Cannabis Extract Has a Positive–Immunostimulating Effect through Proteolytic System and Metabolic Compounds of Honey Bee (Apis mellifera) Workers
Abstract
:Simple Summary
Abstract
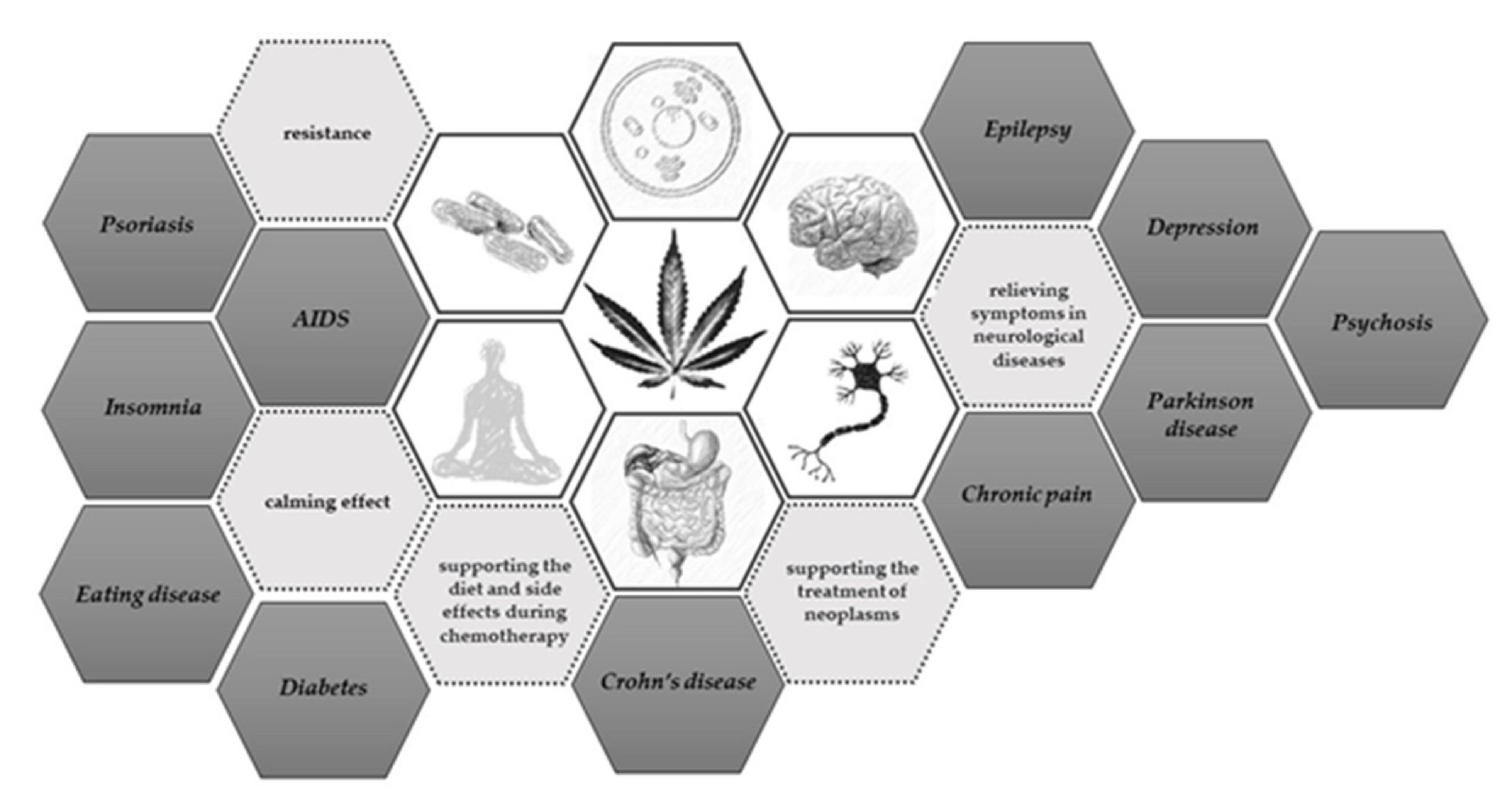
1. Introduction
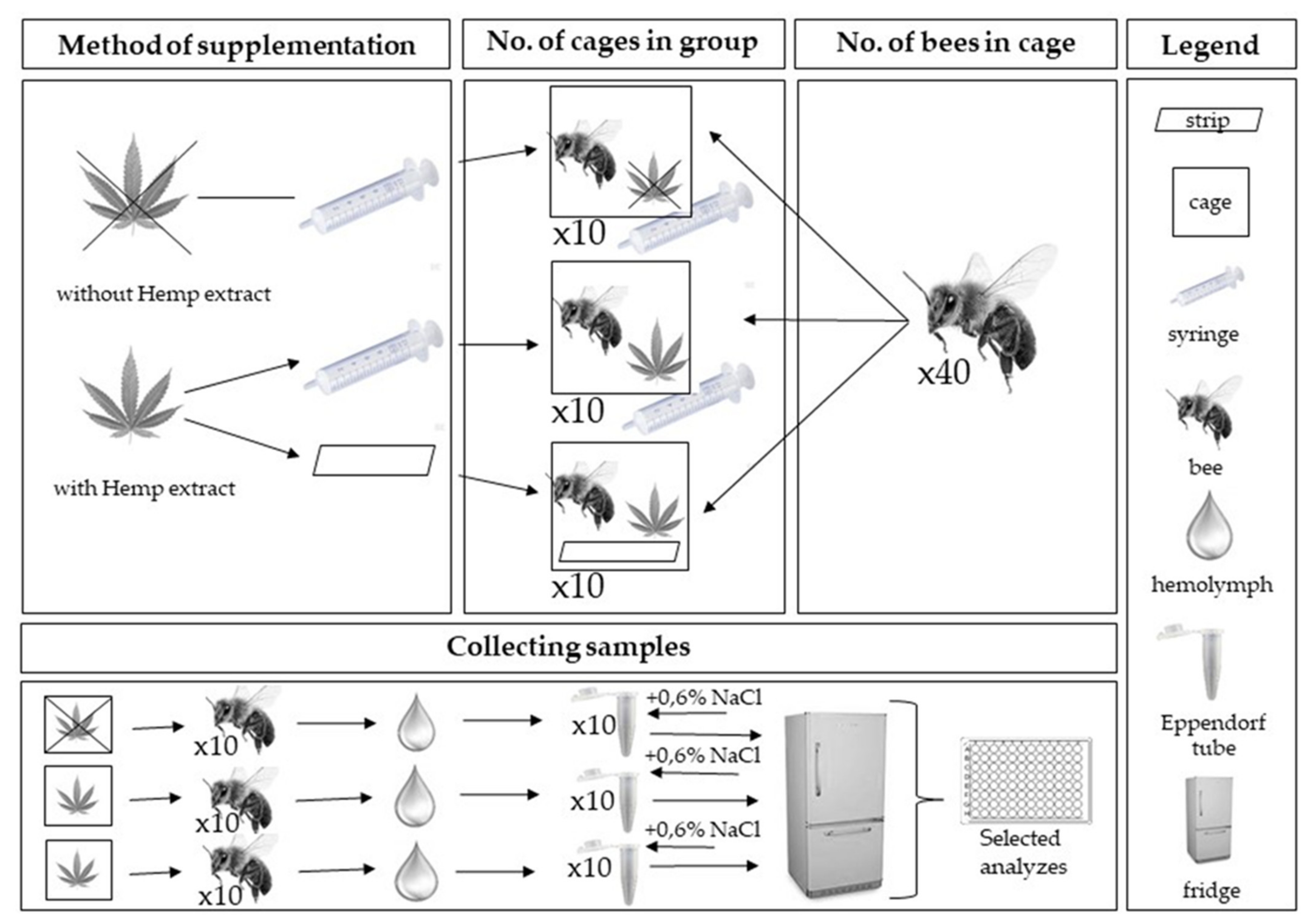
2. Materials and Methods
2.1. Cages Part
- (1)
- control group fed with mixture of sugar and water-glycerine solution in a 1:1 ratio ad libidum
- (2)
- experimental group with 1:1 pure sugar syrup ad libidum and inside with cotton strips soaked with 3 mL hemp extract (0.25 g pure hemp paste extract +3 mL water-glycerine solution)
- (3)
- experimental group with a mixture of sugar syrup 1:1 with hemp extract ad libidum (500 mL water-glycerine solution with 4.38 g hemp paste extract)
2.2. Analytical Part
- (1)
- (2)
- Proteolytic system activity was determined as follows:
- (3)
- Activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) using monotests from Cormay (Lublin, Poland) according to the manufacturer’s procedure (methodological details in: File S1);
