K.; Ahmad, A. Effect of Carcass Electrical Stimulation and Suspension Methods on Meat Quality Characteristics of Longissimus lumborum of Young Buffalo (Bubalus bubalis) Bulls. Food Sci. Anim. Resour. 2021, 41, 34. [Google Scholar] [CrossRef] [PubMed]
- El Debaky, H.A.; Kutchy, N.A.; Ul-Husna, A.; Indriastuti, R.; Akhter, S.; Purwantara, B.; Memili, E. Potential of water buffalo in world agriculture: Challenges and opportunities. Appl. Ani. Sci. 2019, 35, 255–268. [Google Scholar] [CrossRef]
- Hamid, M.A.; Siddiky, M.N.A.; Rahman, M.A.; Hossain, K.M. Scopes and opportunities of buffalo farming in Bangladesh: A review. SAARC J. Agric. 2016, 14, 63–77. [Google Scholar] [CrossRef]
- Naveena, B.M.; Kiran, M. Buffalo meat quality, composition, and processing characteristics: Contribution to the global economy and nutritional security. Anim. Front. 2014, 4, 18–24. [Google Scholar] [CrossRef]
- Medek, D.E.; Schwartz, J.; Myers, S.S. Estimated effects of future atmospheric CO2 concentrations on protein intake and the risk of protein deficiency by country and region. Environ. Health Perspect. 2017, 125, 087002. [Google Scholar] [CrossRef]
- Kausar, T.; Hanan, E.; Ayob, O.; Praween, B.; Azad, Z.R.A.A. A review on functional ingredients in red meat products. Bioinformation 2019, 15, 358. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Jaspal, M.H.; Hayat, Z.; Yar, M.K.; Badar, I.H.; Ullah, S.; Hussain, Z.; Ali, S.; Farid, M.U.; Farooq, M.Z.; et al. Effect of animal age, postmortem chilling rate, and aging time on meat quality attributes of water buffalo and humped cattle bulls. Anim. Sci. J. 2020, 91, 13354. [Google Scholar] [CrossRef]
- Nair, M.N.; Canto, A.C.; Rentfrow, G.; Suman, S.P. Muscle-specific effect of aging on beef tenderness. LWT 2019, 100, 250–252. [Google Scholar] [CrossRef]
- Colle, M.J.; Nasados, J.A.; Rogers, J.M.; Kerby, D.M.; Colle, M.M.; Van Buren, J.B.; Richard, R.P.; Murdoch, G.K.; Williams, C.J.; Doumit, M.E. Strategies to improve beef tenderness by activating calpain-2 earlier postmortem. Meat Sci. 2018, 135, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Gagaoua, M.; Micol, D.; Cassar-Malek, I.; Hocquette, J.F.; Terlouw, C.E. Inverse relationships between biomarkers and beef tenderness according to contractile and metabolic properties of the muscle. J. Agric. Food Chem. 2014, 62, 9808–9818. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.M.; Phelps, K.J. United States beef quality as chronicled by the National Beef Quality Audits, Beef Consumer Satisfaction Projects, and National Beef Tenderness Surveys—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 1036. [Google Scholar] [CrossRef]
- Suman, S.P.; Joseph, P. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Neethling, N.E.; Suman, S.P.; Sigge, G.O.; Hoffman, L.C.; Hunt, M.C. Exogenous and endogenous factors influencing color of fresh meat from ungulates. Meat Muscle Biol. 2017, 1, 253–275. [Google Scholar] [CrossRef]
- Naveena, B.M.; Mendiratta, S.K.; Anjaneyulu, A.S.R. Tenderization of buffalo meat using plant proteases from Cucumis trigonus Roxb (Kachri) and Zingiber officinale roscoe (Ginger rhizome). Meat Sci. 2004, 68, 363–369. [Google Scholar] [CrossRef]
