Effects of a Maternal Essential Fatty Acid and Conjugated Linoleic Acid Supplementation during Late Pregnancy and Early Lactation on Hematologic and Immunological Traits and the Oxidative and Anti-Oxidative Status in Blood Plasma of Neonatal Calves
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals, Experimental Design, and Husbandry
2.2. Sample Collection
2.3. Milk Analyses
2.4. Blood Analyses
2.5. Statistical Analyses
3. Results
3.1. Hematology
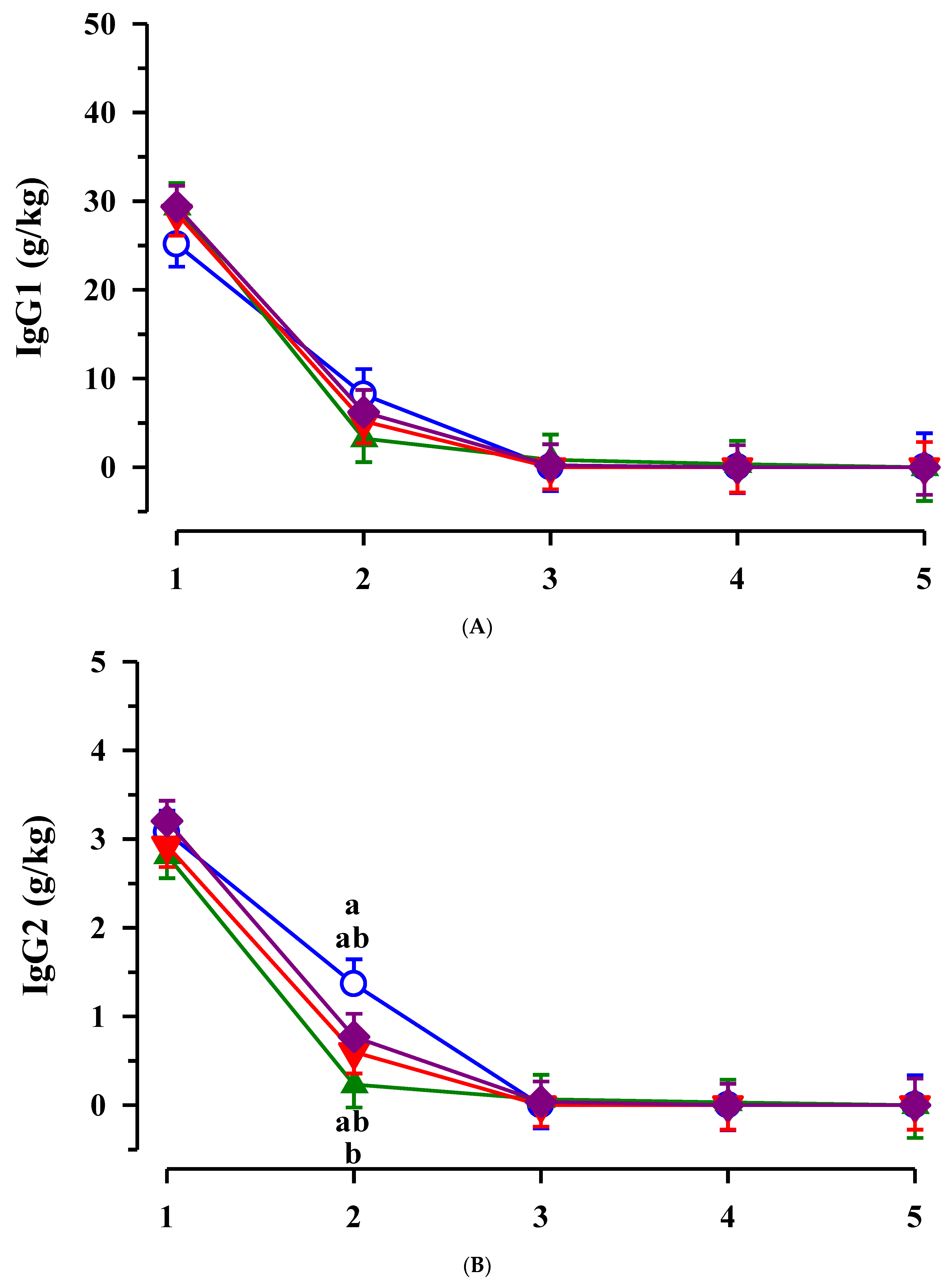
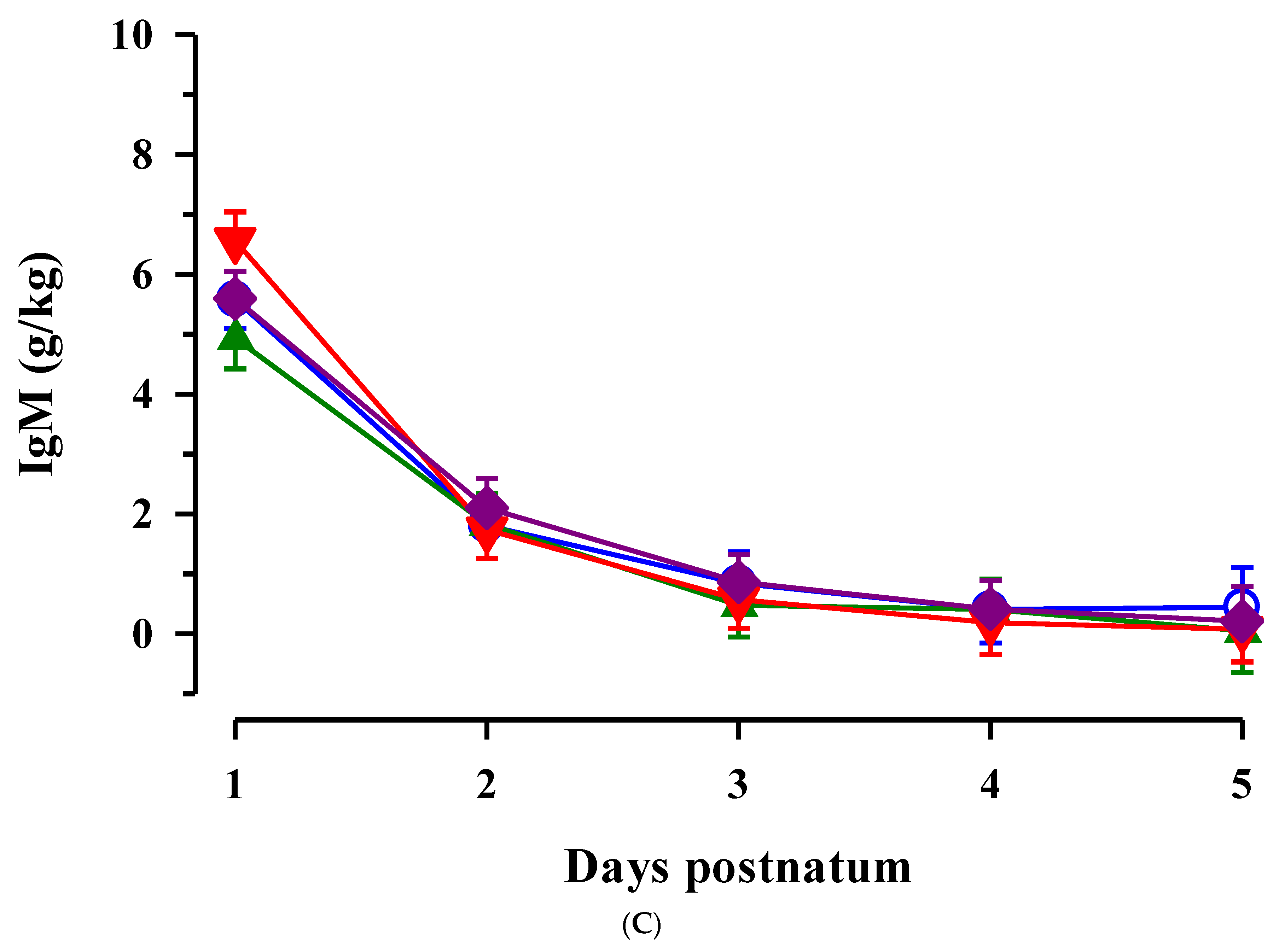
3.2. Immunoglobulins in Colostrum and Transition Milk
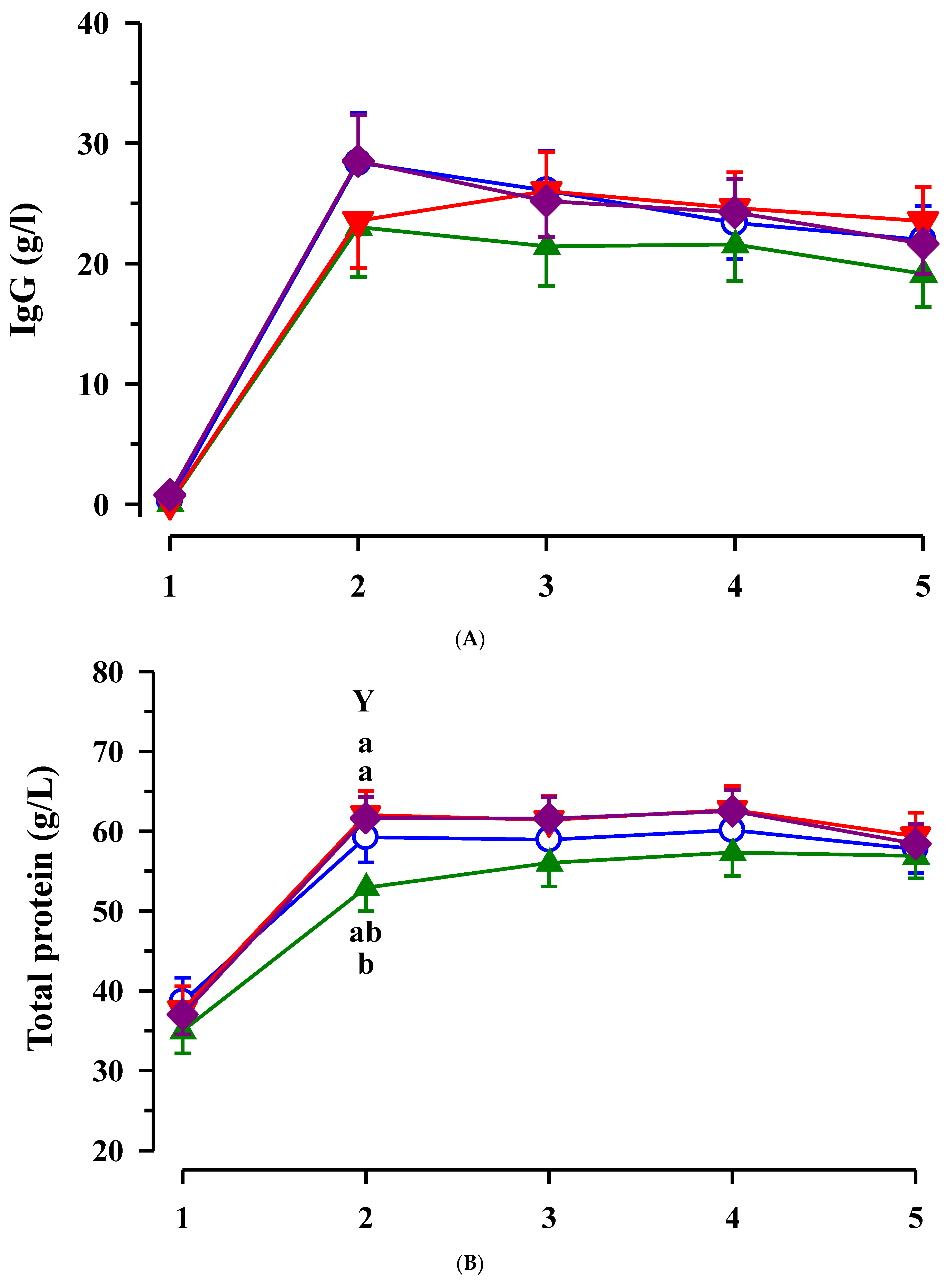
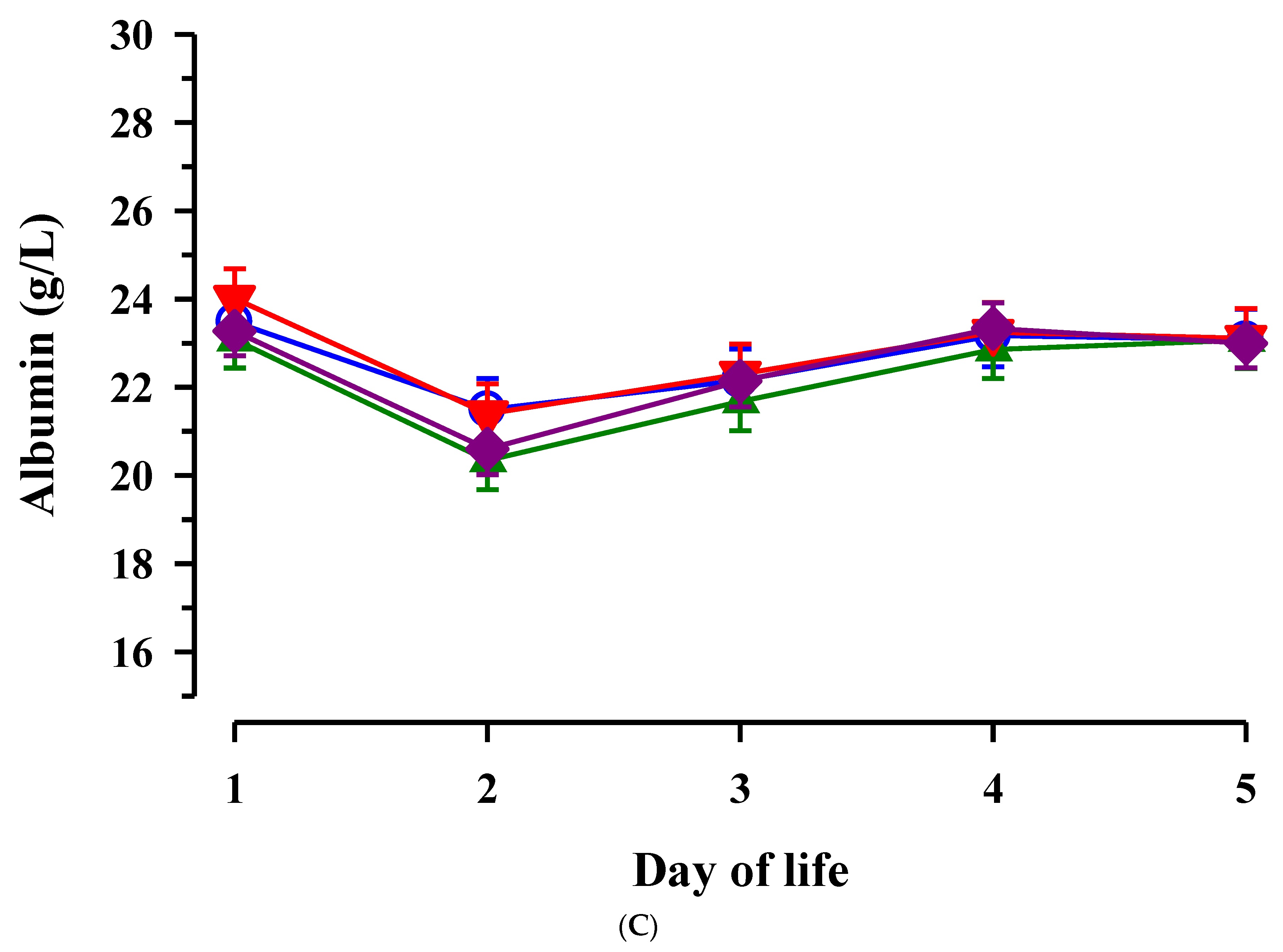
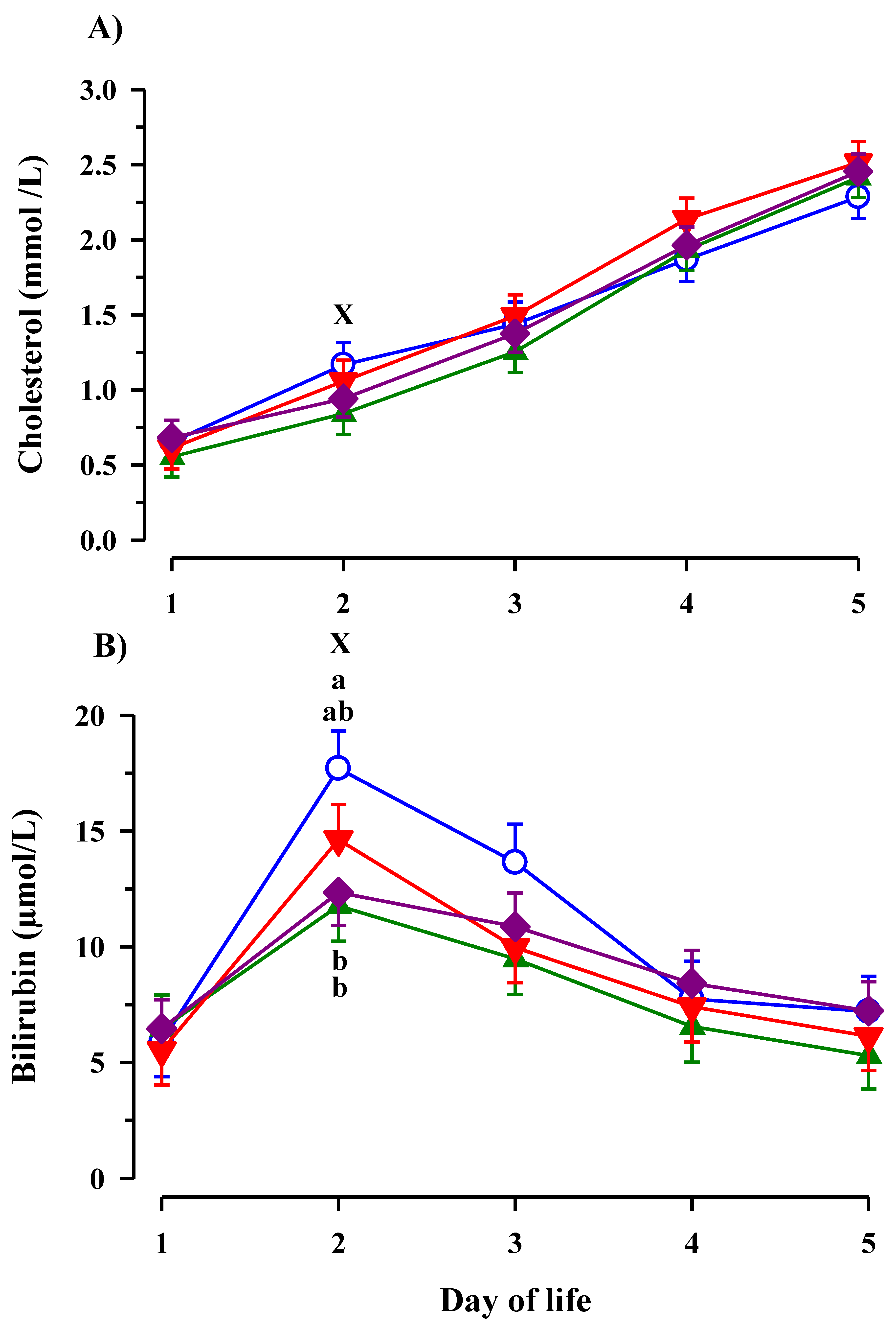
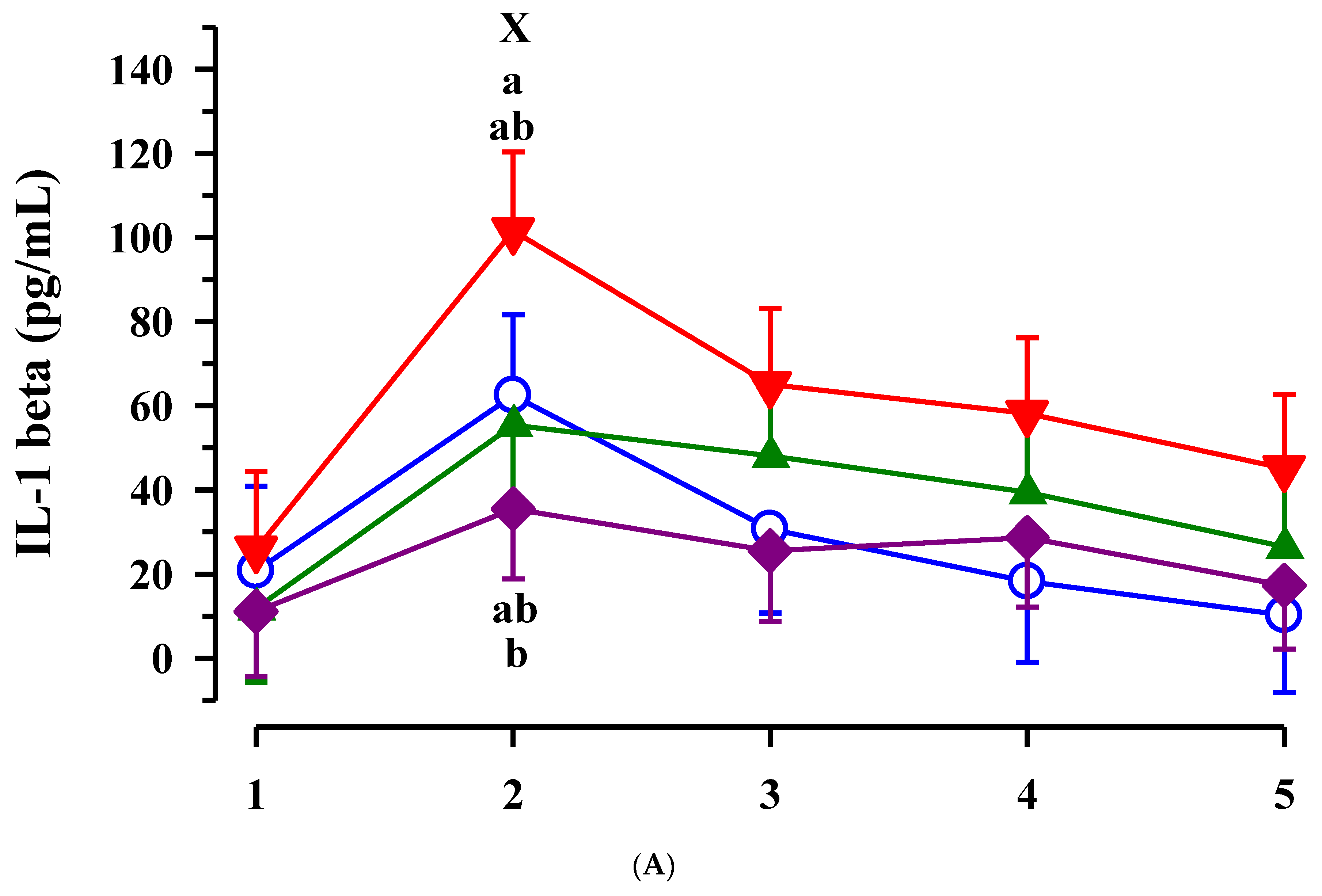
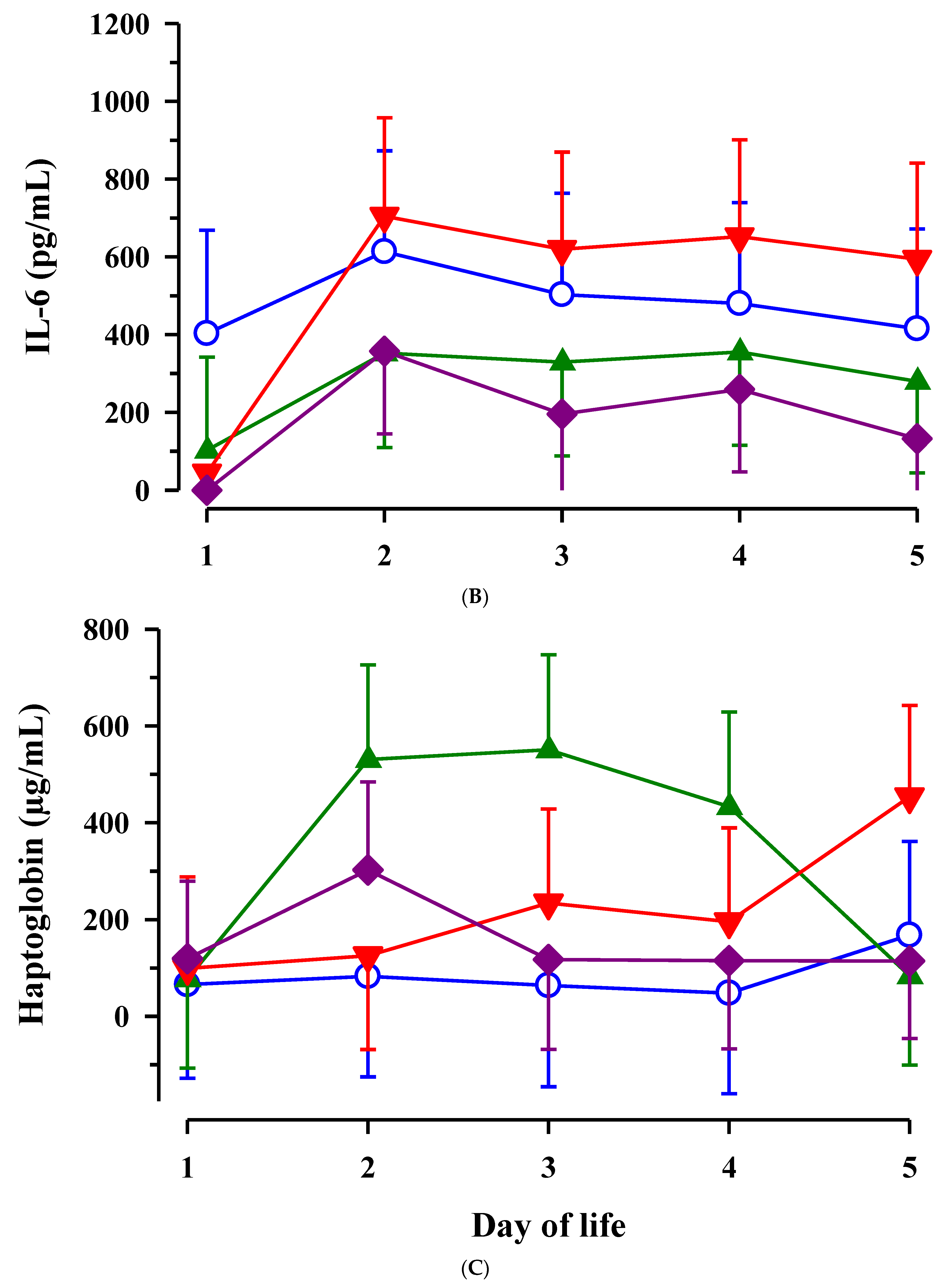
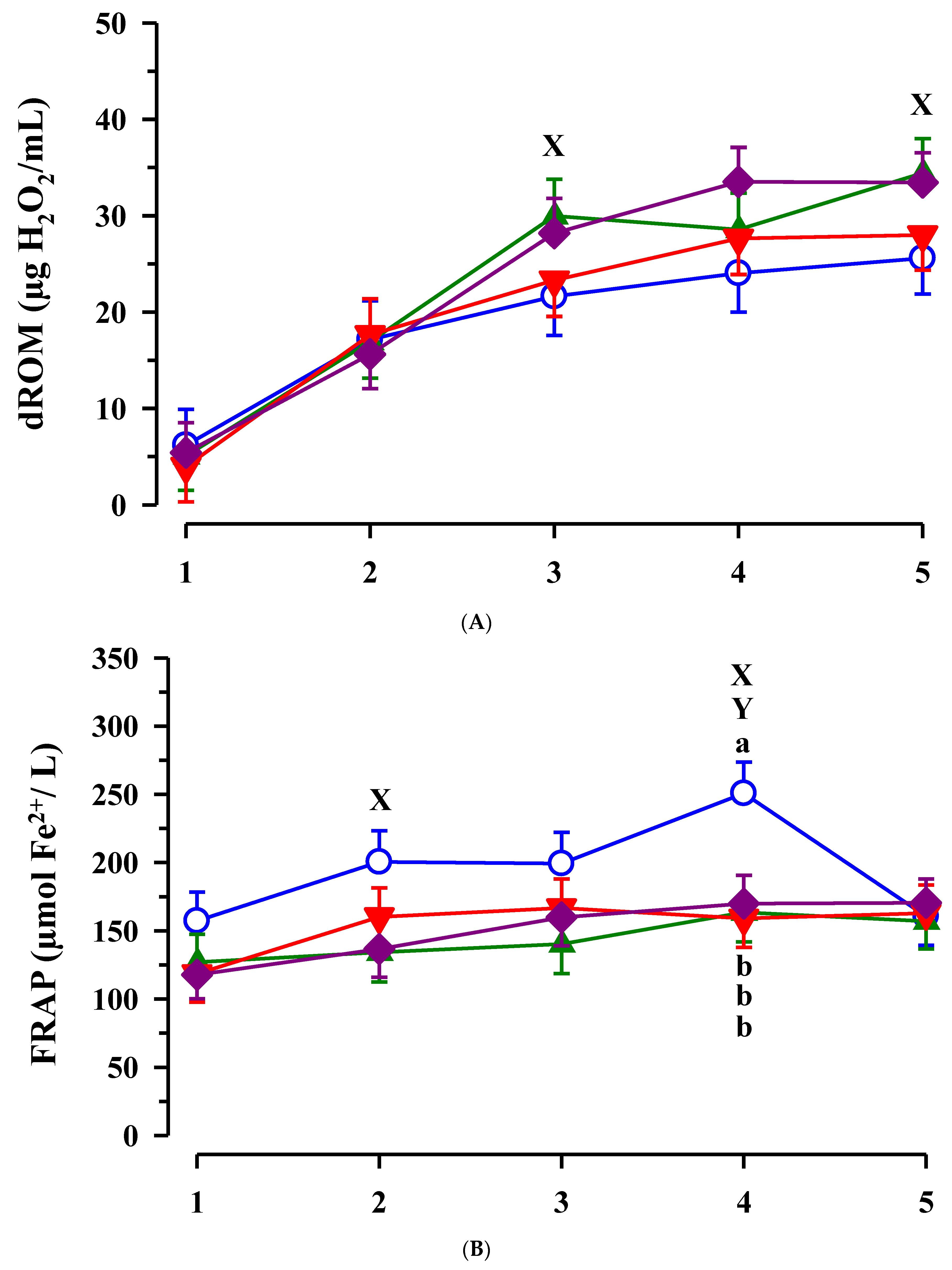
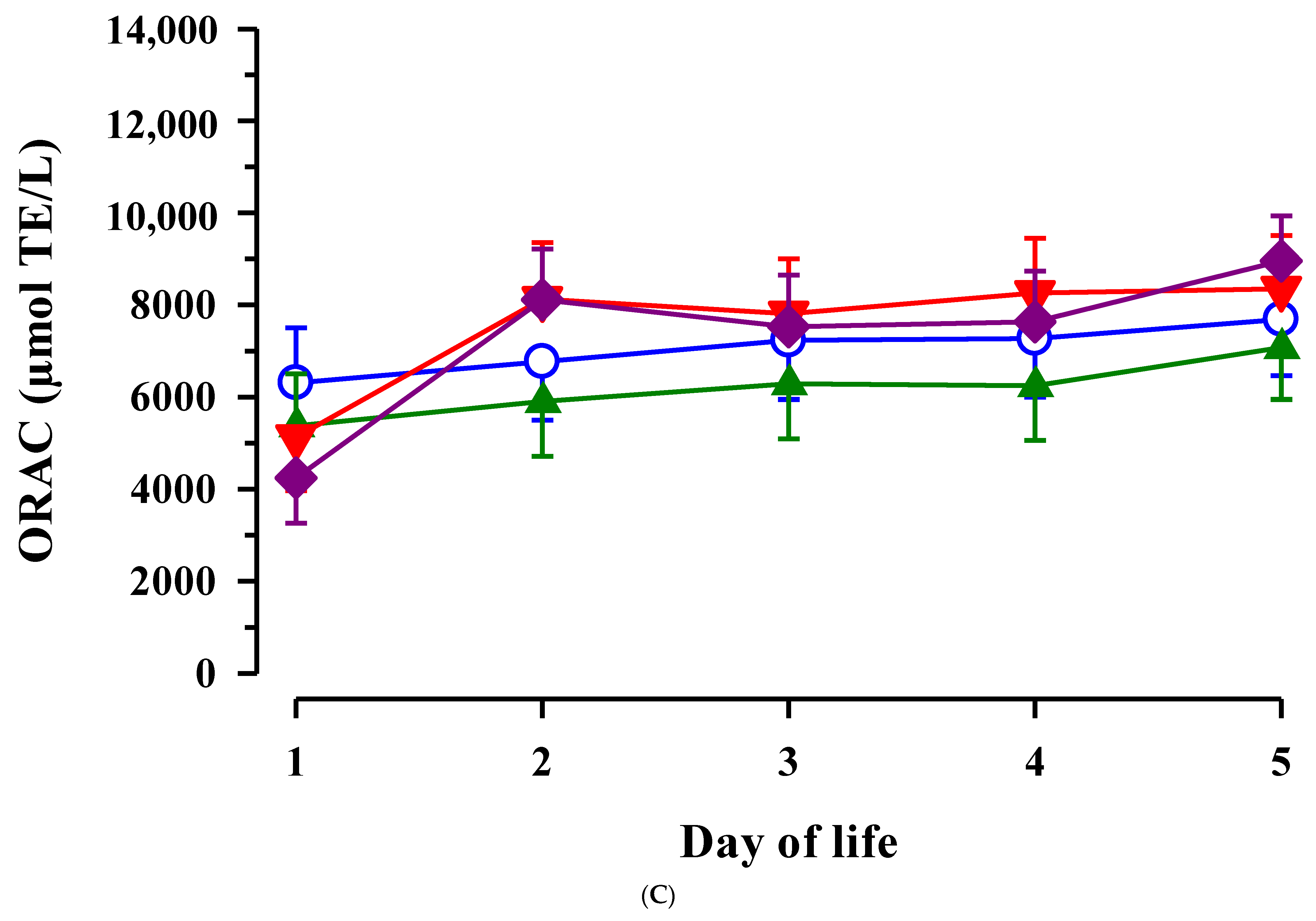
3.3. Immunoglobulin G, Metabolites, Interleukins, Vitamins and Oxidative and Anti-Oxidative Traits in Plasma of Calves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hulbert, L.E.; Moisa, S.J. Stress, immunity, and the management of calves. J. Dairy Sci. 2016, 99, 3199–3216. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernandez, J.; Benedito, J.L.; Castillo, C. Redox biology in transition periods of dairy cattle: Role in the health of periparturient and neonatal animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.L.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Godden, S. Colostrum management for dairy calves. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 535–556. [Google Scholar] [CrossRef] [PubMed]
- Urie, N.J.; Lombard, J.E.; Shivley, C.B.; Kopral, C.A.; Adams, A.E.; Earleywine, T.J.; Olson, J.D.; Garry, F.B. Preweaned heifer management on US dairy operations: Part V. Factors associated with morbidity and mortality in preweaned dairy heifer calves. J. Dairy Sci. 2018, 101, 9229–9244. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, E.; Grossi, P.; Cappelli, F.P.; Cogrossi, S.; Bertoni, G. Attenuation of inflammatory response phenomena in periparturient dairy cows by the administration of an omega 3 rumen protected supplement containing vitamin E. Ital. J. Anim. Sci. 2011, 10, e61. [Google Scholar] [CrossRef]
- Ling, T.; Hernandez-Jover, M.; Sordillo, L.M.; Abuelo, A. Maternal late-gestation metabolic stress is associated with changes in immune and metabolic responses of dairy calves. J. Dairy Sci. 2018, 101, 6568–6580. [Google Scholar] [CrossRef]
- Bertoni, G.; Ferrari, A.; Gubbiotti, A.; Trevisi, E. Blood indices calves: Relationship with mother values and changes in the first days of life. Ital. J. Anim. Sci. 2009, 8 (Suppl. 2), 595–597. [Google Scholar] [CrossRef]
