Etiology of Colitis-Complex Diarrhea in Growing Pigs: A Review
Abstract
Simple Summary
Abstract
1. Introduction
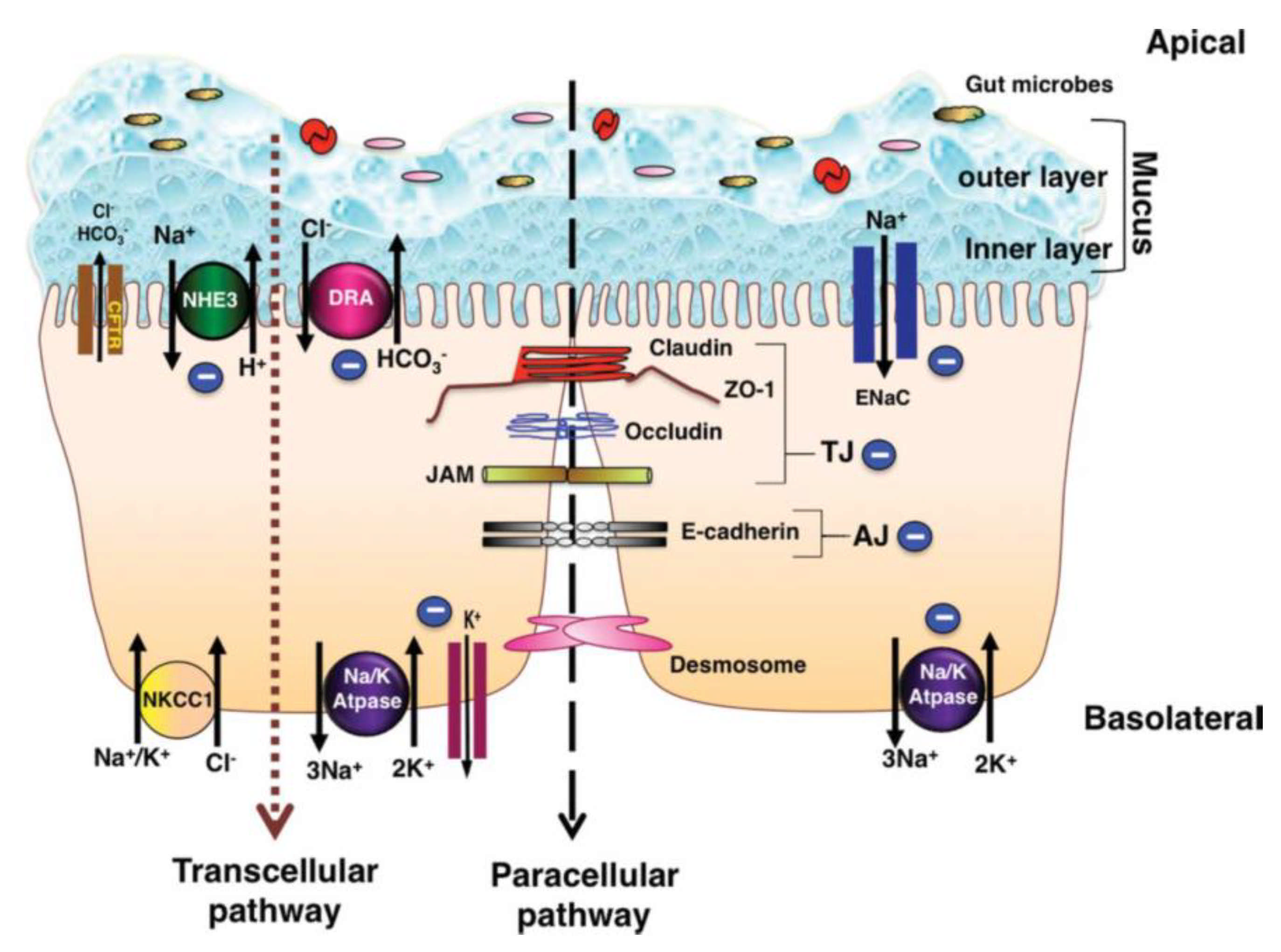
2. Colonic Epithelium Function
2.1. Colonic Ion Exchange
2.2. Cytokines and Luminal Function
2.3. Mucins
3. Colitis
3.1. Specific Colitis
3.1.1. Swine Dysentery
3.1.2. Spirochetal Colitis
3.1.3. Parasitic Colitis
3.2. Non-Specific Colitis and Role of Dietary Factors in Inducing Colitis
3.2.1. Dietary Crude Protein and NSC
3.2.2. Dietary Fiber and Non-Starch Polysaccharides
3.2.3. Pelleted Feedstuff
4. Role of Dietary Factors in Preventing CCD
4.1. Polyunsaturated Fatty Acids and Polyphenols
4.2. Dietary Protein and Essential Amino Acids
4.3. Dietary Fiber
4.4. Resistant Starch
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Constable, P.; Hinchcliff, K.; Done, S.; Grünberg, W. 7—Diseases of the Alimentary Tract: Nonruminant. In Veterinary Medicine, 11st ed.; Constable, P.D., Hinchcliff, K.W., Done, S.H., Grünberg, W., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2017; pp. 175–435. [Google Scholar]
- Zachary, J.F. Chapter 4—Mechanisms of Microbial Infections1. In Pathologic Basis of Veterinary Disease, 6th ed.; Zachary, J.F., Ed.; Mosby: Maryland Heights, MO, USA, 2017; pp. 132–241.e1. [Google Scholar]
- Field, M. Intestinal ion transport and the pathophysiology of diarrhea. J. Clin. Investig. 2003, 111, 931–943. [Google Scholar] [CrossRef]
- Moeser, A.J.; Blikslager, A.T. Mechanisms of porcine diarrheal disease. J. Am. Vet. Med. Assoc. 2007, 231, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Chase-Topping, M.E.; Gunn, G.; Strachan, W.D.; Edwards, S.A.; Smith, W.J.; Hillman, K.; Stefopoulou, S.N.; Thomson, J.R. Epidemiology of porcine non-specific colitis on Scottish farms. Vet. J. 2007, 173, 353–360. [Google Scholar] [CrossRef]
- Grave, K.; Jensen, V.F.; Odensvik, K.; Wierup, M.; Bangen, M. Usage of veterinary therapeutic antimicrobials in Denmark, Norway and Sweden following termination of antimicrobial growth promoter use. Prev. Vet. Med. 2006, 75, 123–132. [Google Scholar] [CrossRef]
- Jacobson, M.; Hård af Segerstad, C.; Gunnarsson, A.; Fellström, C.; Klingenberg, K.; Wallgren, P.; Jensen-Waern, M. Diarrhoea in the growing pig—A comparison of clinical, morphological and microbial findings between animals from good and poor performance herds. Res. Vet. Sci. 2003, 74, 163–169. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Johansen, M.; Angen, O.; Jorsal, S.E.; Nielsen, J.P.; Jensen, T.K.; Guedes, R.; Ståhl, M.; Bækbo, P. Herd diagnosis of low pathogen diarrhoea in growing pigs—A pilot study. Ir. Vet. J. 2014, 67, 24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [PubMed]
- Hampson, D.J.; Burrough, E.R. Swine Dysentery and Brachyspiral Colitis. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 951–970. [Google Scholar]
- Jensen, T.; Boye, M.; Møller, K. Extensive intestinal spirochaetosis in pigs challenged with Brachyspira pilosicoli. J. Med. Microbiol. 2004, 53, 309–312. [Google Scholar] [CrossRef]
- Wills, R.W. Diarrhea in Growing-Finishing Swine. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 135–161. [Google Scholar] [CrossRef]
- Carr, J.; Chen, S.-P.; Connor, J.F.; Kirkwood, R.; Segalés, J. Pig Health; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Karuppannan, A.K.; Opriessnig, T. Lawsonia intracellularis: Revisiting the Disease Ecology and Control of This Fastidious Pathogen in Pigs. Front. Vet. Sci. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Lawhorn, D.B. Diarrheal disease in show swine. Tex. FARMER Collect. 2007, 3, 439. [Google Scholar]
- Strobel, D.; Goertz, R.S.; Bernatik, T. Diagnostics in inflammatory bowel disease: Ultrasound. World J. Gastroenterol. 2011, 17, 3192. [Google Scholar]
