Anti-Aging Effect of Urolithin A on Bovine Oocytes In Vitro
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Previous Assay—Dose-Response Study
2.1.2. Experiment 1
2.1.3. Experiment 2
2.2. Oocyte Collection and In Vitro Maturation
2.3. Granulosa Cells Collection and Culture
2.4. Oocyte Nuclear Maturation
2.5. Assessment of Mitochondrial Membrane Potential
2.6. In Vitro Fertilization and Embryo Culture
2.7. DNA and RNA Extraction and Quantification
2.7.1. Complementary DNA Synthesis
2.7.2. Primer Design
2.7.3. Quantitative Reverse-Transcription Polymerase Chain Reaction
2.8. Statistical Analysis
3. Results
3.1. Previous Assay—Dose-Response Study
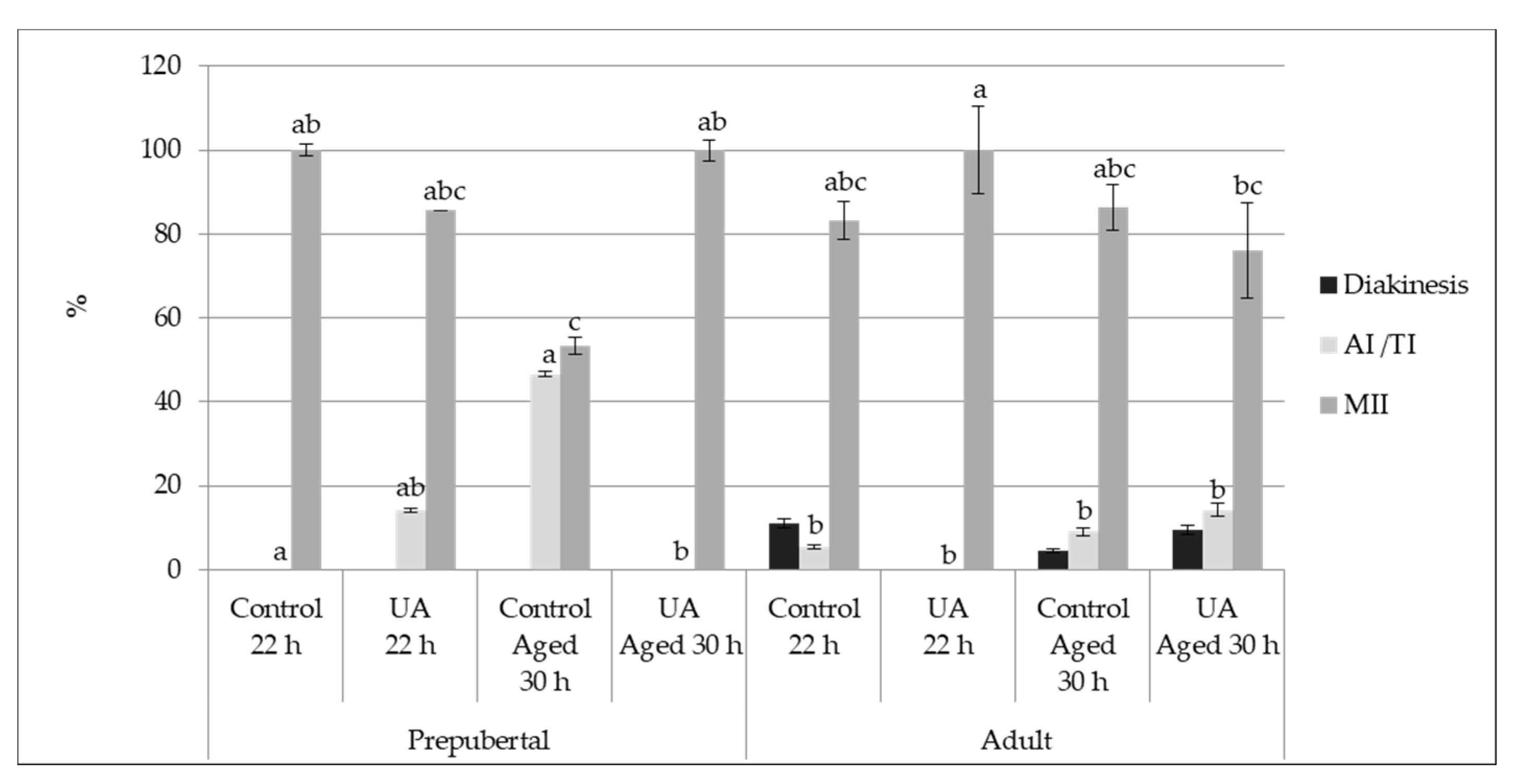
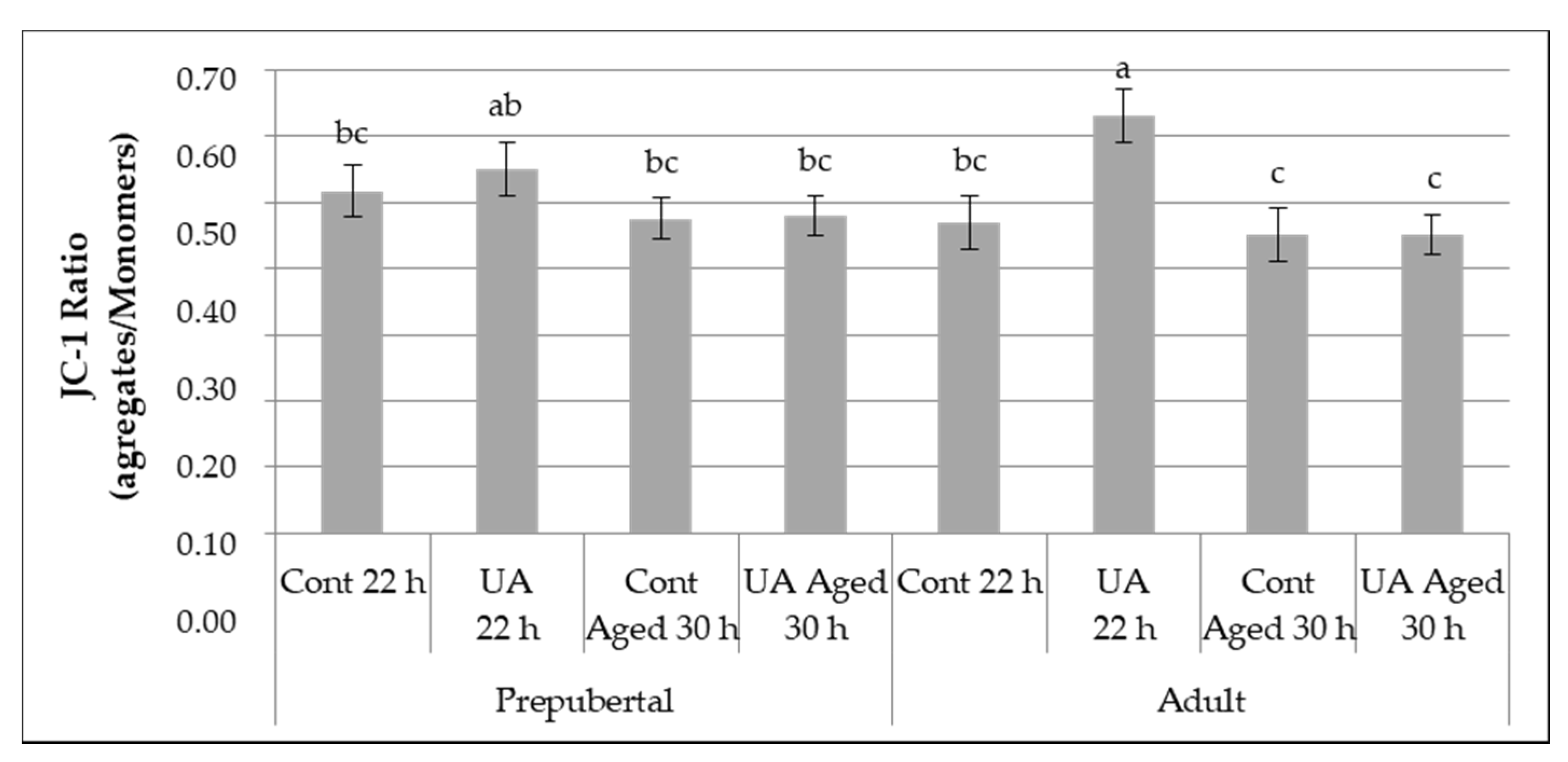
3.2. Experiment 1—Oocyte Quality and Developmental Potential of Aged and Physiologically Matured Oocytes
3.2.1. Nuclear Maturation
3.2.2. Mitochondrial Membrane Potential
3.2.3. Embryonic Development
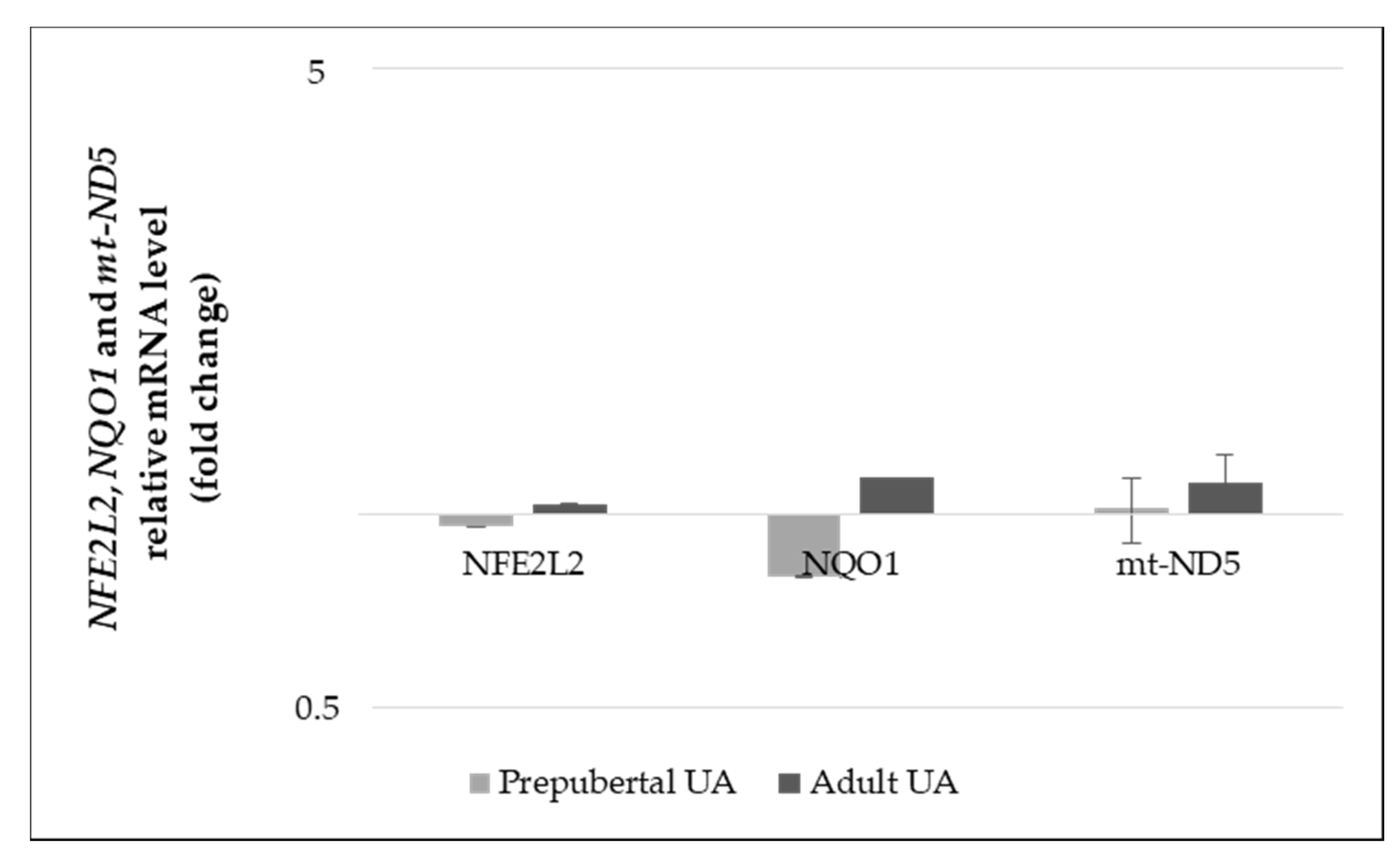
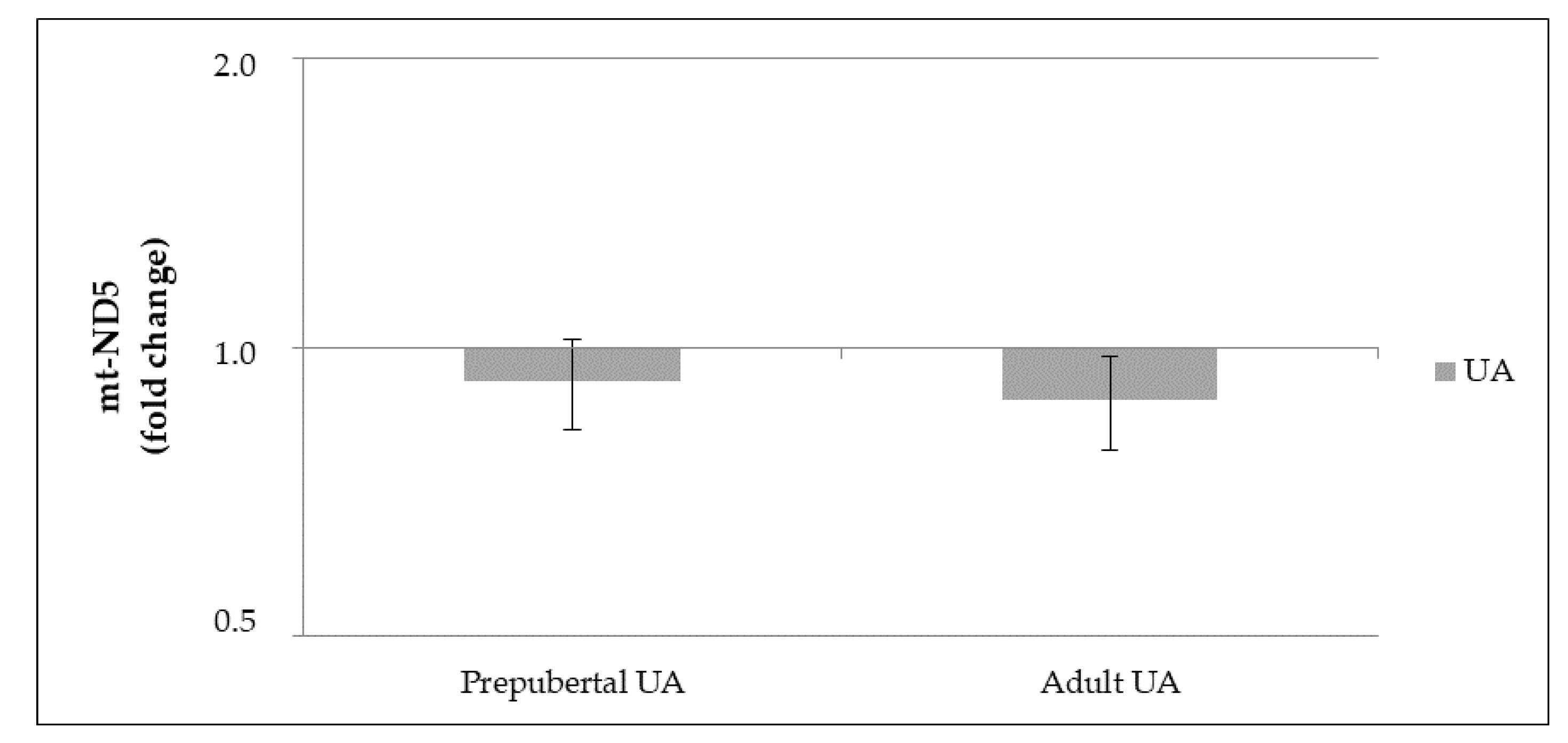
3.3. Experiment 2
3.3.1. Gene Expression Levels
3.3.2. mt-DNA Copy Number
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Malhi, P.S.; Adams, G.P.; Mapletoft, R.J.; Singh, J. Oocyte Developmental Competence in a Bovine Model of Reproductive Aging. Reproduction 2007, 134, 233–239. [Google Scholar] [CrossRef]
- Hui, L.; Shuangshuang, G.; Jianning, Y.; Zhendan, S. Systemic Analysis of Gene Expression Profiles in Porcine Granulosa Cells during Aging. Oncotarget 2017, 8, 96588–96603. [Google Scholar] [CrossRef] [PubMed]
- del Collado, M.; da Silveira, J.C.; Oliveira, M.L.F.; Alves, B.M.S.M.; Simas, R.C.; Godoy, A.T.; Coelho, M.B.; Marques, L.A.; Carriero, M.M.; Nogueira, M.F.G.; et al. In Vitro Maturation Impacts Cumulus–Oocyte Complex Metabolism and Stress in Cattle. Reproduction 2017, 154, 881–893. [Google Scholar] [CrossRef]
- Modina, S.C.; Tessaro, I.; Lodde, V.; Franciosi, F.; Corbani, D.; Luciano, A.M. Reductions in the Number of Mid-Sized Antral Follicles Are Associated with Markers of Premature Ovarian Senescence in Dairy Cows. Reprod. Fertil. Dev. 2014, 26, 235. [Google Scholar] [CrossRef]
- Ter Keurst, A.; Boivin, J.; Gameiro, S. Women’s Intentions to Use Fertility Preservation to Prevent Age-Related Fertility Decline. Reprod. Biomed. Online 2016, 32, 121–131. [Google Scholar] [CrossRef]
- Zhang, M.; ShiYang, X.; Zhang, Y.; Miao, Y.; Chen, Y.; Cui, Z.; Xiong, B. Coenzyme Q10 Ameliorates the Quality of Postovulatory Aged Oocytes by Suppressing DNA Damage and Apoptosis. Free Radic. Biol. Med. 2019, 143, 84–94. [Google Scholar] [CrossRef]