- Jacometo, C.; Osorio, J.; Socha, M.; Correa, M.; Piccioli-Cappelli, F.; Trevisi, E.; Loor, J.J. Maternal consumption of organic trace minerals alters calf systemic and neutrophil mRNA and microRNA indicators of inflammation and oxidative stress. J. Dairy Sci. 2015, 98, 7717–7729. [Google Scholar] [CrossRef]
- Jacometo, C.; Zhou, Z.; Louchini, D.; Trevisi, E.; Loor, J. Maternal rumen-protected methionine supplementation and its impact on blood and liver biomarkers of energy metabolism, inflammation, and oxidative stress in neonatal Holstein calves. J. Dairy Sci. 2016, 99, 6753–6763. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Long-chain fatty acids and inflammation. Proc. Nutr. Soc. 2012, 71, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Greco, L.F.; Favoreto, M.G.; Marsola, R.S.; Wang, D.; Shin, J.H.; Block, E.; Thatcher, W.W.; Santos, J.E.P.; Staples, C.R. Effect of supplementing essential fatty acids to pregnant non lactating Holstein cows and their preweaned calves on calf performance, immune response, and health. J. Dairy Sci. 2014, 97, 5045–5064. [Google Scholar] [CrossRef]
- Ballou, M.A.; DePeters, E.J. Supplementing milk replacer with omega-3 fatty acids from fish oil on immunocompetence and health of Jersey calves. J. Dairy Sci. 2008, 91, 3488–3500. [Google Scholar] [CrossRef]
- Yao, W.; Li, J.; Wang, J.J.; Zhou, W.; Wang, Q.; Zhu, R.; Wang, F.; Thacker, P. Effects of dietary ratio of n-6 to n-3 polyunsaturated fatty acids on immunoglobulins, cytokines, fatty acid composition, and performance of lactating sows and suckling piglets. J. Anim. Sci. Biotechnol. 2012, 3, 43. [Google Scholar] [CrossRef]
- Nagao, K.; Yanagita, N. Conjugated fatty acids in food and their health benefits. J. Biosci. Bioeng. 2005, 100, 152–157. [Google Scholar] [CrossRef]
- Sugano, M.; Tsujita, A.; Yamasaki, M.; Noguchi, M.; Yamada, K. Conjugated linoleic acid modulates tissue levels of chemical mediators and immunoglobulins in rats. Lipids 1998, 33, 521–527. [Google Scholar] [CrossRef]
- Renner, L.; Kersten, S.; Duevel, A.; Schuberth, H.J.; Dänicke, S. Effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid, linoleic acid, phytanic acid and the combination of various fatty acids on proliferation and cytokine expression of bovine peripheral blood mononuclear cells. Nutrients 2013, 5, 2667–2683. [Google Scholar] [CrossRef] [PubMed]
- Gnott, M.; Vogel, L.; Kröger-Koch, C.; Dannenberger, D.; Tuchscherer, A.; Tröscher, A.; Trevisi, E.; Stefaniak, T.; Bajzert, J.; Starke, A.; et al. Changes in fatty acids in plasma and association with the inflammatory response in dairy cows abomasally infused with essential fatty acids and conjugated linoleic acid during late and early lactation. J. Dairy Sci. 2020, 103, 11889–11910. [Google Scholar] [CrossRef]