- Ramanathan, R.; Suman, S.P.; Faustman, C. Biomolecular interactions governing fresh meat color in post-mortem skeletal muscle: A review. J. Agric. Food Chem. 2020, 68, 12779–12787. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Applied and emerging methods for meat tenderization: A comparative perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Lana, A.; Zolla, L. Proteolysis in meat tenderization from the point of view of each single protein: A proteomic perspective. J. Proteom. 2016, 147, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, G. Colour stability of steaks from large beef cuts aged under vacuum or high oxygen modified atmosphere. Meat Sci. 2011, 87, 428–435. [Google Scholar] [CrossRef]
- Ijaz, M.; Li, X.; Zhang, D.; Hussain, Z.; Ren, C.; Bai, Y.; Zheng, X. Association between meat color of DFD beef and other quality attributes. Meat Sci. 2020, 161, 107954. [Google Scholar] [CrossRef]
- Xiao, X.; Hou, C.; Zhang, D.; Li, X.; Ren, C.; Ijaz, M.; Hussain, Z.; Liu, D. Effect of pre-and post-rigor on texture, flavor, heterocyclic aromatic amines and sensory evaluation of roasted lamb. Meat Sci. 2020, 169, 108220. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Sabikun, N.; Ismail, I.; Joo, S.T. Comparison of meat quality characteristics of wet-and dry-aging pork belly and shoulder blade. Korean J. Food Sci. Anim. Resour. 2018, 38, 950. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.H.; Ji, D.S.; Lee, H.J.; Yoon, D.K.; Lee, C.H. Effect of aging process and time on physicochemical and sensory evaluation of raw beef top round and shank muscles using an electronic tongue. Korean J. Food Sci. Anim. Resour. 2017, 37, 823. [Google Scholar]
- Wright, S.A.; Ramos, P.; Johnson, D.D.; Scheffler, J.M.; Elzo, M.A.; Mateescu, R.G.; Bass, A.L.; Carr, C.C.; Scheffler, T.L. Brahman genetics influence muscle fiber properties, protein degradation, and tenderness in an Angus-Brahman multibreed herd. Meat Sci. 2018, 135, 84–93. [Google Scholar] [CrossRef]
- Ma, D.; Kim, Y.H.B. Proteolytic changes of myofibrillar and small heat shock proteins in different bovine muscles during aging: Their relevance to tenderness and water-holding capacity. Meat Sci. 2020, 163, 108090. [Google Scholar] [CrossRef]
- Manzoor, A.; Jaspal, M.H.; Yaqub, T.; Haq, A.U.; Nasir, J.; Avais, M.; Asghar, B.; Badar, I.H.; Ahmad, S.; Yar, M.K. Effect of lactic acid spray on microbial and quality parameters of buffalo meat. Meat Sci. 2020, 159, 107923. [Google Scholar] [CrossRef]
- Wyrwisz, J.; Moczkowska, M.; Kurek, M.; Stelmasiak, A.; Półtorak, A.; Wierzbicka, A. Influence of 21 days of vacuum-aging on color, bloom development, and WBSF of beef semimembranosus. Meat Sci. 2016, 122, 48–54. [Google Scholar] [CrossRef]
- Smaoui, S.; Ennouri, K.; Chakchouk-Mtibaa, A.; Karray-Rebai, I.; Hmidi, M.; Bouchaala, K.; Mellouli, L. Relationships between textural modifications, lipid and protein oxidation and sensory attributes of refrigerated turkey meat sausage treated with bacteriocin BacTN635. Food Bioproc. Tech. 2017, 10, 1655–1667. [Google Scholar] [CrossRef]
- Li, C.; Liu, D.; Zhou, G.; Xu, X.; Qi, J.; Shi, P.; Xia, T. Meat quality and cooking attributes of thawed pork with different low field NMR T21. Meat Sci. 2012, 92, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Luz, P.A.C.D.; Jorge, A.M.; Francisco, C.D.L.; Mello, J.L.M.D.; Santos, C.T.; Andrighetto, C. Chemical-physical characteristics of buffalo (Bubalus bubalis) meat subjected to different aging times. Acta Sci. 2017, 39, 419–428. [Google Scholar] [CrossRef][Green Version]