- (4)
- Concentration of urea and glucose using monotests from Cormay (Lublin, Poland) according to the manufacturer’s procedure (methodological details in: File S1).
3. Statistical Analysis
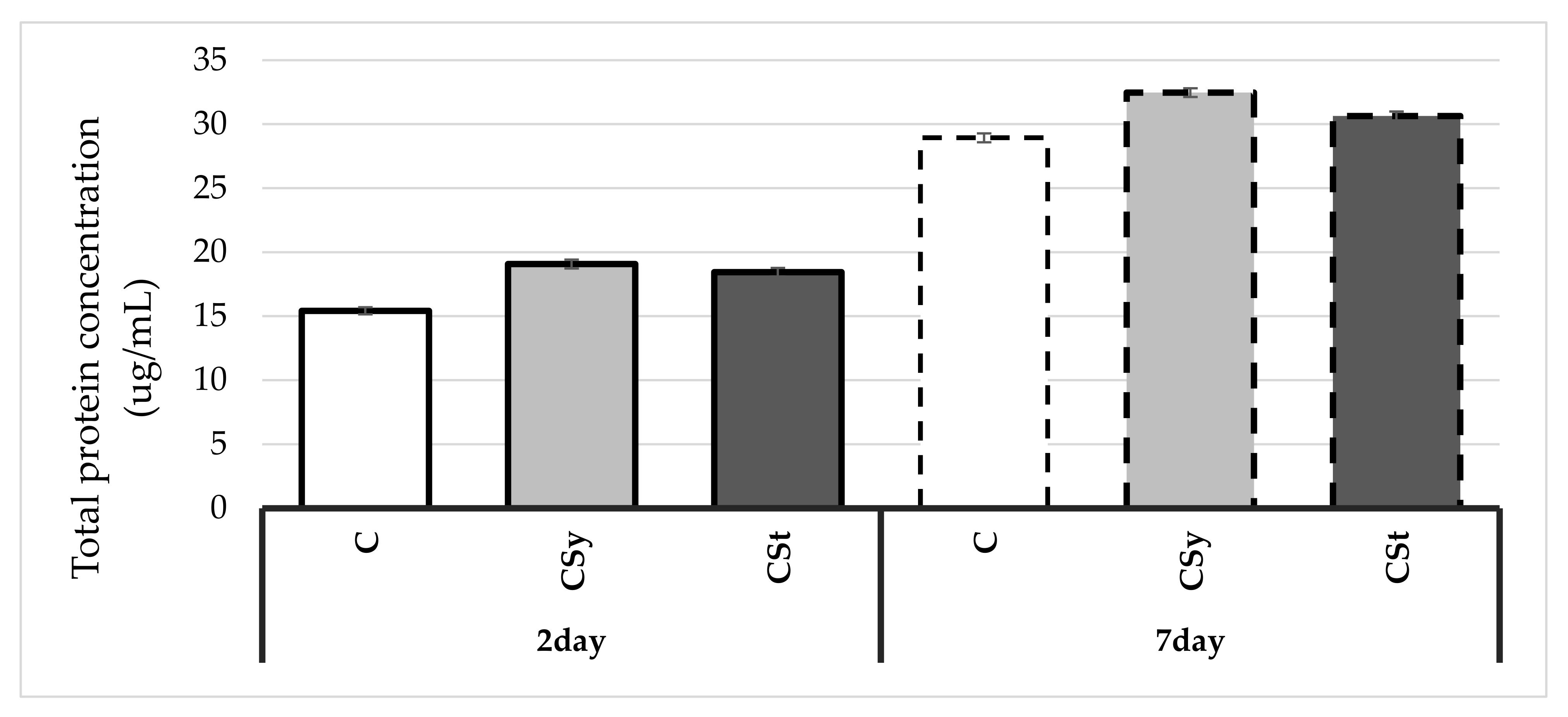
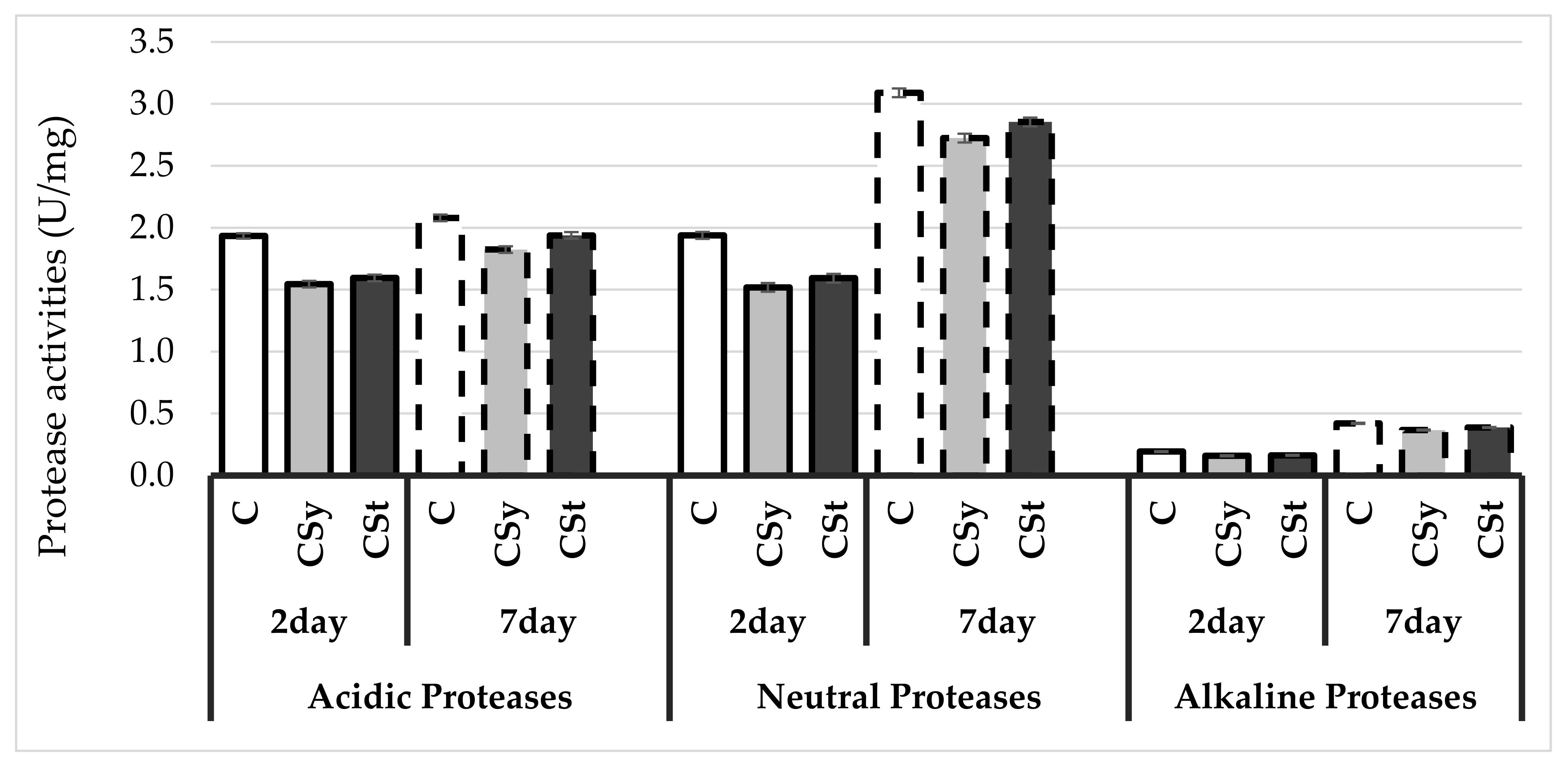
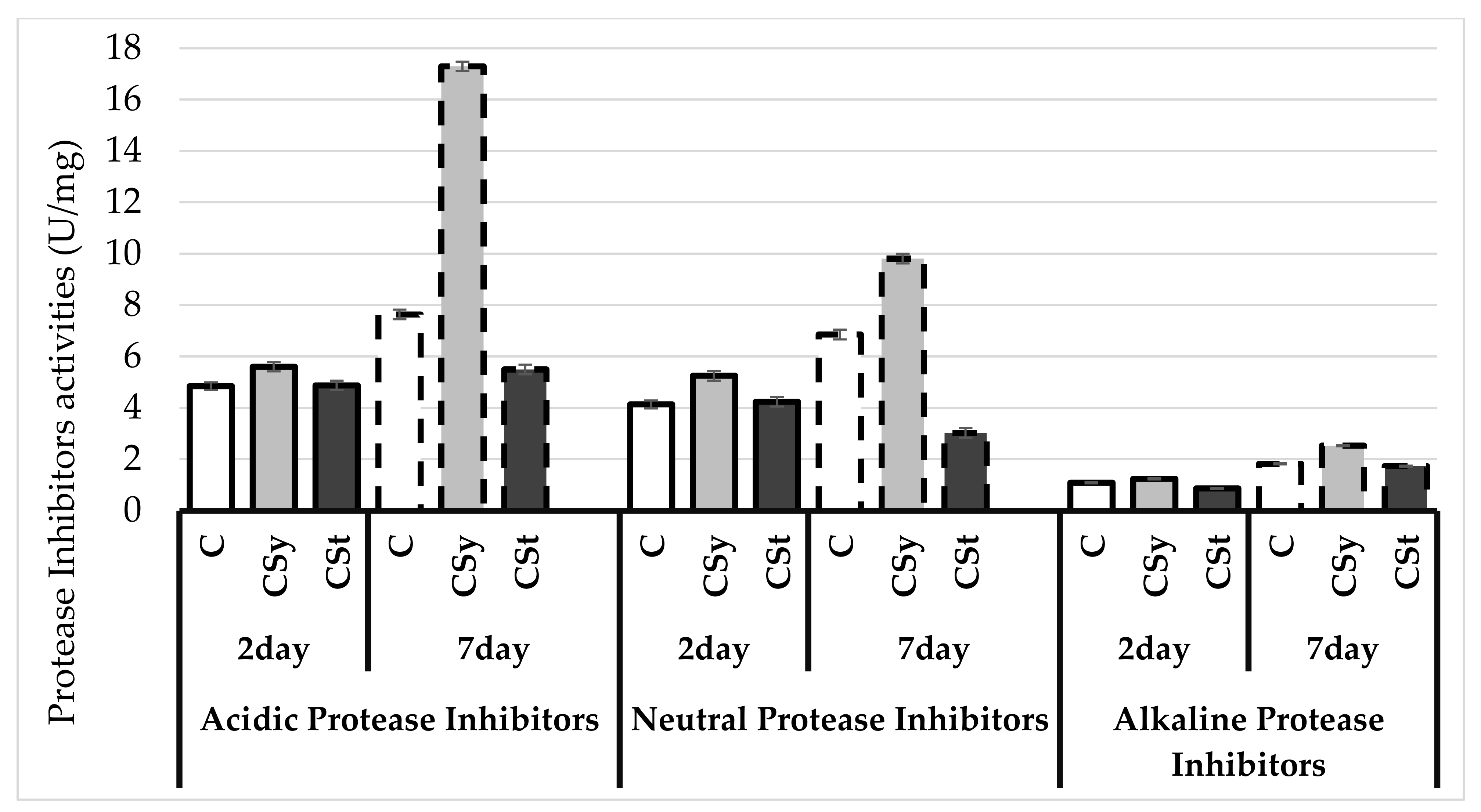
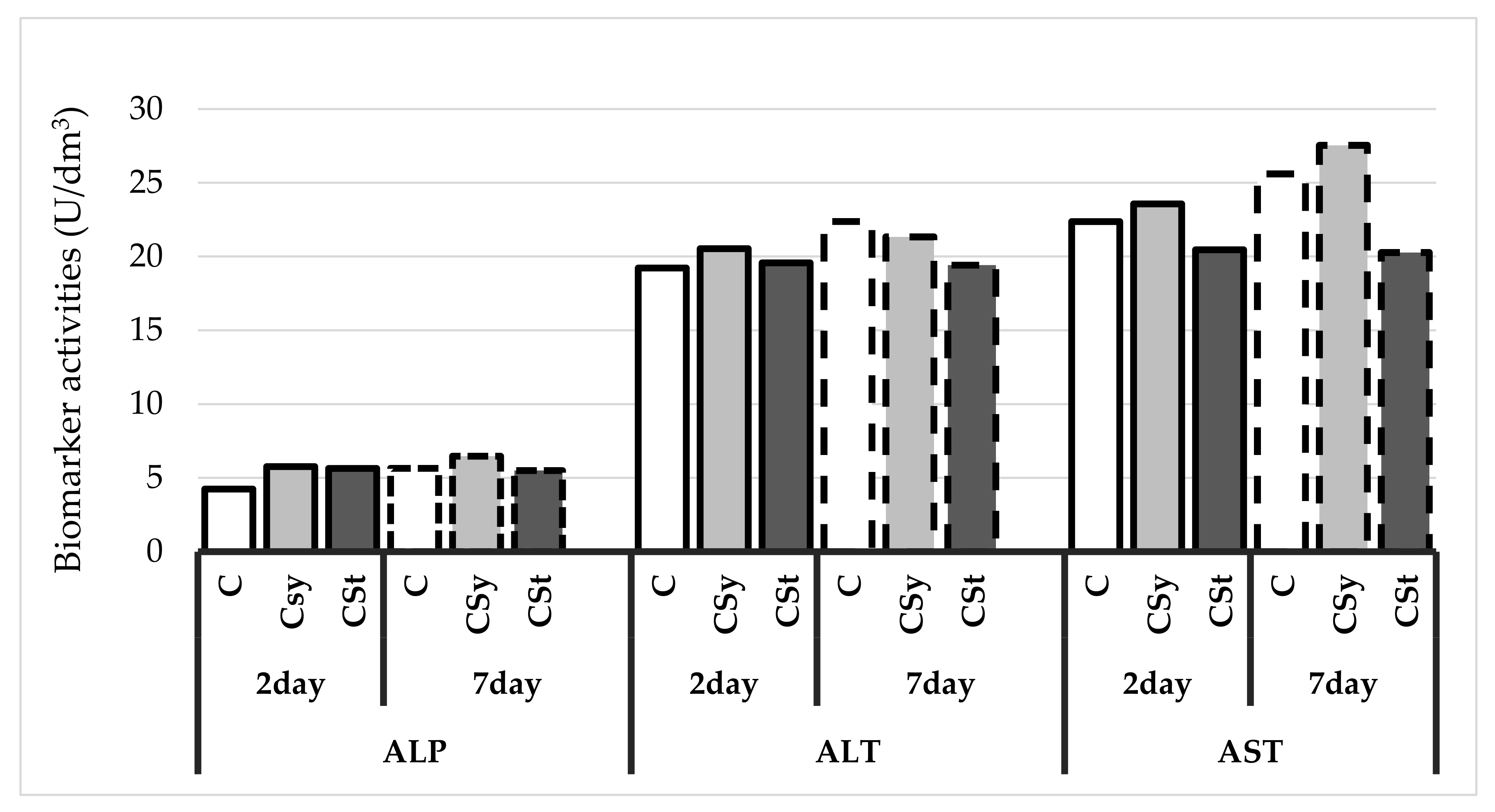
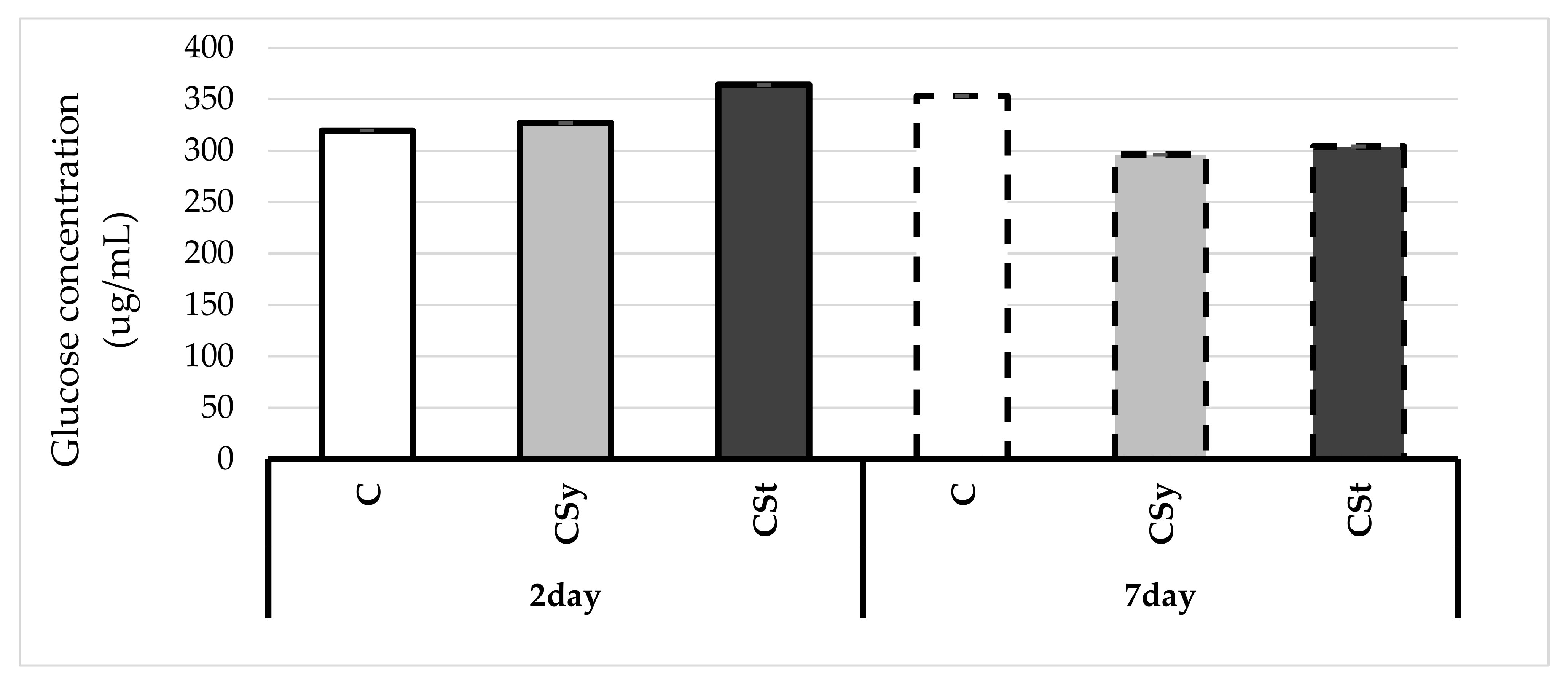
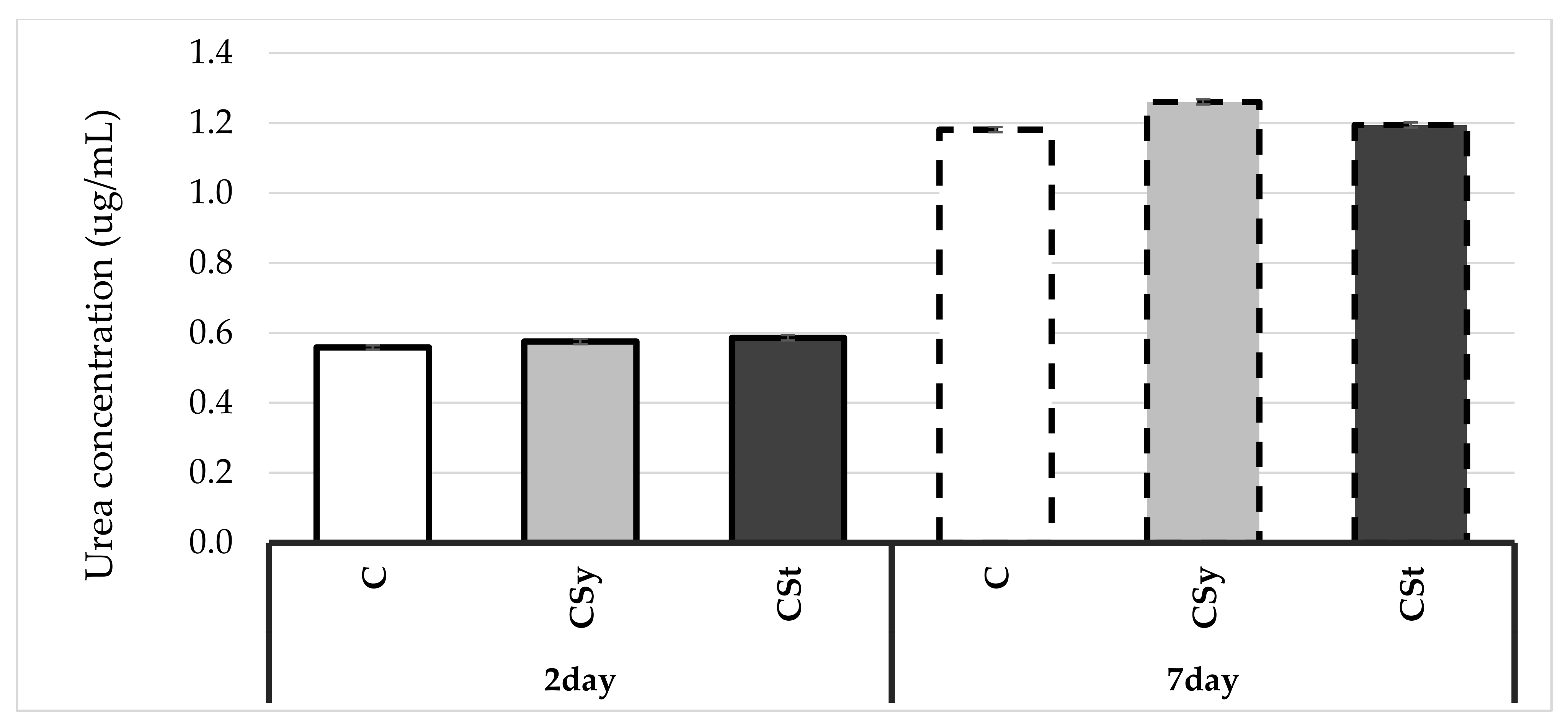
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mizrahi, A.; Lensky, Y. Bee Products; Springer: New York, NY, USA, 2013. [Google Scholar]
- Aizen, M.A.; Harder, L.D. The Global Stock of Domesticated Honey Bees Is Growing Slower Than Agricultural Demand for Pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rascón, B.; Hubbard, B.P.; Sinclair, D.A.; Amdam, G.V. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging 2012, 4, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Ricigliano, V.A.; Simone-Finstrom, M. Nutritional and prebiotic efficacy of the microalga Arthrospira platensis (spirulina) in honey bees. Apidologie 2020, 51, 898–910. [Google Scholar] [CrossRef]
- Farjan, M.; Dmitryjuk, M.; Lipiński, Z.; Biernat-Łopieńska, E.; Zółtowska, K. Supplementation of the honey bee diet with vitamin C: The effect on the antioxidative system of Apis mellifera carnica brood at different stages. J. Apic. Res. 2012, 51, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Strachecka, A.; Krauze, M.; Olszewski, K.; Borsuk, G.; Paleolog, J.; Merska, M.; Chobotow, J.; Bajda, M.; Grzywnowicz, K. Unexpectedly strong effect of caffeine on the vitality of western honeybees (Apis mellifera). Biochem 2014, 79, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Van der Steen, J. Effect of a home-made pollen substitute on honey bee colony development. J. Apic. Res. 2007, 46, 114–119. [Google Scholar] [CrossRef]
- Kaznowski, A.; Szymas, B.; Jazdzinska, E.; Kazimierczak, M.; Paetz, H.; Mokracka, J. The effects of probiotic supplementation on the content of intestinal microflora and chemical composition of worker honey bees (Apis mellifera). J. Apic. Res. 2005, 44, 10–14. [Google Scholar] [CrossRef]
- Suwannapong, G.; Maksong, S.; Phainchajoen, M.; Benbow, M.E.; Mayack, C. Survival and health improvement of Nosema infected Apis florea (Hymenoptera: Apidae) bees after treatment with propolis extract. J. Asia. Pac. Entomol. 2018, 21, 437–444. [Google Scholar] [CrossRef]
- Benson, M.J.; Abelev, S.V.; Connor, S.J.; Corte, C.J.; Martin, L.J.; Gold, L.K.; Suraev, A.S.; McGregor, I.S. Medicinal cannabis for inflammatory bowel disease: A survey of perspectives, experiences, and current use in australian patients. Crohn’s Colitis 360 2020, 2. [Google Scholar] [CrossRef] [Green Version]