- Dipasquale, D.; Basirico, L.; Morera, P.; Primi, R.; Tröscher, A.; Bernabucci, U. Anti-inflammatory effects of conjugated linoleic acid isomers and essential fatty acids in bovine mammary epithelial cells. Animal 2018, 12, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Kowalczyk, J.; Renner, L.; Pappritz, J.; Meyer, U.; Kramer, R.; Weber, E.M.; Döll, S.; Rehage, J.; Jahreis, G. Effects of conjugated linoleic acids fed to dairy cows during early gestation on hematological, immunological, and metabolic characteristics of cows and their calves. J. Dairy Sci. 2012, 95, 3938–3953. [Google Scholar] [CrossRef]
- Kay, J.K.; Roche, J.R.; Kolver, E.S.; Thomson, N.A.; Baumgard, L.H. A comparison between feeding systems (pasture and TMR) and the effect of vitamin E supplementation on plasma and milk fatty acid profiles in dairy cows. J. Dairy Res. 2005, 72, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, S.; Hurtaud, C.; Lopez, C.; Delaby, L.; Peyraud, J.L. The linear relationship between the proportion of fresh grass in the cow diet, milk fatty acid composition, and butter properties. J. Dairy Sci. 2006, 89, 1956–1969. [Google Scholar] [CrossRef]
- Ferlay, A.; Martin, B.; Pradel, P.; Coulon, J.; Chilliard, Y. Influence of grass-based diets on milk fatty acid composition and milk lipolytic system in Tarentaise and Montbéliarde cow breeds. J. Dairy Sci. 2006, 89, 4026–4041. [Google Scholar] [CrossRef]
- Garcia, M.; Greco, L.F.; Favoreto, M.G.; Marsola, R.S.; Martins, L.T.; Bisinotto, R.S.; Shin, J.H.; Lock, A.L.; Block, E.; Thatcher, W.W.; et al. Effect of supplementing fat to pregnant nonlactating cows on colostral fatty acid profile and passive immunity of the newborn calf. J. Dairy Sci. 2014, 97, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Uken, K.L.; Schäff, C.T.; Vogel, L.; Gnott, M.; Dannenberger, D.; Görs, S.; Tuchscherer, A.; Tröscher, A.; Liermann, W.; Hammon, H.M. Modulation of colostrum composition and fatty acid status in neonatal calves by maternal supplementation with essential fatty acids and conjugated linoleic acid starting in late lactation. J. Dairy Sci. 2021, 104, 4950–4969. [Google Scholar] [CrossRef]
- Vogel, L.; Gnott, M.; Kröger-Koch, C.; Dannenberger, D.; Tuchscherer, A.; Tröscher, A.; Kienberger, H.; Rychlik, M.; Starke, A.; Bachmann, L.; et al. Effects of abomasal infusion of essential fatty acids together with conjugated linoleic acid in late and early lactation on performance, milk and body composition, and plasma metabolites in dairy cows. J. Dairy Sci. 2020, 103, 7431–7450. [Google Scholar] [CrossRef]
- Gerbert, C.; Frieten, D.; Koch, C.; Dusel, G.; Eder, K.; Stefaniak, T.; Bajzert, J.; Jawor, P.; Tuchscherer, A.; Hammon, H.M. Effects of ad libitum milk replacer feeding and butyrate supplementation on behavior, immune status, and health of Holstein calves in the postnatal period. J. Dairy Sci. 2018, 101, 7348–7360. [Google Scholar] [CrossRef]