- Kim, J.H.; Kim, J.H.; Yoon, D.K.; Ji, D.S.; Jang, H.J.; Lee, C.H. A comparison of dry and wet aging on physicochemical and sensory characteristics of pork loin with two aging times. Food Sci. Biotechnol. 2018, 27, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Stenström, H.; Li, X.; Hunt, M.C.; Lundström, K. Consumer preference and effect of correct or misleading information after ageing beef longissimus muscle using vacuum, dry ageing, or a dry ageing bag. Meat Sci. 2014, 96, 661–666. [Google Scholar] [CrossRef]
- Davis, G.W.; Dutson, T.R.; Smith, G.C.; Carpenter, Z.L. Fragmentation procedure for bovine longissimus muscle as an index of cooked steak tenderness. J. Food Sci. 1980, 45, 880–884. [Google Scholar] [CrossRef]
- Caine, W.R.; Aalhus, J.L.; Best, D.R.; Dugan, M.E.R.; Jeremiah, L.E. Relationship of texture profile analysis and Warner-Bratzler shear force with sensory characteristics of beef rib steaks. Meat Sci. 2003, 64, 333–339. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Stanišić, N.; Petričević, M.; Živković, D.; Petrović, M.M.; Ostojić-Andrić, D.; Aleksić, S.; Stajić, S. Changes of physical-chemical properties of beef during 14 days of chilling. Biotechnol. Anim. Husb. 2012, 28, 77–85. [Google Scholar] [CrossRef]
- Vitale, M.; Pérez-Juan, M.; Lloret, E.; Arnau, J.; Realini, C.E. Effect of aging time in vacuum on tenderness, and color and lipid stability of beef from mature cows during display in high oxygen atmosphere package. Meat Sci. 2014, 96, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Faustman, C.; Hoagland, T.A.; Mancini, R.A.; Seyfert, M.; Hunt, M.C. Postmortem oxygen consumption by mitochondria and its effects on myoglobin form and stability. J. Agric. Food Chem. 2005, 53, 1223–1230. [Google Scholar] [CrossRef]
- Jacob, R. Implications of the variation in bloom properties of red meat: A review. Meat Sci. 2020, 162, 108040. [Google Scholar] [CrossRef]
- Seideman, S.C.; Cross, H.R.; Smith, G.C.; Durland, P.R. Factors associated with fresh meat color: A review. J. Food Qual. 1984, 6, 211–237. [Google Scholar] [CrossRef]
- Daszkiewicz, T.; Wajda, S.; Matusevicius, P. Changing of beef quality in the process of storage. Vet. Zootech. 2003, 21, 62–65. [Google Scholar]
- Lawrie, R.; Ledward, D. Lawrie’s Meat Science, 7th ed.; Woodhead Publishing: Cambridge, UK, 2006; pp. 279–341. [Google Scholar]
- Gašperlin, L.; Žlender, B.; Abram, V. Colour of beef heated to different temperatures as related to meat ageing. Meat Sci. 2001, 59, 23–30. [Google Scholar] [CrossRef]
- Florek, M.; Litwinczuk, A.; Skalecki, P.; Ryszkowska-Siwko, M. Changes of physicochemical properties of bullocks and heifers meat during 14 days of ageing under vacuum. Polish J. Food Nutr. Sci. 2007, 57, 281–287. [Google Scholar]
- Purslow, P.P.; Oiseth, S.; Hughes, J.; Warner, R.D. The structural basis of cooking loss in beef: Variations with temperature and ageing. Food Res. Int. 2016, 89, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kang, S.M.; Seong, P.; Kang, G.; Kim, Y.; Kim, J.; Lee, S.; Kim, S. Effect of aging time on physicochemical meat quality and sensory property of Hanwoo bull beef. Korean J. Food Sci. Anim. Resour. 2016, 36, 68. [Google Scholar] [CrossRef] [PubMed]
- Huff-Lonergan, E.; Sosnicki, A. Water-holding capacity of fresh meat. Fact Sheet 2002, 4669. Available online: https://porkgateway.org/wp-content/uploads/2015/07/water-holding-capacity-of-fresh-meat.pdf (accessed on 28 July 2021).
