The Goat as a Risk Factor for Parasitic Infections in Ovine Flocks
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval Statement
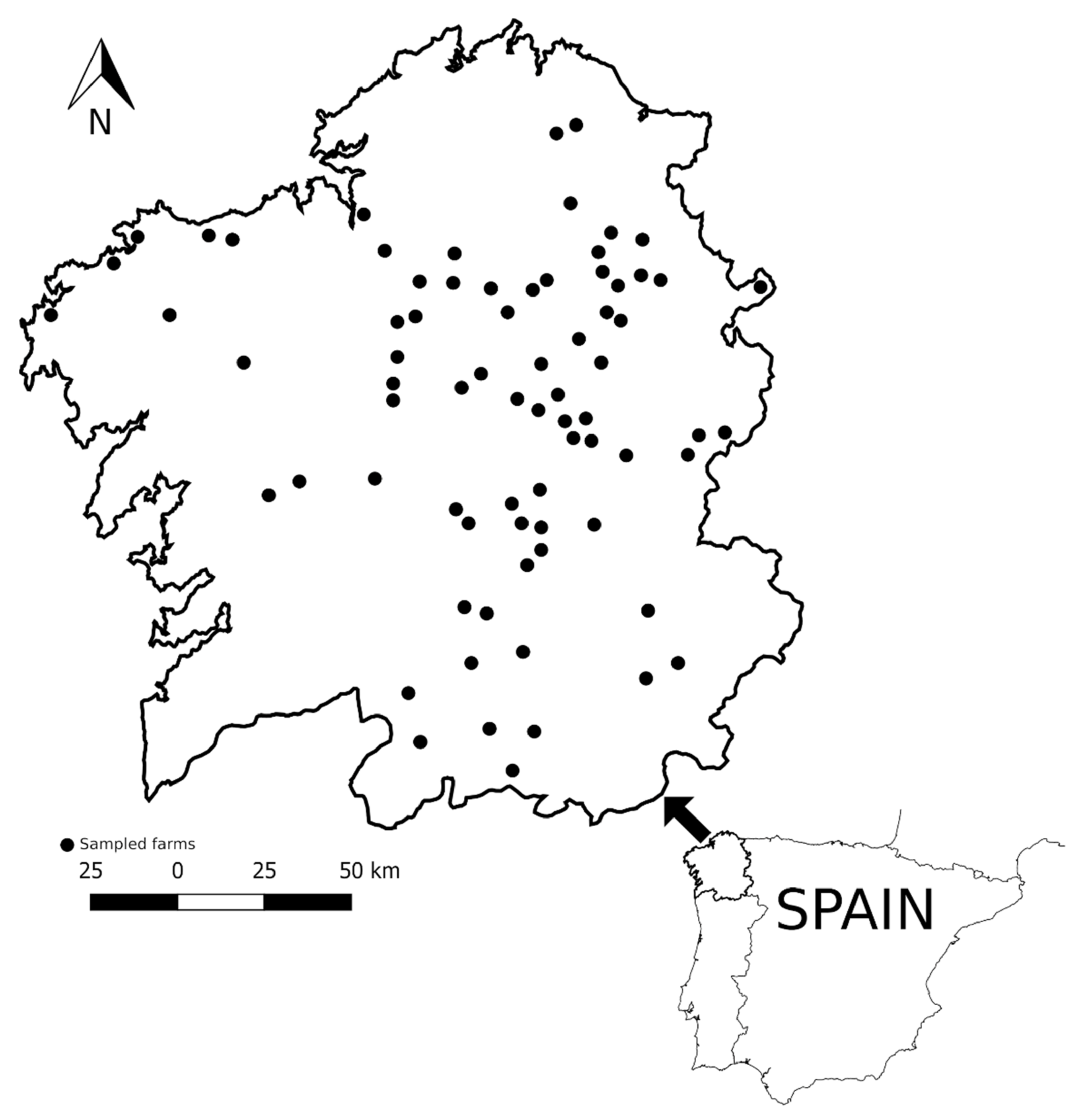
2.2. Animals and Flocks Surveyed
2.3. Examination of Fecal Samples
2.4. Statistical Analysis
3. Results
3.1. Prevalence and Intensity of Infection in Sheep and Goats
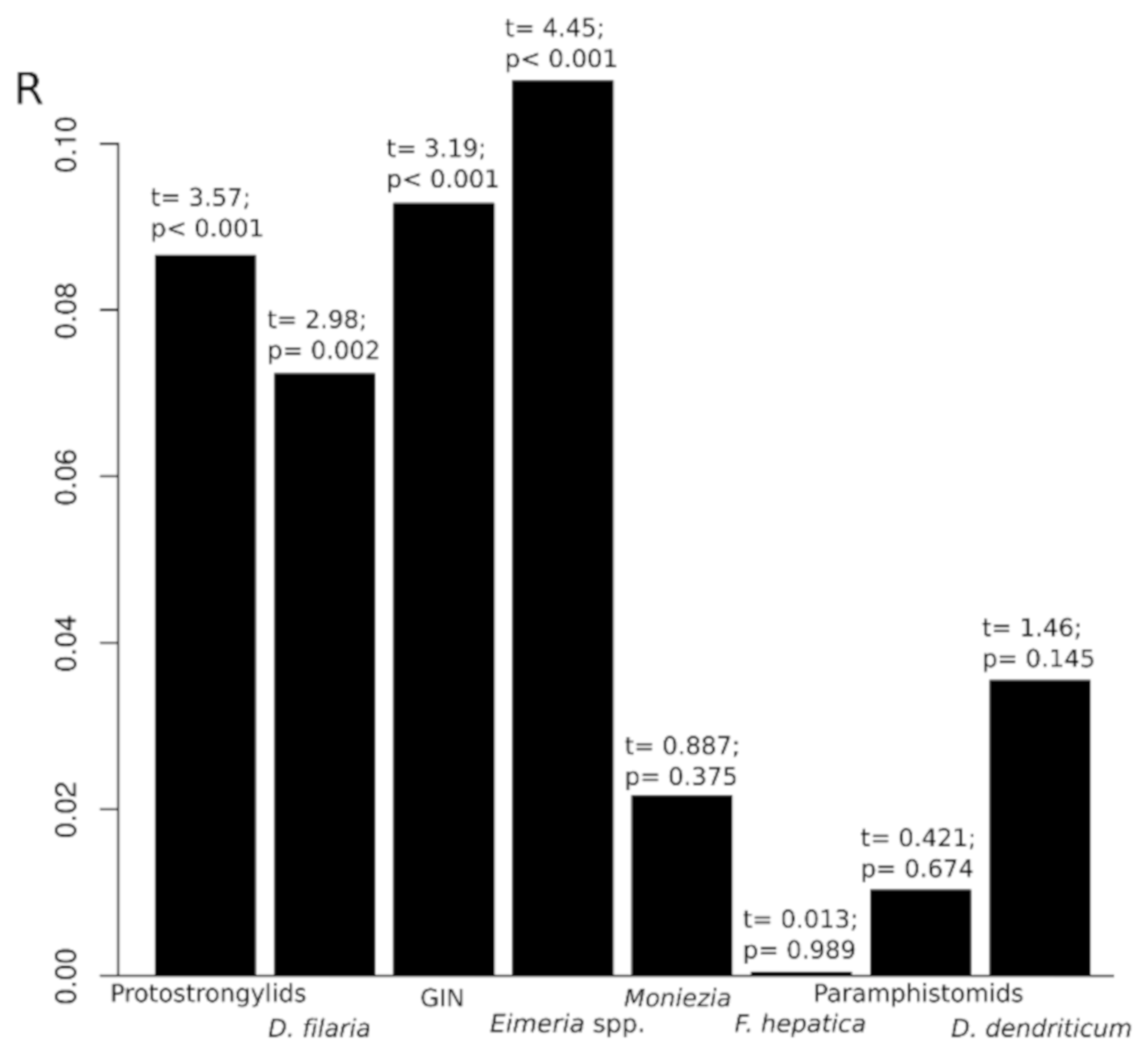
3.2. Effect of Sheep–Goat Contact on Parasitic Prevalences
3.3. Intensity of Infection and Goat Contact Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xunta de Galicia. Consellería do Medio Rural. 2014. Available online: http://www.medioruralemar.xunta.es/fileadmin/arquivos/estatisticas/2010/31204/31204_Estrutura_produtiva_por_tipoloxia_2014.xlsx (accessed on 1 December 2015).