- Igarashi, H.; Takahashi, T.; Nagase, S. Oocyte Aging Underlies Female Reproductive Aging: Biological Mechanisms and Therapeutic Strategies. Reprod. Med. Biol. 2015, 14, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Marques, C.C.; Pimenta, J.; Barbas, J.; Baptista, M.; Torres, A.; Costa, L. Assisted Reproductive Technologies (ART) Directed to Germplasm Preservation. In Advances in Animal Health, Medicine and Production: A Research Portrait of the Centre for Interdisciplinary Research in Animal Health (CIISA), University of Lisbon; Springer: Berlin/Heidelberg, Germany, 2020; pp. 199–215. [Google Scholar] [CrossRef]
- Gonçalves, S.C.; Marques, C.C.; Stöckemann, K.; Wang, W.; Horta, A.E.M. Influence of an Antiprogestin (Onapristone) on in Vivo and in Vitro Fertilization. Anim. Reprod. Sci. 1997, 46, 55–67. [Google Scholar] [CrossRef]
- Davies Morel, M.C.G.; Newcombe, J.R. The Efficacy of Different HCG Dose Rates and the Effect of HCG Treatment on Ovarian Activity: Ovulation, Multiple Ovulation, Pregnancy, Multiple Pregnancy, Synchrony of Multiple Ovulation; in the Mare. Anim. Reprod. Sci. 2008, 109, 189–199. [Google Scholar] [CrossRef]
- Song, C.; Peng, W.; Yin, S.; Zhao, J.; Fu, B.; Zhang, J.; Mao, T.; Wu, H.; Zhang, Y. Melatonin Improves Age-Induced Fertility Decline and Attenuates Ovarian Mitochondrial Oxidative Stress in Mice. Sci. Rep. 2016, 6, 35165. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.T.; Greig, M.M.; Sontakke, A.A.; Peloquin, A.S.; McPeek, M.A.; Bickel, S.E. Increased Levels of Superoxide Dismutase Suppress Meiotic Segregation Errors in Aging Oocytes. Chromosoma 2019, 128, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Carbone, M.C.; Falone, S.; Aimola, P.; Giardinelli, A.; Caserta, D.; Marci, R.; Pandolfi, A.; Ragnelli, A.M.; Amicarelli, F. Age-Dependent Changes in the Expression of Superoxide Dismutases and Catalase Are Associated with Ultrastructural Modifications in Human Granulosa Cells. Mol. Hum. Reprod. 2006, 12, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.; Hoelker, M.; Besenfelder, U.; Havlicek, V.; Cinar, U.; Rings, F.; Held, E.; Dufort, I.; Sirard, M.-A.; Schellander, K.; et al. Molecular Mechanisms and Pathways Involved in Bovine Embryonic Genome Activation and Their Regulation by Alternative In Vivo and In Vitro Culture Conditions. Biol. Reprod. 2012, 87, 100. [Google Scholar] [CrossRef]