- Yang, H.; Jiang, W.; Furth, E.E.; Wen, X.; Katz, J.P.; Sellon, R.K.; Silberg, D.G.; Antalis, T.M.; Schweinfest, C.W.; Wu, G.D. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am. J. Physiol. Gastrointest. Liver Physiol. 1998, 275, G1445–G1453. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M. Mechanisms of diarrhea. Curr. Gastroenterol. Rep. 2010, 12, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.J. Mechanisms of diarrhea in inflammatory bowel diseases. Ann. N. Y. Acad. Sci. 2009, 1165, 285–293. [Google Scholar] [CrossRef]
- Anbazhagan, A.N.; Priyamvada, S.; Alrefai, W.A.; Dudeja, P.K. Pathophysiology of IBD associated diarrhea. Tissue Barriers 2018, 6, e1463897. [Google Scholar] [CrossRef]
- Musch, M.W.; Arvans, D.L.; Wu, G.D.; Chang, E.B. Functional coupling of the downregulated in adenoma Cl-/base exchanger DRA and the apical Na+/H+ exchangers NHE2 and NHE3. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G202–G210. [Google Scholar] [CrossRef][Green Version]
- Khurana, S.; Ganguly, N.K.; Khullar, M.; Panigrahi, D.; Walia, B.N.S. Studies on the mechanism of Salmonella typhimurium enterotoxin-induced diarrhoea. Biochim. Biophys. Acta Mol. Basis Dis. 1991, 1097, 171–176. [Google Scholar] [CrossRef]
- Kaur, T.; Singh, S.; Gorowara, S.; Ganguly, N.K. Role of enteric nervous system in Shigella dysenteriae type 1 toxin-induced fluid secretion in rabbit ileum. J. Diarrhoeal Dis. Res. 1995, 159–165. [Google Scholar]
- Vernia, P.; Gnaedinger, A.; Hauck, W.; Breuer, R.I. Organic anions and the diarrhea of inflammatory bowel disease. Dig. Dis. Sci. 1988, 33, 1353–1358. [Google Scholar] [CrossRef]
- Madara, J.L.; Stafford, J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J. Clin. Investig. 1989, 83, 724–727. [Google Scholar] [CrossRef]
- Amasheh, S.; Barmeyer, C.; Koch, C.S.; Tavalali, S.; Mankertz, J.; Epple, H.-J.; Gehring, M.M.; Florian, P.; Kroesen, A.-J.; Zeitz, M.; et al. Cytokine-dependent transcriptional down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology 2004, 126, 1711–1720. [Google Scholar] [CrossRef]
- Liu, Y. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 2015, 6, 41. [Google Scholar] [CrossRef]
- Schulzke, J.D.; Ploeger, S.; Amasheh, M.; Fromm, A.; Zeissig, S.; Troeger, H.; Richter, J.; Bojarski, C.; Schumann, M.; Fromm, M. Epithelial tight junctions in intestinal inflammation. Ann. N. Y. Acad. Sci. 2009, 1165, 294–300. [Google Scholar] [CrossRef]
- Caruso, R.; Lo, B.C.; Núñez, G. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 20, 411–426. [Google Scholar] [CrossRef]
- Faure, M.; Mettraux, C.; Moennoz, D.; Godin, J.-P.; Vuichoud, J.; Rochat, F.; Breuillé, D.; Obled, C.; Corthésy-Theulaz, I. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium–treated rats. J. Nutr. 2006, 136, 1558–1564. [Google Scholar] [CrossRef]
- Puiman, P.J.; Jensen, M.; Stoll, B.; Renes, I.B.; de Bruijn, A.C.J.M.; Dorst, K.; Schierbeek, H.; Schmidt, M.; Boehm, G.; Burrin, D.G.; et al. Intestinal Threonine Utilization for Protein and Mucin Synthesis Is Decreased in Formula-Fed Preterm Pigs. J. Nutr. 2011, 141, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenteritis 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Specian, R.D.; Oliver, M.G. Functional biology of intestinal goblet cells. Am. J. Physiol. Cell Physiol. 1991, 260, C183–C193. [Google Scholar] [CrossRef]
- Fuss, I.J.; Strober, W. Chapter 81—Ulcerative Colitis. In Mucosal Immunology, 4th ed.; Mestecky, J., Strober, W., Russell, M.W., Kelsall, B.L., Cheroutre, H., Lambrecht, B.N., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 1573–1612. [Google Scholar]
- Martin, C.R.; Walker, W.A. Chapter 70—Innate and Mucosal Immunity in the Developing Gastrointestinal Tract: Relationship to Early and Later Disease. In Avery’s Diseases of the Newborn, 9th ed.; Gleason, C.A., Devaskar, S.U., Eds.; W.B. Saunders: Philadelpia, PA, USA, 2012; pp. 994–1006. [Google Scholar]