- López, C.M.; Fernández, G.; Viña, M.; Cienfuegos, S.; Panadero, R.; Vázquez, L.; Díaz, P.; Pato, J.; Lago, N.; Dacal, V.; et al. Protostrongylid infection in meat sheep from Northwestern Spain: Prevalence and risk factors. Vet. Parasitol. 2011, 178, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Lago, N.; López, C.; Panadero, R.; Cienfuegos, S.; Pato, J.; Prieto, A.; Díaz, P.; Mourazos, N.; Fernández, G. Seroprevalence and risk factors associated with Visna/Maedi virus in semi-intensive lamb-producing flocks in northwestern Spain. Prev. Vet. Med. 2012, 103, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Baermann, G. Eine einfache Methode zur Auffindung von Ancyclostomum (Nematode) Larven in Erdproben. Geneesk. Tijaschr. Nederl. Indië. 1917, 57, 131–137. [Google Scholar]
- Wetzel, R. Zur Diagnose der Lungenwurminvasionen bei Rind und Schaf. Dtsch. Tierärztl. Wschr. 1930, 38, 49–50. [Google Scholar]
- MAFF. Manual of Veterinary Parasitological Laboratory Techniques; Her Majesty’s Stationary Office: London, UK, 1971; 131p. [Google Scholar]
- Virasakdi, C. Epicalc: Epidemiological Calculator, R Package Version 2.15.1.0; Available online: http://CRAN.R-project.org/package=epicalc.2011.URLbook;https://cran.r-project.org/doc/contrib/Epicalc_Book.pdf (accessed on 13 June 2016).
- Virasakdi, C. epiDisplay: Epidemiological Data Display Package. R Package. 2015. Available online: https://CRAN.R-project.org/package=epiDisplay (accessed on 13 June 2016).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 20 April 2019).
- Sanchez, G.; Trinchera, L.; Russolillo, G. plspm: Tools for Partial Least Squares Path Modeling (PLS-PM), R Package Version 0.4.7; 2015. Available online: https://CRAN.R-project.org/package=plspm (accessed on 30 March 2017).
- Mangeon, M.; Cabaret, J. Infestation comparée des ovins et des caprins en pâturages mixtes. Bull. Des. GTV 1987, 4, 43–48. [Google Scholar]
- Alemu, S.; Leykun, E.G.; Ayelet, G.; Zeleke, A. Study on small ruminant lungworms in northeastern Ethiopia. Vet. Parasitol. 2006, 142, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Berrag, B.; Urquhart, G.M. Epidemiological aspects of lungworm infections of goats in Morocco. Vet. Parasitol. 1996, 61, 81–95. [Google Scholar] [CrossRef]
- Huntley, J.; Patterson, M.; Mackellar, A.; Jackson, F.; Stevenson, L.; Coop, R. A comparison of the mast cell and eosinophil responses of sheep and goats to gastrointestinal nematode infections. Res. Vet. Sci. 1995, 58, 5–10. [Google Scholar] [CrossRef]
- Macaldowie, C.; Jackson, F.; Huntley, J.; Mackellar, A.; Jackson, E. A comparison of larval development and mucosal mast cell responses in worm-naïve goat yearlings, kids and lambs undergoing primary and secondary challenge with Teladorsagia circumcincta. Vet. Parasitol. 2003, 114, 1–13. [Google Scholar] [CrossRef]