- Sindan, N.; Bhandari, A.; Zhao, Y.; Lu, X.; Lv, J. Expression and Localization of Nuclear Factor Erythroid 2-related Factor 2 in the Ovarian Tissues of Mice at Different Ages. Exp. Ther. Med. 2018, 16, 3546–3552. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, S.; Xu, C.; Li, L.; Zhang, Y.; Guo, Y.; Zhang, C.; Li, P.; Long, M.; He, J. Proanthocyanidins Protect against β-Hydroxybutyrate-Induced Oxidative Damage in Bovine Endometrial Cells. Molecules 2019, 24, 400. [Google Scholar] [CrossRef] [PubMed]
- Akino, N.; Wada-Hiraike, O.; Terao, H.; Honjoh, H.; Isono, W.; Fu, H.; Hirano, M.; Miyamoto, Y.; Tanikawa, M.; Harada, M.; et al. Activation of Nrf2 Might Reduce Oxidative Stress in Human Granulosa Cells. Mol. Cell. Endocrinol. 2018, 470, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Akino, N.; Wada-Hiraike, O.; Isono, W.; Terao, H.; Honjo, H.; Miyamoto, Y.; Tanikawa, M.; Sone, K.; Hirano, M.; Harada, M.; et al. Activation of Nrf2/Keap1 Pathway by Oral Dimethylfumarate Administration Alleviates Oxidative Stress and Age-Associated Infertility Might Be Delayed in the Mouse Ovary. Reprod. Biol. Endocrinol. 2019, 17, 23. [Google Scholar] [CrossRef]
- Babayev, E.; Wang, T.; Szigeti-Buck, K.; Lowther, K.; Taylor, H.S.; Horvath, T.; Seli, E. Reproductive Aging Is Associated with Changes in Oocyte Mitochondrial Dynamics, Function, and MtDNA Quantity. Maturitas 2016, 93, 121–130. [Google Scholar] [CrossRef]
- Simsek-Duran, F.; Li, F.; Ford, W.; Swanson, R.J.; Jones, H.W.; Castora, F.J. Age-Associated Metabolic and Morphologic Changes in Mitochondria of Individual Mouse and Hamster Oocytes. PLoS ONE 2013, 8, e64955. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, H.; Liang, S.; Shen, X.; Gao, Q.; Xu, Y.N.; Kim, N.-H. Laminarin Enhances the Quality of Aged Pig Oocytes by Reducing Oxidative Stress. J. Reprod. Dev. 2018, 64, 489–494. [Google Scholar] [CrossRef]
- Peters, A.E.; Mihalas, B.P.; Bromfield, E.G.; Roman, S.D.; Nixon, B.; Sutherland, J.M. Autophagy in Female Fertility: A Role in Oxidative Stress and Aging. Antioxid. Redox Signal. 2020, 32, 550–568. [Google Scholar] [CrossRef] [PubMed]
- May-Panloup, P.; Boguenet, M.; El Hachem, H.; Bouet, P.-E.; Reynier, P. Embryo and Its Mitochondria. Antioxidants 2021, 10, 139. [Google Scholar] [CrossRef]
- Lin, X.; Dai, Y.; Tong, X.; Xu, W.; Huang, Q.; Jin, X.; Li, C.; Zhou, F.; Zhou, H.; Lin, X.; et al. Excessive Oxidative Stress in Cumulus Granulosa Cells Induced Cell Senescence Contributes to Endometriosis-Associated Infertility. Redox Biol. 2020, 30, 101431. [Google Scholar] [CrossRef]
- Hammond, E.R.; Green, M.P.; Shelling, A.N.; Berg, M.C.; Peek, J.C.; Cree, L.M. Oocyte Mitochondrial Deletions and Heteroplasmy in a Bovine Model of Ageing and Ovarian Stimulation. Mol. Hum. Reprod. 2016, 22, 261–271. [Google Scholar] [CrossRef]