- Bengtsson, R.J.; MacIntyre, N.; Guthrie, J.; Wilson, A.D.; Finlayson, H.; Matika, O.; Pong-Wong, R.; Smith, S.H.; Archibald, A.L.; Ait-Ali, T. Lawsonia intracellularis infection of intestinal crypt cells is associated with specific depletion of secreted MUC2 in goblet cells. Vet. Immunol. Immunopathol. 2015, 168, 61–67. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Zheng, C.; Wang, L.; Zhu, C.; Yang, X.; Wen, X.; Ma, X. Effects of protein sources and levels in antibiotic-free diets on diarrhea, intestinal morphology, and expression of tight junctions in weaned piglets. Anim. Nutr. 2015, 1, 170–176. [Google Scholar] [CrossRef]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Stege, H.; Jensen, T.K.; Møller, K.; Baekbo, P.; Jorsal, S. Risk factors for intestinal pathogens in Danish finishing pig herds. Prev. Vet. Med. 2001, 50, 153–164. [Google Scholar] [CrossRef]
- Thomson, J.; Smith, W.J.; Fowler, V.R.; Edwards, S.; Hazzledine, M. Non-specific colitis in pigs: Defining the condition. In Proceedings of the 17th International Pig Veterinary Society Congress, Ames, IA, USA, 2–5 June 2002. [Google Scholar]
- Luise, D.; Lauridsen, C.; Bosi, P.; Trevisi, P. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J. Anim. Sci. Biotechnol. 2019, 10, 53. [Google Scholar] [CrossRef]
- Burrough, E.R. Swine Dysentery: Etiopathogenesis and Diagnosis of a Reemerging Disease. Vet. Pathol. 2016, 54, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.B. The Genus Brachyspira. Prokar 2006, 7, 330–356. [Google Scholar]
- Trott, D.J.; Huxtable, C.R.; Hampson, D.J. Experimental infection of newly weaned pigs with human and porcine strains of Serpulina pilosicoli. Infect. Immun. 1996, 64, 4648–4654. [Google Scholar] [CrossRef]
- Kringel, H.; Iburg, T.; Dawson, H.; Aasted, B.; Roepstorff, A. A time course study of immunological responses in Trichuris suis infected pigs demonstrates induction of a local type 2 response associated with worm burden. Int. J. Parasitol. 2006, 36, 915–924. [Google Scholar] [CrossRef]
- Pittman, J.S.; Shepherd, G.; Thacker, B.J.; Myers, G.H. Trichuris suis in finishing pigs: Case report and review. J. Swine Health Prod. 2010, 18, 306–313. [Google Scholar]
- Roepstorff, A.; Mejer, H.; Nejsum, P.; Thamsborg, S.M. Helminth parasites in pigs: New challenges in pig production and current research highlights. Vet. Parasitol. 2011, 180, 72–81. [Google Scholar] [CrossRef]
- Leroux, L.-P.; Nasr, M.; Valanparambil, R.; Tam, M.; Rosa, B.A.; Siciliani, E.; Hill, D.E.; Zarlenga, D.S.; Jaramillo, M.; Weinstock, J.V.; et al. Analysis of the Trichuris suis excretory/secretory proteins as a function of life cycle stage and their immunomodulatory properties. Sci. Rep. 2018, 8, 15921. [Google Scholar] [CrossRef]
- Thomson, J. Feed-associated colitis of growing pigs and its interaction with enteric infections. Acta Sci. Vet. 2009, 37, s1–s9. [Google Scholar]
- Gelberg, H.B. Chapter 7—Alimentary System and the Peritoneum, Omentum, Mesentery, and Peritoneal Cavity1. In Pathologic Basis of Veterinary Disease, 6th ed.; Zachary, J.F., Ed.; Mosby: Maryland Heights, MO, USA, 2017; pp. 324–411.e1. [Google Scholar]
- Argenzio, R.A.; Whipp, S.C.; Glock, R.D. Pathophysiology of Swine Dysentery: Colonic Transport and Permeability Studies. J. Infect. Dis. 1980, 142, 676–684. [Google Scholar] [CrossRef]
- Stege, H.; Jensen, T.K.; Møller, K.; Bækbo, P.; Jorsal, S.E. Prevalence of intestinal pathogens in Danish finishing pig herds. Prev. Vet. Med. 2000, 46, 279–292. [Google Scholar] [CrossRef]
- Quintana-Hayashi, M.P.; Mahu, M.; De Pauw, N.; Boyen, F.; Pasmans, F.; Martel, A.; Premaratne, P.; Fernandez, H.R.; Teymournejad, O.; Maele, L.V. The levels of Brachyspira hyodysenteriae binding to porcine colonic mucins differ between individuals, and binding is increased to mucins from infected pigs with de novo MUC5AC synthesis. Infect. Immun. 2015, 83, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Reynolds, L.P.; Redmer, D.A.; Caton, J.S.; Crenshaw, J.D. Effects of dietary fiber on intestinal growth, cell proliferation, and morphology in growing pigs2. J. Anim. Sci. 1994, 72, 2270–2278. [Google Scholar] [CrossRef]