- Madsen, B.D.; Rasmussen, M.D.; Nielsen, M.O.; Wiking, L.; Larsen, L.B. Physical properties of mammary secretions in relation to chemical changes during transition from colostrum to milk. J. Dairy Res. 2004, 71, 263–272. [Google Scholar] [CrossRef]
- Hiss, S.; Weinkauf, C.; Hachenberg, S.; Sauerwein, H. Short communication: Relationship between metabolic status and the milk concentrations of haptoglobin and lactoferrin in dairy cows during early lactation. J. Dairy Sci. 2009, 92, 4439–4443. [Google Scholar] [CrossRef]
- Regenhard, P.; Nakov, D.; Sauerwein, H. Applicability of a spectrophotometric method for assessment of oxidative stress in poultry. Maced. Vet. Rev. 2014, 37, 43–47. [Google Scholar] [CrossRef][Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Barrington, G.M.; Parish, S.M. Bovine neonatal immunology. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 463–476. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Wolinski, J.; Westrom, B.; Jazwiec, R.; Shmigel, H.; Pierzynowski, S.G. Absorption of polyunsaturated fatty acid (PUFA) is related to IgG blood levels of neonatal pigs during the first 48 hours postpartum. J. Immunol. Res. 2020, 2020, 3813250. [Google Scholar] [CrossRef]
- Jolazadeh, A.R.; Mohammadabadi, T.; Dehghan-Banadaky, M.; Chaji, M.; Garcia, M. Effect of supplementation fat during the last 3 weeks of uterine life and the preweaning period on performance, ruminal fermentation, blood metabolites, passive immunity and health of the newborn calf. Br. J. Nutr. 2019, 122, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Panousis, N.; Siachos, N.; Kitkas, G.; Kalaitzakis, E.; Kritsepi-Konstantinou, M.; Valergakis, G.E. Hematology reference intervals for neonatal Holstein calves. Res. Vet. Sci. 2018, 118, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.G.; Edwards, J.E.; Bazeley, K.J.; Brown, S.N.; Butterworth, A.; Warriss, P.D. Changes in the blood biochemical and haematological profile of neonatal calves with age. Vet. Rec. 2000, 147, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Brun-Hansen, H.C.; Kampen, A.H.; Lund, A. Hematologic values in calves during the first 6 months of life. Vet. Clin. Pathol. 2006, 35, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Lacetera, N.; Kuzminsky, G.; Morera, P.; Basiricò, L. Fatty acids affect proliferation of peripheral blood mononuclear cells in dairy cows. Ital. J. Anim. Sci. 2007, 6, 434–436. [Google Scholar] [CrossRef]
- Li, T.G.; Chiang, J.Y.L. Regulation of Bile Acid and Cholesterol Metabolism by PPARs. PPAR Res. 2009, 2009, 501739. [Google Scholar] [CrossRef]