- Liang, S.; Guo, J.; Choi, J.-W.; Kim, N.-H.; Cui, X.-S. Effect and Possible Mechanisms of Melatonin Treatment on the Quality and Developmental Potential of Aged Bovine Oocytes. Reprod. Fertil. Dev. 2017, 29, 1821. [Google Scholar] [CrossRef]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The Mitophagy Activator Urolithin A Is Safe and Induces a Molecular Signature of Improved Mitochondrial and Cellular Health in Humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A Induces Mitophagy and Prolongs Lifespan in C. Elegans and Increases Muscle Function in Rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Identification of Novel Urolithin Metabolites in Human Feces and Urine after the Intake of a Pomegranate Extract. J. Agric. Food Chem. 2019, 67, 11099–11107. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cui, Y.; Yang, F.; Xu, Z.; Da, L.-T.; Zhang, Y. Inhibition of Polypeptide N-Acetyl-α-Galactosaminyltransferases Is an Underlying Mechanism of Dietary Polyphenols Preventing Colorectal Tumorigenesis. Bioorgan. Med. Chem. 2019, 27, 3372–3382. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Saleem, Y.I.; Albassam, H.; Selim, M. Urolithin A Induces Prostate Cancer Cell Death in P53-Dependent and in P53-Independent Manner. Eur. J. Nutr. 2020, 59, 1607–1618. [Google Scholar] [CrossRef]
- Gong, Z.; Huang, J.; Xu, B.; Ou, Z.; Zhang, L.; Lin, X.; Ye, X.; Kong, X.; Long, D.; Sun, X.; et al. Urolithin A Attenuates Memory Impairment and Neuroinflammation in APP/PS1 Mice. J. Neuroinflamm. 2019, 16, 62. [Google Scholar] [CrossRef]
- Kang, I.; Kim, Y.; Tomás-Barberán, F.A.; Espín, J.C.; Chung, S. Urolithin A, C, and D, but Not Iso-Urolithin A and Urolithin B, Attenuate Triglyceride Accumulation in Human Cultures of Adipocytes and Hepatocytes. Mol. Nutr. Food Res. 2016, 60, 1129–1138. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; Choya-Foces, C.; Hugo, M.; López, V. The Metabolite Urolithin-A Ameliorates Oxidative Stress in Neuro-2a Cells, Becoming a Potential Neuroprotective Agent. Antioxidants 2020, 9, 177. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Zhang, Z.; Qiu, L.; Ding, S.; Xue, J.; Zhao, G.; Li, J. Antiaging Effects of Urolithin A on Replicative Senescent Human Skin Fibroblasts. Rejuvenation Res. 2019, 22, 191–200. [Google Scholar] [CrossRef]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J.; et al. Long-Term Melatonin Treatment Delays Ovarian Aging. J. Pineal Res. 2017, 62, e12381. [Google Scholar] [CrossRef]