- Pothoulakis, C.; Castagliuolo, I.; LaMont, J.T. Nerves and intestinal mast cells modulate responses to enterotoxins. Physics 1998, 13, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Kristensen, C.S.; Nielsen, J.P. Demonstration of non-specific colitis and increased crypt depth in colon of weaned pigs with diarrhea. Vet. Q. 2012, 32, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Casas, V.; Vadillo, S.; San Juan, C.; Carrascal, M.; Abian, J. The Exposed Proteomes of Brachyspira hyodysenteriae and B. pilosicoli. Front. Microbiol. 2016, 7, 1103. [Google Scholar] [CrossRef]
- Wyns, H.; Plessers, E.; De Backer, P.; Meyer, E.; Croubels, S. In vivo porcine lipopolysaccharide inflammation models to study immunomodulation of drugs. Vet. Immunol. Immunopathol. 2015, 166, 58–69. [Google Scholar] [CrossRef]
- Gessner, D.K.; Fiesel, A.; Most, E.; Dinges, J.; Wen, G.; Ringseis, R.; Eder, K. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet. Scand. 2013, 55, 18. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Fagerhol, M.K. Faecal calprotectin: A non invasive marker of inflammation in pigs. ISAH 2005, 1, 405–408. [Google Scholar]
- Lamb, C.A.; Mansfield, J.C. Measurement of faecal calprotectin and lactoferrin in inflammatory bowel disease. Frontline Gastroenterol. 2011, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Shiraki, M.; Bamba, T.; Umegae, S.; Matsumoto, K. Faecal calprotectin and lactoferrin as markers for monitoring disease activity and predicting clinical recurrence in patients with Crohn’s disease after ileocolonic resection: A prospective pilot study. United Eur. Gastroenterol. J. 2013, 1, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Bogere, P.; Choi, Y.J.; Heo, J. Optimization of Fecal Calprotectin Assay for Pig Samples. J. Agric. Life Sci. 2019, 53, 93–104. [Google Scholar] [CrossRef]
- Jobin, C.; Hellerbrand, C.; Licato, L.L.; Brenner, D.A.; Sartor, R.B. Mediation by NF-kappa B of cytokine induced expression of intercellular adhesion molecule 1 (ICAM-1) in an intestinal epithelial cell line, a process blocked by proteasome inhibitors. Gut 1998, 42, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Schölmerich, J.; Gross, V. Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef]
- Ungaro, F.; Rubbino, F.; Danese, S.; D’Alessio, S. Actors and Factors in the Resolution of Intestinal Inflammation: Lipid Mediators as a New Approach to Therapy in Inflammatory Bowel Diseases. Front. Immunol. 2017, 8, 1331. [Google Scholar] [CrossRef]
- Pistol, G.C.; Marin, D.E.; Rotar, M.C.; Ropota, M.; Taranu, I. Bioactive compounds from dietary whole grape seed meal improved colonic inflammation via inhibition of MAPKs and NF-κB signaling in pigs with DSS induced colitis. J. Funct. Foods 2020, 66, 103708. [Google Scholar] [CrossRef]
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S.; Peng, J. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. BioMed Res. Int. 2015, 2015, 525218. [Google Scholar] [CrossRef]
- Schuh, S.; Muller, L.K.; Campos, L.P.; Moresco, R.N.; Baldissera, M.D.; de Oliveira, S.C.; Campigotto, G.; Da Silva, A.S.; Paiano, D. Effect of supplementation of newborn piglets with spray dry blood plasma on weight gain and serum biochemical variables. Comp. Clin. Path. 2016, 25, 1029–1033. [Google Scholar] [CrossRef]
- Rubio, C.P.; Mainau, E.; Cerón, J.J.; Contreras-Aguilar, M.D.; Martínez-Subiela, S.; Navarro, E.; Tecles, F.; Manteca, X.; Escribano, D. Biomarkers of oxidative stress in saliva in pigs: Analytical validation and changes in lactation. BMC Vet. Res. 2019, 15, 144. [Google Scholar] [CrossRef]
- Argenzio, R.A. Glucose-stimulated fluid absorption in the pig small intestine during the early stage of swine dysentery. Am. J. Vet. Res. 1980, 41, 2000–2006. [Google Scholar]