- Koohmaraie, M. Biochemical factors regulating the toughening and tenderization processes of meat. Meat Sci. 1996, 43, 193–201. [Google Scholar] [CrossRef]
- Devine, C.E.; Graafhuis, A.E. The basal toughness of unaged lamb. Meat Sci. 1995, 39, 285–291. [Google Scholar] [CrossRef]
- Dransfield, E. Optimisation of tenderisation, ageing and tenderness. Meat Sci. 1994, 36, 105–121. [Google Scholar] [CrossRef]
- Vaskoska, R.; Ha, M.; Naqvi, Z.B.; White, J.D.; Warner, R.D. Muscle, ageing and temperature influence the changes in texture, cooking loss and shrinkage of cooked beef. Foods 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Shanks, B.C.; Wulf, D.M.; Maddock, R.J. The effect of freezing on Warner-Bratzler shear force values of beef longissimus steaks across several postmortem aging periods. J. Anim. Sci. 2002, 80, 2122–2125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Monsón, F.; Sañudo, C.; Sierra, I. Influence of cattle breed and ageing time on textural meat quality. Meat Sci. 2004, 68, 595–602. [Google Scholar] [CrossRef]
- Koohmaraie, M.; Seidemann, S.C.; Schollmeyer, J.E.; Dutson, T.R.; Crouse, J.D. Effect of post-mortem storage on Ca++-dependent proteases, their inhibitor and myofibril fragmentation. Meat Sci. 1987, 19, 187–196. [Google Scholar] [CrossRef]
- Koohmaraie, M.; Shackelford, S.D.; Wheeler, T.L.; Lonergan, S.M.; Doumit, M.E. A muscle hypertrophy condition in lamb (callipyge): Characterization of effects on muscle growth and meat quality traits. J. Anim. Sci. 1995, 73, 3596–3607. [Google Scholar] [CrossRef]
- Kemp, C.M.; Sensky, P.L.; Bardsley, R.G.; Buttery, P.J.; Parr, T. Tenderness—An enzymatic view. Meat Sci. 2010, 84, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Irurueta, M.; Cadoppi, A.; Langman, L.; Grigioni, G.; Carduza, F. Effect of aging on the characteristics of meat from water buffalo grown in the Delta del Paraná region of Argentina. Meat Sci. 2008, 79, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Ramanathan, R. Effects of postmortem storage time on color and mitochondria in beef. Meat Sci. 2014, 98, 65–70. [Google Scholar] [CrossRef]
- Ramanathan, R.; Mancini, R.A. Role of mitochondria in beef color: A review. Meat Muscle Biol. 2018, 2, 309–320. [Google Scholar] [CrossRef]
- Monsón, F.; Sañudo, C.; Sierra, I. Influence of breed and ageing time on the sensory meat quality and consumer acceptability in intensively reared beef. Meat Sci. 2005, 71, 471–479. [Google Scholar] [CrossRef]
| Aging (Days) | Muscle Type | Color | pH | ||||
|---|---|---|---|---|---|---|---|
| L * | a * | b * | C * | h | |||