- Teixeira, C.; Marques, C.; Baptista, M.; Pimenta, J.; Teixeira, J.; Cagide, F.; Borges, F.; Montezinho, L.; Oliveira, P.; Pereira, R. Effect of a New Mitochondriotropic Antioxidant on Oocyte Maturation and Embryo Production. In Book of Abstracts of the 71st Annual Meeting of the European Federation of Animal Science; Wageningen Academic Publishers: Wageningen, The Netherlands, 2020. [Google Scholar]
- Wang, T.; Gao, Y.-Y.; Chen, L.; Nie, Z.-W.; Cheng, W.; Liu, X.; Schatten, H.; Zhang, X.; Miao, Y.-L. Melatonin Prevents Postovulatory Oocyte Aging and Promotes Subsequent Embryonic Development in the Pig. Aging 2017, 9, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the Gut Barrier Integrity by a Microbial Metabolite through the Nrf2 Pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.; Mesquita, P.; Marques, C.C.; Baptista, M.C.; Pimenta, J.; Matos, J.E.; Soveral, G.; Pereira, R.M.L.N. Modulation of P2Y2 Receptors in Bovine Cumulus Oocyte Complexes: Effects on Intracellular Calcium, Zona Hardening and Developmental Competence. Purinergic Signal. 2020, 16, 85–96. [Google Scholar] [CrossRef]
- Pereira, R.M.; Marques, C.C.; Baptista, M.C.; Vasques, M.I.; Horta, A.E.M. Embryos and Culture Cells: A Model for Studying the Effect of Progesterone. Anim. Reprod. Sci. 2009, 111, 31–40. [Google Scholar] [CrossRef]
- Lapa, M.; Marques, C.; Alves, S.; Vasques, M.; Baptista, M.; Carvalhais, I.; Silva Pereira, M.; Horta, A.; Bessa, R.; Pereira, R. Effect of Trans-10 Cis-12 Conjugated Linoleic Acid on Bovine Oocyte Competence and Fatty Acid Composition: Effects of T10,C12 CLA on Bovine Oocytes. Reprod. Domest. Anim. 2011, 46, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.; Marques, C.C.; da Conceição Baptista, M.; Vasques, M.I.; Horta, A.E. Effect of Arachidonic Acid Supplementation and Cyclooxygenase/Lipoxygenase Inhibition on the Development of Early Bovine Embryos. Rev. Bras. Zootec. 2006, 35, 422–427. [Google Scholar] [CrossRef][Green Version]
- Bó, G.A.; Mapletoft, R.J. Evaluation and Classification of Bovine Embryos. Anim. Reprod. 2013, 10, 344–348. [Google Scholar]
- Endo, M.; Kimura, K.; Kuwayama, T.; Monji, Y.; Iwata, H. Effect of Estradiol during Culture of Bovine Oocyte–Granulosa Cell Complexes on the Mitochondrial DNA Copies of Oocytes and Telomere Length of Granulosa Cells. Zygote 2014, 22, 431–439. [Google Scholar] [CrossRef]
- Moore, S.G.; Hasler, J.F. A 100-Year Review: Reproductive Technologies in Dairy Science. J. Dairy Sci. 2017, 100, 10314–10331. [Google Scholar] [CrossRef] [PubMed]
- Romão, R.; Marques, C.C.; Bettencourt, E.; Pereira, R.M.L.N. Cryopreservation of Sheep Produced Embryos—Current and Future Perspectives. In Insights from Animal Reproduction; Payan Carreira, R., Ed.; InTech: Rijeka, Croatia, 2016; ISBN 978-953-51-2268-5. [Google Scholar]