- Laber, K.E.; Whary, M.T.; Bingel, S.A.; Goodrich, J.A.; Smith, A.C.; Swindle, M.M. Chapter 15—Biology and Diseases of Swine. In Laboratory Animal Medicine, 2nd ed.; Fox, J.G., Anderson, L.C., Loew, F.M., Quimby, F.W., Eds.; Academic Press: Burlington, NJ, USA, 2002; pp. 615–673. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.E.; Romanowski, R.D.; Urban, J.F. A Trichuris specific diagnostic antigen from culture fluids of Trichuris suis adult worms. Vet. Parasitol. 1997, 68, 91–102. [Google Scholar] [CrossRef]
- Taylor-Pickard, J.A.; Stevenson, Z.; Glebocka, K. Formula for the Future: Nutrition or Pathology? Elevating Performance and Health in Pigs and Poultry; Wageningen Academic Pub: Wageningen, The Netherlands, 2008. [Google Scholar]
- Luecke, R.; McMillen, W.; Thorp, F., Jr.; Tull, C. The relationship of nicotinic acid, tryptophane and protein in the nutrition of the pig. J. Nutr. 1947, 33, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Zhang, Y.; Zhao, J.; Guo, S.; Zhai, L.; Yao, S.; Sang, H.; Yang, N.; Song, G.; Gu, J.; et al. Niacin Inhibits Vascular Inflammation via Downregulating Nuclear Transcription Factor-B Signaling Pathway. Mediat. Inflamm. 2014, 2014, 263786. [Google Scholar] [CrossRef]
- Salem, H.; Wadie, W. Effect of Niacin on Inflammation and Angiogenesis in a Murine Model of Ulcerative Colitis. Sci. Rep. 2017, 7, 7139. [Google Scholar] [CrossRef]
- Thérond, P.; Bonnefont-Rousselot, D.; Davit-Spraul, A.; Conti, M.; Legrand, A. Biomarkers of oxidative stress: An analytical approach. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Lowe, F. Biomarkers of Oxidative Stress. Syst. Biol. Free Radic. Antioxid. 2014, 22. [Google Scholar] [CrossRef]
- Toledo, J.B.; Furlan, A.C.; Pozza, P.C.; Piano, L.M.; Carvalho, P.L.O.; Peñuela-Sierra, L.M.; Huepa, L.M.D. Effect of the reduction of the crude protein content of diets supplemented with essential amino acids on the performance of piglets weighing 6–15kg. Livest. Sci. 2014, 168, 94–101. [Google Scholar] [CrossRef]
- Nyachoti, C.M.; Omogbenigun, F.O.; Rademacher, M.; Blank, G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J. Anim. Sci. 2006, 84, 125–134. [Google Scholar] [CrossRef]
- Molist, F.; van Oostrum, M.; Pérez, J.F.; Mateos, G.G.; Nyachoti, C.M.; van der Aar, P.J. Relevance of functional properties of dietary fibre in diets for weanling pigs. Anim. Feed Sci. Technol. 2014, 189, 1–10. [Google Scholar] [CrossRef]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef]
- Regassa, A.; Nyachoti, C.M. Application of resistant starch in swine and poultry diets with particular reference to gut health and function. Anim. Nutr. 2018, 4, 305–310. [Google Scholar] [CrossRef]
- Souza da Silva, C.; van den Borne, J.J.G.C.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.E. Effects of dietary fibers with different physicochemical properties on feeding motivation in adult female pigs. Physiol. Behav. 2012, 107, 218–230. [Google Scholar] [CrossRef]
- Haenen, D.; Zhang, J.; Souza da Silva, C.; Bosch, G.; van der Meer, I.M.; van Arkel, J.; van den Borne, J.J.G.C.; Pérez Gutiérrez, O.; Smidt, H.; Kemp, B.; et al. A Diet High in Resistant Starch Modulates Microbiota Composition, SCFA Concentrations, and Gene Expression in Pig Intestine. J. Nutr. 2013, 143, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Darko, K.O.; Huang, Y.; He, C.; Yang, H.; He, S.; Li, J.; Li, J.; Hocher, B.; Yin, Y. Resistant Starch Regulates Gut Microbiota: Structure, Biochemistry and Cell Signalling. Cell. Physiol. Biochem. 2017, 42, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.R.; Smith, W.J.; Murray, B.P. Investigations into field cases of porcine colitis with particular reference to infection with Serpulina pilosicoli. Vet. Rec. 1998, 142, 235. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. J. Virt. Lib. 2009, 14, 2765. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef]