- Celaya, R.; Benavides, R.; García, U.; Ferreira, L.M.; Ferre, I.; Martínez, A.; Ortega-Mora, L.M.; Osoro, K. Grazing behaviour and performance of lactating suckler cows, ewes and goats on partially improved heathlands. Animal 2008, 2, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Hoste, H.; Torres-Acosta, J.F.J.; Aguilar-Caballero, A.J. Nutrition–parasite interactions in goats: Is immunoregulation involved in the control of gastrointestinal nematodes? Parasite Immunol. 2008, 30, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Levine, N.D.; Ivens, V. The Coccidian Parasites (Protozoa, Sporozoa) of Ruminants; Illinois Biological Monographs, 44; University of Illinois Press Urbana: Chicago, IL, USA, 1970; 278p. [Google Scholar]
- McDougald, L.R. Attempted cross-transmission of coccidia between sheep and goats and description of Eimeria ovinoidalis sp. n. J. Protozool. 1979, 26, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bakunzi, F.R.; Thwane, S.N.; Motsei, L.E.; Dzoma, B.M. Diversity and seasonal occurrence of Eimeria species in a mixed flock of communally reared sheep and goats in Mafikeng in the North West Province, South Africa. J. S. Afr. Vet. Assoc. 2010, 81, 48–150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rankins, D.L., Jr.; Pugh, D.G. Feeding and Nutrition. In Sheep and Goat Medicine, 2nd ed.; Pugh, D.G., Baird, A.N., Eds.; ELSEVIER Saunders: Philadelphia, PA, USA, 2012; pp. 18–49. [Google Scholar]
- Smith, M.C.; Sherman, D.M. Goat Medicine, 2nd ed.; A John Wiley & Sons, Inc. Publication: Ames, IA, USA, 2009; pp. 3–8. [Google Scholar]
- Taylor, M. Diagnosis and control of coccidiosis in sheep. Practice 1995, 17, 172–177. [Google Scholar] [CrossRef]
| Parasitic Infection | Prevalence Positive/Total (%, 95% C.I. 1) | Intensity of Infection Mean (Parasitic Forms per g); sd | ||
|---|---|---|---|---|
| Goat | Sheep | Goat | Sheep | |
| Protostrongylid nematodes | 81/103 (78.6, 69.2–85.9) | 242/2093 (11.6, 10.2–13.0) | 283.2; 787.40 | 11.9; 30.85 |
| Dictyocaulus filaria | 11/103 (10.7, 5.7–18.7) | 223/2093 (10.6, 9.4–12.1) | 3.7; 6.36 | 8.5; 41.89 |
| Gastrointestinal nematodes | 75/103 (72.8, 63.0–80.9) | 1233/1914 (64.4, 62.2–66.6) | 634.7; 1507.07 | 465.7; 930.61 |
| Eimeria spp. | 86/103 (83.5, 74.6–89.8) | 1415/1914 (73.9, 71.9–75.9) | 2808.7; 6924.40 | 1393.3; 10,369.73 |
| Moniezia spp. | 0/103 (0, 0–0.04) | 96/1914 (5.0, 4.1–6.1) | - | 358.6; 700.37 |
| Fasciola hepatica | 0/103 (0, 0–0.04) | 104/1697 (6.1, 5.1–7.4) | - | 128.2; 209.23 |
| D. dendriticum | 0/103 (0, 0–0.04) | 14/1697 (0.8, 0.5–1.4) | - | 73.7; 64.97 |
| Paramphistomidae | 0/103 (0, 0–0.04) | 12/1697 (0.7, 0.4–1.3) | - | 89.2; 65.30 |
| Parasitic Infection | Mixed Flocks Positive/Total (%, 95% C.I. 1) | Pure Sheep Flocks Positive/Total (%, 95% C.I.) | Chi-Squared Result | OR (95% C.I.) |
|---|---|---|---|---|
| Protostrongylid nematodes | 83/468 (17.7, 14.4–21.6) | 159/1625 (9.8, 8.4–11.4) | χ2 = 21.690; p < 0.001 ***2 | 1.99 (1.47–2.67) |