- Takeo, S.; Kimura, K.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Age-Associated Deterioration in Follicular Fluid Induces a Decline in Bovine Oocyte Quality. Reprod. Fertil. Dev. 2017, 29, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Mattern, F.; Herrmann, D.; Heinzmann, J.; Hadeler, K.G.; Bernal-Ulloa, S.M.; Haaf, T.; Niemann, H. DNA Methylation and MRNA Expression of Developmentally Important Genes in Bovine Oocytes Collected from Donors of Different Age Categories. Mol. Reprod. Dev. 2016, 83, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Iwata, H.; Goto, H.; Shiratuki, S.; Tanaka, H.; Monji, Y.; Kuwayama, T. Effect of Maternal Age on the Developmental Competence and Progression of Nuclear Maturation in Bovine Oocytes. Mol. Reprod. Dev. 2010, 77, 595–604. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Roura, M.; Catalá, M.G.; Menéndez-Blanco, I.; Izquierdo, D.; Fouladi-Nashta, A.A.; Paramio, M.T. Beneficial Effects of Melatonin on in Vitro Embryo Production from Juvenile Goat Oocytes. Reprod. Fertil. Dev. 2018, 30, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Cunha, M.C.; Mesquita, L.G.; Bressan, F.; Del Collado, M.; Balieiro, J.C.C.; Schwarz, K.R.L.; de Castro, F.C.; Watanabe, O.Y.; Watanabe, Y.F.; de Alencar Coelho, L.; et al. Effects of Melatonin during IVM in Defined Medium on Oocyte Meiosis, Oxidative Stress, and Subsequent Embryo Development. Theriogenology 2016, 86, 1685–1694. [Google Scholar] [CrossRef]
- Mukherjee, A.; Malik, H.; Saha, A.P.; Dubey, A.; Singhal, D.K.; Boateng, S.; Saugandhika, S.; Kumar, S.; De, S.; Guha, S.K.; et al. Resveratrol Treatment during Goat Oocytes Maturation Enhances Developmental Competence of Parthenogenetic and Hand-Made Cloned Blastocysts by Modulating Intracellular Glutathione Level and Embryonic Gene Expression. J. Assist. Reprod. Genet. 2014, 31, 229–239. [Google Scholar] [CrossRef]
- Sovernigo, T.C.; Adona, P.R.; Monzani, P.S.; Guemra, S.; Barros, F.; Lopes, F.G.; Leal, C. Effects of Supplementation of Medium with Different Antioxidants during in Vitro Maturation of Bovine Oocytes on Subsequent Embryo Production. Reprod. Domest. Anim. 2017, 52, 561–569. [Google Scholar] [CrossRef]
- Goto, H.; Iwata, H.; Takeo, S.; Nisinosono, K.; Murakami, S.; Monji, Y.; Kuwayama, T. Effect of Bovine Age on the Proliferative Activity, Global DNA Methylation, Relative Telomere Length and Telomerase Activity of Granulosa Cells. Zygote 2013, 21, 256–264. [Google Scholar] [CrossRef]