- Berger, A.L.; Ikuma, M.; Welsh, M.J. Normal gating of CFTR requires ATP binding to both nucleotide-binding domains and hydrolysis at the second nucleotide-binding domain. Proc. Natl. Acad. Sci. USA. 2005, 102, 455–460. [Google Scholar] [CrossRef]
- Wilfart, A.; Montagne, L.; Simmins, H.; Noblet, J.; Milgen, J.v. Digesta transit in different segments of the gastrointestinal tract of pigs as affected by insoluble fibre supplied by wheat bran. Brit. J. Nutr. 2007, 98, 54–62. [Google Scholar] [CrossRef]
- Canibe, N.; Bach Knudsen, K.E. Degradation and physicochemical changes of barley and pea fibre along the gastrointestinal tract of pigs. J. Sci. Food Agric. 2002, 82, 27–39. [Google Scholar] [CrossRef]
- Högberg, A.; Lindberg, J.E.; Leser, T.; Wallgren, P. Influence of cereal non-starch polysaccharides on ileo-caecal and rectal microbial populations in growing pigs. Acta Vet. Scand. 2004, 45, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Kiarie, E.G.; Mills, A. Role of Feed Processing on Gut Health and Function in Pigs and Poultry: Conundrum of Optimal Particle Size and Hydrothermal Regimens. Front. Vet. Sci. 2019, 6, 19. [Google Scholar] [CrossRef]
- Inborr, J.; Bedford, M.R. Stability of feed enzymes to steam pelleting during feed processing. Anim. Feed Sci. Technol. 1994, 46, 179–196. [Google Scholar] [CrossRef]
- Amezcua, R.; Friendship, R.; Dewey, C.; Gyles, C. A case-control study investigating risk factors associated with postweaning Escherichia coli diarrhea in southern Ontario. J. Swine Health Prod. 2002, 10, 245–249. [Google Scholar]
- Somani, S.J.; Modi, K.P.; Majumdar, A.S.; Sadarani, B.N. Phytochemicals and Their Potential Usefulness in Inflammatory Bowel Disease. Phytother. Res. 2015, 29, 339–350. [Google Scholar] [CrossRef]
- Hontecillas, R.; Wannemeulher, M.J.; Zimmerman, D.R.; Hutto, D.L.; Wilson, J.H.; Ahn, D.U.; Bassaganya-Riera, J. Nutritional Regulation of Porcine Bacterial-Induced Colitis by Conjugated Linoleic Acid. J. Nutr. 2002, 132, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Dodd, G.; Thomas, S.; Zhang, X.; Wasserman, M.A.; Rovin, B.H.; Kunsch, C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am. J. Physiol. Heart. Circ. Physiol. 2006, 290, H1862–H1870. [Google Scholar] [CrossRef] [PubMed]
- Bacou, E.; Walk, C.; Rider, S.; Litta, G.; Perez-Calvo, E. Dietary Oxidative Distress: A Review of Nutritional Challenges as Models for Poultry, Swine and Fish. Antioxidants 2021, 10, 525. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.A.; Grisham, M.B.; Twohig, B.; Arfors, K.E.; Harlan, J.M.; Granger, D.N. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am. J. Physiol. Heart. Circ. Physiol. 1987, 253, H699–H703. [Google Scholar] [CrossRef] [PubMed]
- Stuart, L.M.; Ezekowitz, R.A.B. Phagocytosis: Elegant Complexity. Immunity 2005, 22, 539–550. [Google Scholar] [CrossRef]
- Roberts, J.A.; Durnin, L.; Sharkey, K.A.; Mutafova-Yambolieva, V.N.; Mawe, G.M. Oxidative stress disrupts purinergic neuromuscular transmission in the inflamed colon. J. Physiol. 2013, 591, 3725–3737. [Google Scholar] [CrossRef]
- Hirst, G.D.S.; Bywater, R.A.R.; Teramoto, N.; Edwards, F.R. An analysis of inhibitory junction potentials in the guinea-pig proximal colon. J. Physiol. 2004, 558, 841–855. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef]
- Da Silva, E.O.; Gerez, J.R.; Hohmann, M.S.N.; Verri, W.A.; Bracarense, A.P.F.R.L. Phytic Acid Decreases Oxidative Stress and Intestinal Lesions Induced by Fumonisin B1 and Deoxynivalenol in Intestinal Explants of Pigs. Toxins 2019, 11, 18. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Nichols, N.L.; Bertolo, R.F. Luminal Threonine Concentration Acutely Affects Intestinal Mucosal Protein and Mucin Synthesis in Piglets. J. Nutr. 2008, 138, 1298–1303. [Google Scholar] [CrossRef]
- Jørgensen, H.; Zhao, X.-Q.; Eggum, B.O. The influence of dietary fibre and environmental temoperature on the development of the gastrointestinal tract, digestibility, degree of fermentation in the hind-gut and energy metabolism in pigs. Br. J. Nutr. 1996, 75, 365–378. [Google Scholar] [CrossRef]