| 0 | Longissimus lumborum (LL) | 43.15 ± 0.62 | 18.83 ± 0.40 d | 7.18 ± 0.27 | 19.96 ± 0.35 c | 20.73 ± 0.70 | 5.74 ± 0.07 |
| 7 | 44.65 ± 0.75 | 20.22 ± 0.45 abc | 8.21 ± 0.59 | 21.53 ± 0.55 abc | 20.86 ± 0.78 | 5.74 ± 0.04 | |
| 14 | 45.11 ± 0.88 | 19.80 ± 0.44 abcd | 7.93 ± 0.30 | 22.38 ± 1.23 a | 20.93 ± 1.10 | 5.78 ± 0.05 | |
| 21 | 44.13 ± 0.89 | 19.74 ± 0.36 bcd | 8.04 ± 0.52 | 20.73 ± 0.38 bc | 20.07 ± 0.82 | 5.81 ± 0.03 | |
| 28 | 45.24 ± 0.79 | 21.07 ± 0.40 ab | 8.95 ± 0.46 | 21.19 ± 0.53 abc | 21.44 ± 0.84 | 5.84 ± 0.04 | |
| 35 | 44.61 ± 0.37 | 19.53 ± 0.54 cd | 10.53 ± 1.83 | 20.91 ± 0.59 abc | 20.50 ± 0.71 | 5.84 ± 0.03 | |
| 0 | Gluteus medius (GM) | 42.77 ± 0.55 | 19.59 ± 0.42 cd | 8.03 ± 0.55 | 20.83 ± 0.40 abc | 20.91 ± 0.91 | 5.65 ± 0.08 |
| 7 | 43.62 ± 0.86 | 21.17 ± 0.37 a | 9.22 ± 0.36 | 22.24 ± 0.52 ab | 22.38 ± 0.79 | 5.68 ± 0.04 | |
| 14 | 43.78 ± 0.53 | 20.61 ± 0.45 abc | 9.27 ± 0.48 | 22.19 ± 0.60 ab | 22.86 ± 0.96 | 5.71 ± 0.06 | |
| 21 | 44.26 ± 0.68 | 20.13 ± 0.34 abcd | 8.35 ± 0.50 | 21.36 ± 0.39 abc | 21.87 ± 1.38 | 5.75 ± 0.05 | |
| 28 | 43.12 ± 1.80 | 20.34 ± 0.45 abc | 8.79 ± 0.43 | 21.80 ± 0.57 abc | 21.79 ± 0.66 | 5.78 ± 0.03 | |
| 35 | 44.81 ± 0.80 | 20.49 ± 0.45 abc | 9.12 ± 0.57 | 22.48 ± 0.60 a | 23.29 ± 1.04 | 5.82 ± 0.03 | |
| p-value | |||||||
| Muscle type Aging (days) Interactions | 0.133 0.438 0.739 | 0.034 0.005 0.006 | 0.422 0.048 0.110 | 0.030 0.024 0.048 | 0.007 0.762 0.701 | 0.5451 0.0236 0.2302 | |
| Aging (Days) | Muscle Type | Parameters | ||
|---|---|---|---|---|
| Cooking Loss | WBSF | MFI | ||
| 0 | Longissimus lumborum (LL) | 35.67 ± 1.04 | 49.85 ± 3.14 a | 567.78 ± 30.55 c |
| 7 | 38.19 ± 1.05 | 43.38 ± 2.45 ab | 588.39 ± 37.12 c | |
| 14 | 37.24 ± 0.86 | 37.47 ± 2.28 bc | 634.83 ± 33.39 bc | |
| 21 | 37.56 ± 0.69 | 34.37 ± 1.69 cd | 649.44 ± 41.47 abc | |
| 28 | 36.12 ± 1.16 | 29.12 ± 1.44 def | 653.22 ± 51.97 abc | |
| 35 | 36.26 ± 1.30 | 24.75 ± 1.81 f | 768.44 ± 34.32 a | |
| 0 | Gluteus medius (GM) | 36.22 ± 0.71 | 47.81 ± 3.28 a | 544.56 ± 47.41 c |
| 7 | 38.40 ± 0.50 | 38.58 ± 2.73 bc | 568.83 ± 36.15 c | |
| 14 | 38.04 ± 0.43 | 33.48 ± 2.63 cde | 588.06 ± 29.05 c | |
| 21 | 37.85 ± 0.79 | 29.17 ± 1.90 def | 593.11 ± 52.05 c | |
| 28 | 36.15 ± 1.19 | 27.03 ± 1.77 ef | 650.78 ± 43.91 abc | |
| 35 | 36.90 ± 1.04 | 25.96 ± 1.77 f | 736.83 ± 40.31 ab | |
| p-value | ||||
| Muscle type Aging (days) | 0.2969 0.0104 | 0.0361 <0.0001 | 0.2011 <0.0001 | |
| Interactions | 0.0609 | <0.0001 | 0.0016 | |
| Aging (Days) | Muscle Type | Odor | Flavor Intensity | Overall Tenderness | Juiciness | Overall Acceptability |
|---|---|---|---|---|---|---|
| 0 | Longissimus lumborum (LL) | 6.12 ± 0.45 | 5.78 ± 0.60 | 6.25 ± 0.73 f | 5.48 ± 0.61 | 5.42 ± 0.62 c |