- Pasquariello, R.; Ermisch, A.F.; Silva, E.; McCormick, S.; Logsdon, D.; Barfield, J.P.; Schoolcraft, W.B.; Krisher, R.L. Alterations in Oocyte Mitochondrial Number and Function Are Related to Spindle Defects and Occur with Maternal Aging in Mice and Humans. Biol. Reprod. 2019, 100, 971–981. [Google Scholar] [CrossRef]
- Lord, T.; Martin, J.H.; Aitken, R.J. Accumulation of Electrophilic Aldehydes during Postovulatory Aging of Mouse Oocytes Causes Reduced Fertility, Oxidative Stress, and Apoptosis. Biol. Reprod. 2015, 92, 33. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Yao, X.-R.; Zhao, Y.-H.; Gao, Q.-S.; Jin, Q.-G.; Li, Y.-H.; Yan, A.-G.; Xu, Y.-N. L-Carnitine Prevents Bovine Oocyte Aging and Promotes Subsequent Embryonic Development. J. Reprod. Dev. 2019, 65, 499–506. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Y.; Zhao, Y.; Gao, Q.; Jin, Q.; Yan, C.; Xu, Y. L-Carnitine Supplementation during in Vitro Culture Regulates Oxidative Stress in Embryos from Bovine Aged Oocytes. Theriogenology 2020, 143, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.N.; Wason, E.; Edrey, Y.H.; Kristan, D.M.; Nevo, E.; Buffenstein, R. Regulation of Nrf2 Signaling and Longevity in Naturally Long-Lived Rodents. Proc. Natl. Acad. Sci. USA 2015, 112, 3722–3727. [Google Scholar] [CrossRef]
- Ma, R.; Liang, W.; Sun, Q.; Qiu, X.; Lin, Y.; Ge, X.; Jueraitetibaike, K.; Xie, M.; Zhou, J.; Huang, X.; et al. Sirt1/Nrf2 Pathway Is Involved in Oocyte Aging by Regulating Cyclin B1. Aging 2018, 10, 2991–3004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xi, Q.; Wang, D.; Li, J.; Wang, M.; Li, D.; Zhu, L.; Jin, L. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress Involved in Oocyte Aging: An Analysis Using Single-Cell RNA-Sequencing of Mouse Oocytes. J. Ovarian Res. 2019, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Ogino, M.; Tsubamoto, H.; Sakata, K.; Oohama, N.; Hayakawa, H.; Kojima, T.; Shigeta, M.; Shibahara, H. Mitochondrial DNA Copy Number in Cumulus Cells Is a Strong Predictor of Obtaining Good-Quality Embryos after IVF. J. Assist. Reprod. Genet. 2016, 33, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Taugourdeau, A.; Desquiret-Dumas, V.; Hamel, J.F.; Chupin, S.; Boucret, L.; Ferré-L’Hotellier, V.; Bouet, P.E.; Descamps, P.; Procaccio, V.; Reynier, P.; et al. The Mitochondrial DNA Content of Cumulus Cells May Help Predict Embryo Implantation. J. Assist. Reprod. Genet. 2019, 36, 223–228. [Google Scholar] [CrossRef] [PubMed]

| Gene Symbol | Gene ID | Primers Concentration | Primer Pairs (5′–3′) |
|---|---|---|---|
| NFE2L2 | 497024 | 200 nM | F: GTCGTCGGGGAGCCTCAAAG R: ATGTCAATCAAATCCATGTCCTGCT |