- Kurz, M.M.; Willett, L.B. Carbohydrate, enzyme, and hematology dynamics in newborn calves. J. Dairy Sci. 1991, 74, 2109–2118. [Google Scholar] [CrossRef]
- Probo, M.; Giordano, A.; Moretti, P.; Opsomer, G.; Fiems, L.; Paltrinieri, S.; Veronesie, M.C. Serum biochemical profile in Holstein Friesian and Belgian blue calvesin the first 48 hours of life. Ital. J. Anim. Sci. 2019, 18, 657–662. [Google Scholar] [CrossRef]
- Rayyan, M.; Devlieger, H.; Jochum, F.; Allegaert, K. Short-term use of parenteral nutrition with a lipid emulsion containing a mixture of soybean oil, olive oil, medium-chain triglycerides, and fish oil: A randomized double-blind study in preterm infants. JPEN J. Parenter. Enter. Nutr. 2012, 36 (Suppl. 1), 81S–94S. [Google Scholar] [CrossRef]
- Turner, J.M.; Josephson, J.; Field, C.J.; Wizzard, P.R.; Ball, R.O.; Pencharz, P.G.; Wales, P.W. Liver disease, systemic inflammation, and growth using a mixed parenteral lipid emulsion, containing soybean oil, fish oil, and medium chain triglycerides, compared with soybean oil in parenteral nutrition-fed neonatal piglets. JPEN J. Parenter. Enter. Nutr. 2016, 40, 973–981. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Comporti, M.; Signorini, C.; Buonocore, G.; Ciccoli, L. Iron release, oxidative stress and erythrocyte ageing. Free Radic. Biol. Med. 2002, 32, 568–576. [Google Scholar] [CrossRef]
- Opgenorth, J.; Sordillo, L.M.; Lock, A.L.; Gandy, J.C.; VandeHaar, M.J. Colostrum supplementation with n-3 fatty acids alters plasma polyunsaturated fatty acids and inflammatory mediators in newborn calves. J. Dairy Sci. 2020, 103, 11676–11688. [Google Scholar] [CrossRef] [PubMed]
- Tetsuka, T.; Daphna-Iken, D.; Srivastava, S.K.; Baier, L.D.; DuMaine, J.; Morrison, R.A. Cross-talk between cyclooxygenase and nitric oxide pathways: Prostaglandin E2 negatively modulates induction of nitric oxide synthase by interleukin 1. Proc. Natl. Acad. Sci. USA 1994, 91, 12168–12172. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef] [PubMed]
- Hinson, R.M.; Williams, J.A.; Shacter, E. Elevated interleukin 6 is induced by prostaglandin E2 in a murine model of inflammation: Possible role of cyclooxygenase-2. Proc. Natl. Acad. Sci. USA 1996, 93, 4885–4890. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, E.; Amadori, M.; Cogrossi, S.; Razzuoli, E.; Bertoni, G. Metabolic stress and inflammatory response in high-yielding, periparturient dairy cows. Res. Vet. Sci. 2012, 93, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, V.; Miller, N.J.; Milner, A.D.; Rice-Evans, C.A. Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett. 1994, 349, 197–200. [Google Scholar] [CrossRef]