- Prohaszka, L.; Lukács, K. Influence of the diet on the antibacterial effect of volatile fatty acids and on the development of swine dysentery. Zent. für Veterinärmed. Reihe B 1984, 31, 779–785. [Google Scholar] [CrossRef]
- Siba, P.M.; Pethick, D.W.; Hampson, D.J. Pigs experimentally infected with Serpulina hyodysenteriae can be protected from developing swine dysentery by feeding them a highly digestible diet. Epidemiol. Infect. 1996, 116, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R.; Pethick, D.W.; Hopwood, D.E.; Hampson, D.J. Nutritional influences on some major enteric bacterial diseases of pig. Nutr. Res. Rev. 2002, 15, 333–371. [Google Scholar] [CrossRef] [PubMed]
- Åkerberg, A.; Liljeberg, H.; Björck, I. Effects of Amylose/Amylopectin Ratio and Baking Conditions on Resistant Starch Formation and Glycaemic Indices. J. Cereal Sci. 1998, 28, 71–80. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Darcy-Vrillon, B.; Cherbuy, C.; Morel, M.-T.; Durand, M.; Duée, P.-H. Short chain fatty acid and glucose metabolism in isolated pig colonocytes: Modulation by NH4+. Mol. Cell. Biochem. 1996, 156, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Roediger, W.E.W. Utilization of Nutrients by Isolated Epithelial Cells of the Rat Colon. Gastroenterology 1982, 83, 424–429. [Google Scholar] [CrossRef]
- Mangian, H.F.; Tappenden, K.A. Butyrate Increases GLUT2 mRNA Abundance by Initiating Transcription in Caco2-BBe Cells. J. Parenter. Enteral. Nutr. 2009, 33, 607–617. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, 6418. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A. GPR109A is a G-protein–coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.; Foord, S.M.; Fraser, N.J.; Barnes, A.A.; Elshourbagy, N.; Eilert, M.; Ignar, D.M.; Murdock, P.R.; Steplewski, K.; Green, A. Molecular identification of high and low affinity receptors for nicotinic acid. J. Biol. Chem. 2003, 278, 9869–9874. [Google Scholar] [CrossRef]
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.-Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.-J. (D)-β-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Clarke, J.M.; Topping, D.L.; Christophersen, C.T.; Bird, A.R.; Lange, K.; Saunders, I.; Cobiac, L. Butyrate esterified to starch is released in the human gastrointestinal tract. Am. J. Clin. Nutr. 2011, 94, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Prohászka, L. Antibacterial Mechanism of Volatile Fatty Acids in the Intestinal Tract of Pigs agains Escherichia coli. J. Vet. Med. B 1986, 33, 166–173. [Google Scholar] [CrossRef]
- Anguita, M.; Canibe, N.; Pérez, J.F.; Jensen, B.B. Influence of the amount of dietary fiber on the available energy from hindgut fermentation in growing pigs: Use of cannulated pigs and in vitro fermentation. J. Anim. Sci. 2006, 84, 2766–2778. [Google Scholar] [CrossRef]
- Xiao, K.; Zhou, Y.; Guo, C.; Maspolim, Y.; Ng, W.J. Impact of undissociated volatile fatty acids on acidogenesis in a two-phase anaerobic system. J. Environ. Sci. 2016, 42, 196–201. [Google Scholar] [CrossRef] [PubMed]
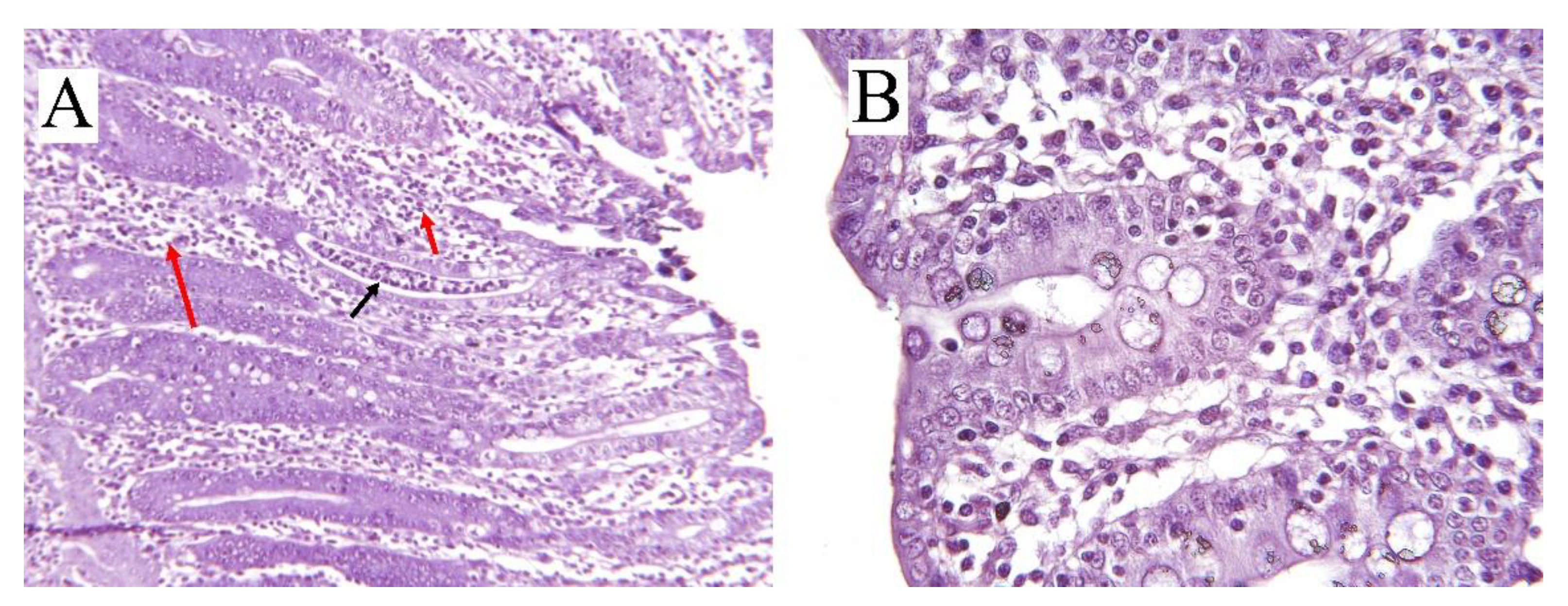
| Type of Colitis | Causative Factor | Affected Site | Pigs Age, Week | Mechanism of Action | Clinical Signs | Gross Lesion | References |
|---|---|---|---|---|---|---|---|
| SC | |||||||
| Swine dysentery | B. hyodysenteriae | Cecum and colon | 6–18 | Absorptive dysfunctionality, hemolysins, and degradative enzymes | Loose stool, mucoid, hemorrhagic diarrhea, dehydration, and retarded growth rate | Inflamed epithelium, mucosal damage, hyperplasia of the crypts, and spirochetal attachment | [2,10,13,43] |
| Spirochetal colitis | B. pilosicoli | Cecum and colon | 4–20 | Absorptive dysfunctionality | Mild non-hemorrhagic, mucoid diarrhea, retarded growth rate | Inflamed epithelium, moderate catarrhal colitis, flaccid and thin luminal wall, appearance of small adherent nodules of digesta | [4,11,13,43,44] |