| 7 | 6.08 ± 0.50 | 5.75 ± 0.53 | 6.66 ± 0.57 e | 5.54 ± 0.59 | 5.44 ± 0.59 c | |
| 14 | 6.05 ± 0.19 | 6.69 ± 0.56 | 6.97 ± 0.65 d | 5.59 ± 0.81 | 5.50 ± 0.81 c | |
| 21 | 6.00 ± 0.26 | 5.72 ± 0.72 | 7.32 ± 0.69 c | 5.65 ± 0.73 | 5.67 ± 0.56 b | |
| 28 | 5.97 ± 0.35 | 5.76 ± 0.51 | 7.47 ± 0.67 b | 5.64 ± 0.83 | 5.69 ± 0.28 b | |
| 35 | 5.99 ± 0.31 | 6.82 ± 0.42 | 7.86 ± 0.40 a | 5.66 ± 0.52 | 5.79 ± 0.57 a | |
| 0 | Gluteus medius (GM) | 6.06 ± 0.29 | 5.69 ± 0.72 | 6.31 ± 0.89 f | 5.31 ± 0.90 | 5.24 ± 0.78 e |
| 7 | 6.03 ± 0.49 | 5.62 ± 0.82 | 6.61 ± 0.69 e | 5.47 ± 0.58 | 5.32 ± 0.71 d | |
| 14 | 5.99 ± 0.66 | 5.69 ± 0.54 | 7.02 ± 0.80 d | 5.48 ± 0.80 | 5.45 ± 0.39 c | |
| 21 | 5.95 ± 0.11 | 5.72 ± 0.32 | 7.20 ± 0.16 c | 5.56 ± 0.50 | 5.51 ± 0.21 c | |
| 28 | 5.90 ± 0.39 | 6.75 ± 0.50 | 7.54 ± 0.42 b | 5.53 ± 0.63 | 5.56 ± 0.43 c | |
| 35 | 5.89 ± 0.19 | 6.70 ± 0.27 | 7.63 ± 0.39 ab | 5.54 ± 0.63 | 5.51 ± 0.45 c | |
| p-value | ||||||
| Muscle type Aging (days) | 0.4692 0.0321 | 0.0221 0.8030 | 0.8322 0.6823 | 0.6726 0.9639 | 0.0182 0.0251 | |
| Interactions | 0.8534 | 0.9353 | 0.0212 | 0.9990 | 0.0158 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaspal, M.H.; Badar, I.H.; Amjad, O.B.; Yar, M.K.; Ijaz, M.; Manzoor, A.; Nasir, J.; Asghar, B.; Ali, S.; Nauman, K.; et al. Effect of Wet Aging on Color Stability, Tenderness, and Sensory Attributes of Longissimus lumborum and Gluteus medius Muscles from Water Buffalo Bulls. Animals 2021, 11, 2248. https://doi.org/10.3390/ani11082248
Jaspal MH, Badar IH, Amjad OB, Yar MK, Ijaz M, Manzoor A, Nasir J, Asghar B, Ali S, Nauman K, et al. Effect of Wet Aging on Color Stability, Tenderness, and Sensory Attributes of Longissimus lumborum and Gluteus medius Muscles from Water Buffalo Bulls. Animals. 2021; 11(8):2248. https://doi.org/10.3390/ani11082248
Chicago/Turabian StyleJaspal, Muhammad Hayat, Iftikhar Hussain Badar, Osama Bin Amjad, Muhammad Kashif Yar, Muawuz Ijaz, Adeel Manzoor, Jamal Nasir, Bilal Asghar, Sher Ali, Kashif Nauman, and et al. 2021. "Effect of Wet Aging on Color Stability, Tenderness, and Sensory Attributes of Longissimus lumborum and Gluteus medius Muscles from Water Buffalo Bulls" Animals 11, no. 8: 2248. https://doi.org/10.3390/ani11082248
APA StyleJaspal, M. H., Badar, I. H., Amjad, O. B., Yar, M. K., Ijaz, M., Manzoor, A., Nasir, J., Asghar, B., Ali, S., Nauman, K., Rahman, A., & Wara, U. U. (2021). Effect of Wet Aging on Color Stability, Tenderness, and Sensory Attributes of Longissimus lumborum and Gluteus medius Muscles from Water Buffalo Bulls. Animals, 11(8), 2248. https://doi.org/10.3390/ani11082248