- Wiedemann, M.; Kontush, A.; Finckh, B.; Hellwege, H.H.; Kohlschutter, A. Neonatal blood plasma is less susceptible to oxidation than adult plasma owing to its higher content of bilirubin and lower content of oxidizable fatty acids. Pediatr. Res. 2003, 53, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Smith, D.L.; Zucker, S.D. Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology 2004, 40, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Christen, S.; Cattin, I.; Knight, I.; Winyard, P.G.; Blum, J.W.; Elsasser, T.H. Plasma S-nitrosothiol status in neonatal calves: Ontogenetic associations with tissue-specific S-nitrosylation and nitric oxide synthase. Exp. Biol. Med. 2007, 232, 309–322. [Google Scholar]
- Dadkhah, A.; Fatemi, F.; Kazemnejad, S.; Rasmi, Y.; Ashrafi-Helan, J.; Allameh, A. Differential effects of acetaminophen on enzymatic and non-enzymatic antioxidant factors and plasma total antioxidant capacity in developing and adult rats. Mol. Cell. Biochem. 2006, 281, 145–152. [Google Scholar] [CrossRef]
- Gaál, T.; Ribiczeyné-Szabó, P.; Stadler, K.; Jakus, J.; Reiczigel, J.; Kóver, P.; Mézes, M.; Sümeghy, L. Free radicals, lipid peroxidation and the antioxidant system in the blood of cows and newborn calves around calving. Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 2006, 143, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Perez-Santos, M.; Hernandez, J.; Castillo, C. Effect of colostrum redox balance on the oxidative status of calves during the first 3 months of life and the relationship with passive immune acquisition. Vet. J. 2014, 199, 295–299. [Google Scholar] [CrossRef]
- Blum, J.W.; Hadorn, U.; Sallmann, H.P.; Schuep, W. Delaying colostrum intake by one day impairs plasma lipid, essential fatty acid, carotene, retinol and alpha-tocopherol status in neonatal calves. J. Nutr. 1997, 127, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Opgenorth, J.; Sordillo, L.M.; VandeHaar, M.J. Colostrum supplementation with n-3 fatty acids and α-tocopherol alters plasma polyunsaturated fatty acid profile and decreases an indicator of oxidative stress in newborn calves. J. Dairy Sci. 2020, 103, 3545–3553. [Google Scholar] [CrossRef] [PubMed]


| Group | Maternal Fatty Acid Supplementation (MFAS) * | Number of Calves (n) | Calf Sex | |
|---|---|---|---|---|
| Male | Female | |||
| Control (CON) | 76 g/day coconut oil † | 9 | 5 | 4 |
| EFA | 78 g/day linseed oil ‡ and 4 g/day safflower oil ⸸ | 9 | 4 | 5 |
| CLA | 38 g/day Lutalin § (CLA; cis-9, trans-11, trans-10, cis-12 CLA, 10 g/day each) | 9 | 1 | 8 |
| EFA + CLA | 78 g/day linseed oil + 4 g/day safflower oil + 38 g/day Lutalin | 11 $ | 4 | 7 |
| Variable | Time # | MFAS | p-Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON α | EFA β | CLA γ | EFA + CLA | EFA | CLA | EFA × CLA | Time | EFA × Time | CLA × Time | ||
| Hematocrit, % | 1 | 30.9 ± 3.8 | 26.6 ± 3.6 | 30.1 ± 3.7 | 27.1 ± 3.2 | 0.21 | 0.95 | 0.38 | <0.001 | 0.37 | 0.49 |