| Dictyocaulus filaria | 71/468 (15.2, 12.1–18.8) | 152/1625 (9.3, 8.0–10.9) | χ2 = 12.312; p < 0.001 *** | 1.73 (1.26–2.37) |
| Gastrointestinal nematodes | 300/399 (75.2, 70.6–79.3) | 933/1515 (61.6, 59.1–64.0) | χ2 = 11.557; p < 0.001 *** | 1.89 (1.46–2.45) |
| Eimeria spp. | 322/399 (80.7, 76.4–84.4) | 1093/1515 (72.1, 69.8–74.4) | χ2 = 11.997; p < 0.001 *** | 1.61 (1.22–2.15) |
| Moniezia spp. | 20/399 (5.0, 3.2–7.8) | 76/1515 (5.0, 4.0–6.3) | χ2 = <0.001; p = 1 | 1 (0.57–1.68) |
| Fasciola hepatica | 21/341 (6.2, 3.9–9.4) | 83/1356 (6.1, 4.9–7.6) | χ2 = 0.004; p = 0.949 | 1.01 (0.58–1.67) |
| D. dendriticum | 5/341 (1.5, 0.5–3.6) | 9/1356 (0.7, 0.3–1.3) | χ2 = 1.276; p = 0.259 | 2.23 (0.58–7.45) |
| Paramphistomidae | 3/341 (0.9, 0.2–2.8) | 9/1356 (0.7, 0.3–1.3) | Fisher’s Test 2 p = 0.716 | 1.33 (0.23–5.36) |
| Parasitic Infection | Mixed Flocks Mean (Parasitic Forms per g); sd | Pure Sheep Flocks Mean (Parasitic Forms per g); sd | Wilcoxon Rank Test Result |
|---|---|---|---|
| Protostrongylid nematodes | 15.9; 36.33 | 9.8; 27.42 | W = 5546.5; p = 0.025 *1 |
| Dictyocaulus filaria | 6.6; 19.53 | 9.4; 49.00 | W = 5844.5; p = 0.318 |
| Gastrointestinal nematodes | 521.9; 1121.16 | 447.7; 860.35 | W = 137,910; p = 0.703 |
| Eimeria spp. | 1119.7; 4392.13 | 1474.0; 11555.95 | W = 171,470; p = 0.484 |
| Moniezia spp. | 442.1; 931.06 | 334.1; 622.63 | W = 839.0; p = 0.906 |
| Fasciola hepatica | 142.9; 160.36 | 124.6; 220.06 | W = 720.5; p = 0.360 |
| Dicrocoelium dendriticum | 82.6; 46.59 | 68.7; 75.48 | W = 17.5; p = 0.545 |
| Paramphistomidae | 168.3; 73.36 | 62.8; 37.24 | W = 1.0; p = 0.026 *1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Dios, D.; Panadero, R.; Díaz, P.; Viña, M.; Remesar, S.; Prieto, A.; López-Lorenzo, G.; Martínez-Calabuig, N.; Díez-Baños, P.; Morrondo, P.; et al. The Goat as a Risk Factor for Parasitic Infections in Ovine Flocks. Animals 2021, 11, 2077. https://doi.org/10.3390/ani11072077
García-Dios D, Panadero R, Díaz P, Viña M, Remesar S, Prieto A, López-Lorenzo G, Martínez-Calabuig N, Díez-Baños P, Morrondo P, et al. The Goat as a Risk Factor for Parasitic Infections in Ovine Flocks. Animals. 2021; 11(7):2077. https://doi.org/10.3390/ani11072077
Chicago/Turabian StyleGarcía-Dios, David, Rosario Panadero, Pablo Díaz, Miguel Viña, Susana Remesar, Alberto Prieto, Gonzalo López-Lorenzo, Néstor Martínez-Calabuig, Pablo Díez-Baños, Patrocinio Morrondo, and et al. 2021. "The Goat as a Risk Factor for Parasitic Infections in Ovine Flocks" Animals 11, no. 7: 2077. https://doi.org/10.3390/ani11072077
APA StyleGarcía-Dios, D., Panadero, R., Díaz, P., Viña, M., Remesar, S., Prieto, A., López-Lorenzo, G., Martínez-Calabuig, N., Díez-Baños, P., Morrondo, P., & López, C. M. (2021). The Goat as a Risk Factor for Parasitic Infections in Ovine Flocks. Animals, 11(7), 2077. https://doi.org/10.3390/ani11072077