- Reithmeier, D.; Tang-Wai, R.; Seifert, B.; Lyon, A.W.; Alcorn, J.; Acton, B.; Corley, S.; Prosser-Loose, E.; Mousseau, D.D.; Lim, H.J. The protocol for the Cannabidiol in children with refractory epileptic encephalopathy (CARE-E) study: A phase 1 dosage escalation study. BMC Pediatrics 2018, 18, 221. [Google Scholar] [CrossRef] [Green Version]
- Samarut, É.; Nixon, J.; Kundap, U.P.; Drapeau, P.; Ellis, L.D. Single and synergistic effects of cannabidiol and δ-9-tetrahydrocannabinol on zebrafish models of neuro-hyperactivity. Front. Pharmacol. 2019, 10, 226. [Google Scholar] [CrossRef]
- Hazekamp, A.; Simons, R.; Peltenburg-Looman, A.; Sengers, M.; Van Zweden, R.; Verpoorte, R. Preparative isolation of cannabinoids from Cannabis sativa by centrifugal partition chromatography. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 2421–2439. [Google Scholar] [CrossRef]
- Amin, M.R.; Ali, D.W. Pharmacology of Medical Cannabis. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; Volume 1162, pp. 151–165. [Google Scholar]
- Lopez, H.L.; Cesareo, K.R.; Raub, B.; Kedia, A.W.; Sandrock, J.E.; Kerksick, C.M.; Ziegenfuss, T.N. Effects of Hemp Extract on Markers of Wellness, Stress Resilience, Recovery and Clinical Biomarkers of Safety in Overweight, But Otherwise Healthy Subjects. J. Diet. Suppl. 2020, 17, 561–586. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, V.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Politi, M.; Antolini, M.D.; Acquaviva, A. Metabolomic profile and antioxidant/anti-inflammatory effects of industrial hemp water extract in fibroblasts, keratinocytes and isolated mouse skin specimens. Antioxidants 2021, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Mabou Tagne, A.; Fotio, Y.; Lin, L.; Squire, E.; Ahmed, F.; Rashid, T.I.; Karimian Azari, E.; Piomelli, D. Palmitoylethanolamide and hemp oil extract exert synergistic anti-nociceptive effects in mouse models of acute and chronic pain. Pharmacol. Res. 2021, 167, 105545. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Magi, G.; Ferretti, G.; Bacchetti, T.; Giuliani, A.; Pugnaloni, A.; Rippo, M.R.; Facinelli, B. Attenuation of Listeria monocytogenes virulence by Cannabis sativa L. Essential oil. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef] [Green Version]
- Strachecka, A.; Łoś, A.; Filipczuk, J.; Schulz, M. Individual and social immune mechanisms of the honey bee. Med. Weter. 2018, 74, 426–433. [Google Scholar]
- Wilde, J.; Frączek, R.J.; Siuda, M.; Bąk, B.; Hatjina, F.; Miszczak, A. The influence of sublethal doses of imidacloprid on protein content and proteolytic activity in honey bees (Apis mellifera L.). J. Apic. Res. 2016, 55, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Immunobiologia Pszczoły Miodnej, Zdzisław Gliński, Jan Jarosz. Available online: https://vetbooks.pl/p/7/421/immunobiologia-pszczoly-miodnej-pszczelarstwo-hodowla-zwierzat.html (accessed on 2 July 2021).