| 5 | 23.0 ± 3.6 *** | 19.7 ± 3.3 *** | 23.0 ± 3.5 *** | 20.6 ± 2.9 *** | |||||||
| Hemoglobin, g/dL | 1 | 8.48 ±1.01 | 7.31 ± 0.94 | 8.47 ± 0.97 | 7.57 ± 0.83 | 0.17 | 0.81 | 0.85 | <0.001 | 0.44 | 0.81 |
| 5 | 6.64 ± 0.95 *** | 5.69 ± 0.87 *** | 6.72 ± 0.91 *** | 5.98 ± 0.77 *** | |||||||
| Erythrocytes, 106/mm3 | 1 | 7.20 ± 0.77 | 6.54 ± 0.72 | 7.37 ± 0.75 | 6.66 ± 0.63 | 0.22 | 0.73 | 0.98 | <0.001 | 0.85 | 0.78 |
| 5 | 5.77 ± 0.75 *** | 5.16 ± 0.69 *** | 5.92 ± 0.72 *** | 5.39 ± 0.60 *** | |||||||
| RDW †, % | 1 | 16.9 ± 0.6 | 16.7 ± 0.6 | 15.8 ± 0.6 | 16.8 ± 0.5 | 0.49 | 0.19 | 0.12 | 0.05 | 0.19 | 0.55 |
| 5 | 16.9 ± 0.7 | 16.4 ± 0.6 | 15.6 ± 0.6 | 16.5 ± 0.6 | |||||||
| MCV ‡, µm3 | 1 | 42.8 ± 1.6 | 41.2 ± 1.6 | 40.9 ± 1.6 | 41.0 ± 1.4 | 0.72 | 0.45 | 0.47 | <0.001 | 0.050 | 0.29 |
| 5 | 40.2 ± 1.5 *** | 39.2 ± 1.4 *** | 38.6 ± 1.5 *** | 39.3 ± 1.3 *** | |||||||
| MCH ⸸, pg | 1 | 11.8 ± 0.4 | 11.4 ± 0.4 | 11.5 ± 0.4 | 11.5 ± 0.3 | 0.36 | 0.71 | 0.37 | 0.016 | 0.62 | 0.82 |
| 5 | 11.7 ± 0.4 | 11.2 ± 0.3 | 11.4 ± 0.3 | 11.3 ± 0.3 | |||||||
| MCHC §, g/dL | 1 | 27.5 ± 0.6 | 27.8 ± 0.6 | 28.5 ± 0.6 | 27.9 ± 0.5 | 0.54 | 0.25 | 0.21 | <0.001 | 0.33 | 0.91 |
| 5 | 28.7 ± 0.7 *** | 29.0 ± 0.7 *** | 29.9 ± 0.7 *** | 28.8 ± 0.6 *** | |||||||
| Leucocytes, 103/mm3 | 1 | 9.4 ± 1.4 | 8.9 ± 1.4 | 10.8 ± 1.4 | 9.4 ± 1.2 | 0.029 | 0.14 | 0.44 | 0.01 | 0.09 | 0.61 |
| 5 | 8.4 ± 1.4 ab | 6.2 ± 1.3 b | 10.7 ± 1.4 a | 6.9 ± 1.2 b | |||||||
| Lymphocytes, % | 1 | 34.3 ± 8.3 | 33.0 ± 7.9 | 35.2 ± 8.0 | 42.6 ± 6.9 | 0.44 | 0.84 | 0.98 | 0.002 | 0.68 | 0.029 |
| 5 | 25.6 ± 8.8 | 35.1 ± 8.1 | 22.5 ± 8.3 | 23.7 ± 7.1 * | |||||||
| Atypical Lymphocytes, % | 1 | 4.29 ± 0.54 | 4.19 ± 0.52 | 4.05 ± 0.52 | 3.90 ± 0.46 | 0.80 | 0.95 | 0.74 | <0.001 | 0.85 | 0.27 |
| 5 | 2.30 ± 0.57 * | 2.04 ± 0.53 ** | 2.38 ± 0.53 * | 2.57 ± 0.46 | |||||||
| Basophile Granulocytes, % | 1 | 0.21 ± 0.17 | 0.10 ± 0.17 | 0.21 ± 0.17 | 0.47 ± 0.15 | 0.81 | 0.67 | 0.44 | <0.001 | 0.25 | 0.09 |
| 5 | 0.90 ± 0.16 ** | 0.82 ± 0.15 ** | 0.83 ± 0.15 * | 0.67 ± 0.13 | |||||||
| Thrombocytes, 103/mm3 | 1 | 482 ± 99 | 471 ± 93 | 645 ± 95 | 586 ± 82 | 0.49 | 0.13 | 0.50 | 0.33 | 0.76 | 0.18 |
| 5 | 486 ± 103 | 490 ± 95 | 598 ± 98 | 486 ± 83 | |||||||
| MTV $, µm3 | 1 | 6.36 ± 0.51 | 6.30 ± 0.47 | 6.80 ± 0.49 | 6.68 ± 0.42 | 0.59 | 0.36 | 0.85 | 0.052 | 0.29 | 0.27 |
| 5 | 6.71 ± 0.52 | 6.52 ± 0.48 | 7.01 ± 0.50 | 6.63 ± 0.42 | |||||||
| Thrombocrit, % | 1 | 0.32 ± 0.10 | 0.36 ± 0.09 | 0.48 ± 0.10 | 0.39 ± 0.08 | 0.54 | 0.24 | 0.36 | 0.14 | 0.26 | 0.32 |
| 5 | 0.33 ± 0.11 | 0.33 ± 0.10 | 0.45 ± 0.10 | 0.33 ± 0.09 | |||||||
| Immature cells, % | 1 | 25.4 ± 11.7 | 17.1 ± 11.8 | 16.6 ± 11.5 | 3.5 ± 11.8 | 0.16 | 0.68 | 0.77 | 0.55 | 0.78 | 0.12 |