| NQO1 | 519632 | 100 nM | F: CATGGCTGTCAGAAAAGCACTG R: GGTCTGACACAGTGACCTCC |
| mt-ND5 | - | 75 nM | F: ATTTACAGCAATATGCGCCC R: AAAAGGCGTGGGTACAGATG |
| β-actin | 280979 | 200 nM | F: AGTCGGTTGGATCGAGCATT R: GCTTTTGGGAAGGCAAAGGAC |
| Groups | Oocytes | Cleavage | D7 Embryos | |||
|---|---|---|---|---|---|---|
| (n) | (n) | (%) | (n) | (%) | ||
| Prepubertal | Cont 22 h | 148 | 110 | 74.7 ± 3.6 ab | 16 | 13.8 ± 3.3 bc |
| UA 22 h | 155 | 125 | 80.6 ± 3.2 a | 26 | 20.5 ± 3.6 ab | |
| Cont Aged 30 h | 149 | 99 | 66.9 ± 3.9 b | 8 | 7.7 ± 2.6 c | |
| UA Aged 30 h | 144 | 97 | 67.2 ± 4.0 b | 19 | 18.9 ± 4.0 ab | |
| Adult | Cont 22 h | 155 | 120 | 81.4 ± 3.2 a | 19 | 15.9 ± 3.4 bc |
| UA 22 h | 129 | 111 | 80.9 ± 3.4 a | 30 | 26.8 ± 4.3 a | |
| Cont Aged 30 h | 148 | 112 | 73.0 ± 3.6 ab | 9 | 7.7 ± 2.6 c | |
| UA Aged 30 h | 138 | 84 | 66.5 ± 4.2 b | 14 | 15.9 ± 4.0 bc | |
| Groups | D7 Embryos | ||||||
|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | |||||
| (n) | (%) | (n) | (%) | (n) | (%) | ||
| Prepubertal | Control 22 h | 5 | 31.2 ± 11.8 | 10 | 58.5 ± 12.9 | 1 | 7.3 ± 7.2 |
| UA 22 h | 11 | 43.4 ± 10.4 | 13 | 60.6 ± 10.4 | 1 | 1.5 ± 1.7 | |
| Cont Aged 30 h | 4 | 41.5 ± 16.9 | 4 | 44.3 ± 17.4 | 4 | 0.0 ± 0.0 | |
| UA Aged 30 h | 4 | 20.11 ± 9.2 | 9 | 45.9 ± 11.8 | 6 | 33.8 ± 12.2 | |
| Adult | Control 22 h | 8 | 41.4 ± 11.5 | 9 | 45.5 ± 11.9 | 2 | 10.0 ± 6.6 |
| UA 22 h | 14 | 43.8 ± 9.5 | 12 | 38.9 ± 9.4 | 4 | 13.7 ± 6.7 | |
| Cont Aged 30 h | 4 | 40.7 ± 17.1 | 4 | 46.3 ± 17.5 | 4 | 10.0 ± 10.0 | |
| UA Aged 30 h | 5 | 33.9 ± 13.0 | 7 | 52.0 ± 14.0 | 2 | 11.2 ± 8.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, É.; Marques, C.C.; Pimenta, J.; Jorge, J.; Baptista, M.C.; Gonçalves, A.C.; Pereira, R.M.L.N. Anti-Aging Effect of Urolithin A on Bovine Oocytes In Vitro. Animals 2021, 11, 2048. https://doi.org/10.3390/ani11072048
Fonseca É, Marques CC, Pimenta J, Jorge J, Baptista MC, Gonçalves AC, Pereira RMLN. Anti-Aging Effect of Urolithin A on Bovine Oocytes In Vitro. Animals. 2021; 11(7):2048. https://doi.org/10.3390/ani11072048
Chicago/Turabian StyleFonseca, Élisa, Carla Cruz Marques, Jorge Pimenta, Joana Jorge, Maria Conceição Baptista, Ana Cristina Gonçalves, and Rosa M. L. N. Pereira. 2021. "Anti-Aging Effect of Urolithin A on Bovine Oocytes In Vitro" Animals 11, no. 7: 2048. https://doi.org/10.3390/ani11072048
APA StyleFonseca, É., Marques, C. C., Pimenta, J., Jorge, J., Baptista, M. C., Gonçalves, A. C., & Pereira, R. M. L. N. (2021). Anti-Aging Effect of Urolithin A on Bovine Oocytes In Vitro. Animals, 11(7), 2048. https://doi.org/10.3390/ani11072048