- Strachecka, A.; Demetraki-Paleolog, J. System proteolityczny powierzchni ciała apis mellifera w zachowaniu zdrowotności rodzin pszczelich. Kosmos 2011, 60, 43–51. [Google Scholar]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Łoś, A.; Strachecka, A. Fast and cost-effective biochemical spectrophotometric analysis of solution of insect “blood” and body surface elution. Sensors 2018, 18, 1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strachecka, A.; Olszewski, K.; Paleolog, J. Curcumin stimulates biochemical mechanisms of Apis mellifera resistance and extends the apian life-span. J. Apic. Sci. 2015, 59, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Strachecka, A.; Olszewski, K.; Paleolog, J. Varroa treatment with bromfenvinphos markedly suppresses honeybee biochemical defence levels. Entomol. Exp. Appl. 2016, 160, 57–71. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Paleolog, J.; Borsuk, G.; Bajda, M.; Krauze, M.; Merska, M.; Chobotow, J. Coenzyme Q10 treatments influence the lifespan and key biochemical resistance systems in the honeybee, Apis mellifera. Arch. Insect Biochem. Physiol. 2014, 86, 165–179. [Google Scholar] [CrossRef]
- Schacterle, G.; Pollack, R. A Simplified Method for the Quantitative Assay of Small Amounts of Proteins in Biologic Material. Anal. Biochem. 1973, 51, 654–655. [Google Scholar] [CrossRef]
- Waterborg, J.H. The Lowry Method for Protein Quantitation. In The Protein Protocols Handbook; Humana Press: Totowa, NJ, USA, 2009; pp. 7–10. [Google Scholar]
- Anson, M.L. The Estimation Of Pepsin, Trypsin, Papain, And Cathepsin With Hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef]
- Strachecka Aneta, G.M.M. Influence of Environmental Pollution on the Protective Proteolytic Barrier of the Honey Bee Apis mellifera mellifera. Polish J. Environ. Stud. 2010, 19, 855–859. [Google Scholar]
- Lin, J.; Lee, I.S.; Frey, J.; Slonczewski, J.L.; Foster, J.W. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 1995, 177, 4097–4104. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Salam, O.; Sleem, A.; Youness, E.; Morsy, F. Preventive effects of cannabis on neurotoxic and hepatotoxic activities of malathion in rat. Asian Pac. J. Trop. Med. 2018, 11, 272–279. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [Green Version]
- Dube, K.A.; McDonald, D.G.; O’Donnell, M.J. Calcium homeostasis in larval and adult Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2000, 44, 27–39. [Google Scholar] [CrossRef]
- Park, Y.; Kumar, S.; Kanumuri, R.; Stanley, D.; Kim, Y. A novel calcium-independent cellular PLA2 acts in insect immunity and larval growth. Insect Biochem. Mol. Biol. 2015, 66, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Underwood, R.M.; Traver, B.E.; López-Uribe, M.M. Beekeeping management practices are associated with operation size and beekeepers’ philosophy towards in-hive chemicals. Insects. 2019, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Strachecka, A.; Paleolog, J.; Grzywnowicz, K. The surface proteolytic activity in Apis mellifera. J. Apic. Sci. 2008, 52, 49–56. [Google Scholar]
- Dhule, S.S.; Penfornis, P.; Frazier, T.; Walker, R.; Feldman, J.; Tan, G.; He, J.; Alb, A.; John, V.; Pochampally, R. Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 440–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migdał, P.; Murawska, A.; Strachecka, A.; Bieńkowski, P.; Roman, A. Honey bee proteolytic system and behavior parameters under the influence of an electric field at 50 hz and variable intensities for a long exposure time. Animals 2021, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Bode, W.; Fernandez-Catalan, C.; Tschesche, H.; Grams, F.; Nagase, H.; Maskos, K. Structural properties of matrix metalloproteinases. Cell. Mol. Life Sci. 1999, 55, 639–652. [Google Scholar] [CrossRef]
- Sokol, R. Wybrane wskazniki biochemiczne hemolimfy w przebiegu inwazji Varroa jacobsoni u pszczol. II. Aktywnosc transaminazy asparaginianowej i alaninowej w hemolimfie czerwia, pszczol i trutni. Acta Acad. Agric. Tech. Olstenensis. Vet. 1996, 24, 113–125. [Google Scholar]
- Gilbert, L.I. Lipid Metabolism and Function in Insects. Adv. Insect Phys. 1967, 4, 69–211. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.-P.; Chen, Y.P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M. Standard methods for Nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Schulz, M.; Skowronek, P.; Tyszczuk, J.; Los, A.; Strachecka, A. Nosema apis i Nosema ceranae—Porównanie morfologii, metod identyfikacji oraz przebiegu mikrosporydiozy u pszczół miodnych. Przegląd Hod. 2018, 86, 21–24. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowronek, P.; Wójcik, Ł.; Strachecka, A. Cannabis Extract Has a Positive–Immunostimulating Effect through Proteolytic System and Metabolic Compounds of Honey Bee (Apis mellifera) Workers. Animals 2021, 11, 2190. https://doi.org/10.3390/ani11082190
Skowronek P, Wójcik Ł, Strachecka A. Cannabis Extract Has a Positive–Immunostimulating Effect through Proteolytic System and Metabolic Compounds of Honey Bee (Apis mellifera) Workers. Animals. 2021; 11(8):2190. https://doi.org/10.3390/ani11082190
Chicago/Turabian StyleSkowronek, Patrycja, Łukasz Wójcik, and Aneta Strachecka. 2021. "Cannabis Extract Has a Positive–Immunostimulating Effect through Proteolytic System and Metabolic Compounds of Honey Bee (Apis mellifera) Workers" Animals 11, no. 8: 2190. https://doi.org/10.3390/ani11082190
APA StyleSkowronek, P., Wójcik, Ł., & Strachecka, A. (2021). Cannabis Extract Has a Positive–Immunostimulating Effect through Proteolytic System and Metabolic Compounds of Honey Bee (Apis mellifera) Workers. Animals, 11(8), 2190. https://doi.org/10.3390/ani11082190