| Parasitic colitis | T. suis | Cecum and spiral colon | 4–10 | Stimulation of the epithelium and cascading inflammatory responses by hatched eggs and adult worms | Dark loose stool, mucoid to hemorrhagic diarrhea, dehydration, anorexia, and increased feed conversion ratio | Crypt hyperplasia, goblet cell hyperplasia, a general hypertrophy of mucosa, and presence of bipolar eggs | [15,45,46,47,48] |
| NSC | Dietary factors | Cecum and colon | 4–12 | Absorptive dysfunctionality and increased epithelial permeability | Loose and mucoid, non-hemorrhagic diarrhea | Mucosal hyperplasia, mononuclear cell infiltration, multifocal mucosal erosions, increased crypt depth | [13,37,42,49] |
| Biomarkers | Type | Direction | Recovery Site | Causative Factor | Affected Site | Reference |
|---|---|---|---|---|---|---|
| MUC2 and MUC5AC | Mucin | Increased expression | Feces | Colitis, swine dysentry | Large intestine | [31,35,36] |
| LPS 1 | Saccharide | Increased expression | Serum | Gram-negative pathogens, e.g., B. hyodysenteriae | Small and large intestine | [56,57,58,59] |
| Calprotectin and lactoferrin | Protein | Increased expression | Feces and serum | Colitis and inflammatory factors | Large intestine | [60,61,62,63] |
| Na+, Cl−, HCO3-, and K+ | Ion | Reduced absorption and increased luminal accumulation | Feces | B. hyodysenteriae | Large intestine | [4,20,26,51] |
| TNF-α 2, IFN-γ 3, IL-1β 4, IL-6 5, and IL-10 6 | Cytokines | Increased expression | Serum and mucus | Pathogens | Small and large intestine | [17,20,26,37,58,64] |
| NF-κB 7 | Protein | Increased expression in macrophages and in epithelial cells | Serum | IL-1β and TNF-α, LPS, and ROS 8 | Epithelial cells of inflamed colon | [59,64,65,66,67] |
| CRP 8, HP 9, and pig-MAP 10 | Protein | Increased concentration | Serum | LPS, IL-1β, and TNF-α | Epithelial cells of colon, and hepatic cells | [58] |
| FRAP 11, TBARS 12, and ROS 13 | TAC 14 assay | Increased expression | Serum | Oxidative stress | Epithelial cells of colon | [68,69] |
| TEAC 15, CUPRAC 16, AOPP 17, and H2O2 | TAC assay | Increased expression | Saliva | Oxidative stress | Epithelial cells of colon | [69,70] |
| Factor | Level | Effect | Reference |
|---|---|---|---|
| Trypsin inhibitor | High | Increased undegraded protein in large intestine and inflammation and causing NSC 1 | [13] |
| Vitamin C and E, glutathione, ubiquinol, polyphenols, and β-carotene | Insufficient | Oxidative distress | [13,67,79,80] |
| Essential amino acids | Insufficient | Oxidative stress by reducing antioxidant enzymes, reduced mucin production | [30,31,49,81] |
| Dietary protein | ≥23% | Increased undegraded protein in large intestine and inflammation due to NH4+, reducing gut barrier function and causing NSC | [37,41,49,82] |
| Soluble NSP 2 and RS 3 | Increased | Ameliorating/preventive effect on large intestinal inflammation, increased SCFA 4, and reduced luminal pH | [83,84,85,86,87,88] |
| Pelleted diet | - | Reduced endogenous enzymes in feedstuffs and causing NSC | [5,89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panah, F.M.; Lauridsen, C.; Højberg, O.; Nielsen, T.S. Etiology of Colitis-Complex Diarrhea in Growing Pigs: A Review. Animals 2021, 11, 2151. https://doi.org/10.3390/ani11072151
Panah FM, Lauridsen C, Højberg O, Nielsen TS. Etiology of Colitis-Complex Diarrhea in Growing Pigs: A Review. Animals. 2021; 11(7):2151. https://doi.org/10.3390/ani11072151
Chicago/Turabian StylePanah, Farhad M., Charlotte Lauridsen, Ole Højberg, and Tina Skau Nielsen. 2021. "Etiology of Colitis-Complex Diarrhea in Growing Pigs: A Review" Animals 11, no. 7: 2151. https://doi.org/10.3390/ani11072151
APA StylePanah, F. M., Lauridsen, C., Højberg, O., & Nielsen, T. S. (2021). Etiology of Colitis-Complex Diarrhea in Growing Pigs: A Review. Animals, 11(7), 2151. https://doi.org/10.3390/ani11072151