| 5 | 16.2 ± 8.5 | 2.6 ± 7.8 | 16.2 ± 7.9 | 14.6 ± 6.7 | |||||||
| Variable | Correlated Parameters | Correlation Coefficient (r) | p-Value |
|---|---|---|---|
| Plasma traits | |||
| Ig † G | Bilirubin | 0.255 | <0.001 |
| Interleukin 6 | 0.278 | 0.001 | |
| β- carotene | 0.447 | <0.001 | |
| Tocopherol | 0.245 | 0.001 | |
| dROM | 0.491 | <0.001 | |
| ORAC § | 0.378 | <0.001 | |
| Bilirubin | Interleukin 1-β | 0.263 | 0.001 |
| Retinol | −0.388 | <0.001 | |
| Interleukin 6 | ORAC | 0.270 | 0.001 |
| β- carotene | Retinol | 0.371 | <0.001 |
| Tocopherol | 0.648 | <0.001 | |
| dROM | 0.610 | <0.001 | |
| ORAC | 0.155 | 0.047 | |
| Retinol | Tocopherol | 0.602 | <0.001 |
| dROM | 0.338 | <0.001 | |
| Tocopherol | dROM | 0.520 | <0.001 |
| dROM ‡ | ORAC | 0.238 | 0.002 |
| FRAP ⸸ | ORAC | 0.245 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liermann, W.; Uken, K.L.; Schäff, C.; Vogel, L.; Gnott, M.; Tuchscherer, A.; Trevisi, E.; Stefaniak, T.; Sauerwein, H.; Tröscher, A.; et al. Effects of a Maternal Essential Fatty Acid and Conjugated Linoleic Acid Supplementation during Late Pregnancy and Early Lactation on Hematologic and Immunological Traits and the Oxidative and Anti-Oxidative Status in Blood Plasma of Neonatal Calves. Animals 2021, 11, 2168. https://doi.org/10.3390/ani11082168
Liermann W, Uken KL, Schäff C, Vogel L, Gnott M, Tuchscherer A, Trevisi E, Stefaniak T, Sauerwein H, Tröscher A, et al. Effects of a Maternal Essential Fatty Acid and Conjugated Linoleic Acid Supplementation during Late Pregnancy and Early Lactation on Hematologic and Immunological Traits and the Oxidative and Anti-Oxidative Status in Blood Plasma of Neonatal Calves. Animals. 2021; 11(8):2168. https://doi.org/10.3390/ani11082168
Chicago/Turabian StyleLiermann, Wendy, Katrin Lena Uken, Christine Schäff, Laura Vogel, Martina Gnott, Armin Tuchscherer, Erminio Trevisi, Tadeusz Stefaniak, Helga Sauerwein, Arnulf Tröscher, and et al. 2021. "Effects of a Maternal Essential Fatty Acid and Conjugated Linoleic Acid Supplementation during Late Pregnancy and Early Lactation on Hematologic and Immunological Traits and the Oxidative and Anti-Oxidative Status in Blood Plasma of Neonatal Calves" Animals 11, no. 8: 2168. https://doi.org/10.3390/ani11082168
APA StyleLiermann, W., Uken, K. L., Schäff, C., Vogel, L., Gnott, M., Tuchscherer, A., Trevisi, E., Stefaniak, T., Sauerwein, H., Tröscher, A., & Hammon, H. M. (2021). Effects of a Maternal Essential Fatty Acid and Conjugated Linoleic Acid Supplementation during Late Pregnancy and Early Lactation on Hematologic and Immunological Traits and the Oxidative and Anti-Oxidative Status in Blood Plasma of Neonatal Calves. Animals, 11(8), 2168. https://doi.org/10.3390/ani11082168








