A Review of the Nursery Culture of Mud Crabs, Genus Scylla: Current Progress and Future Directions
Abstract
:Simple Summary
Abstract
1. Introduction
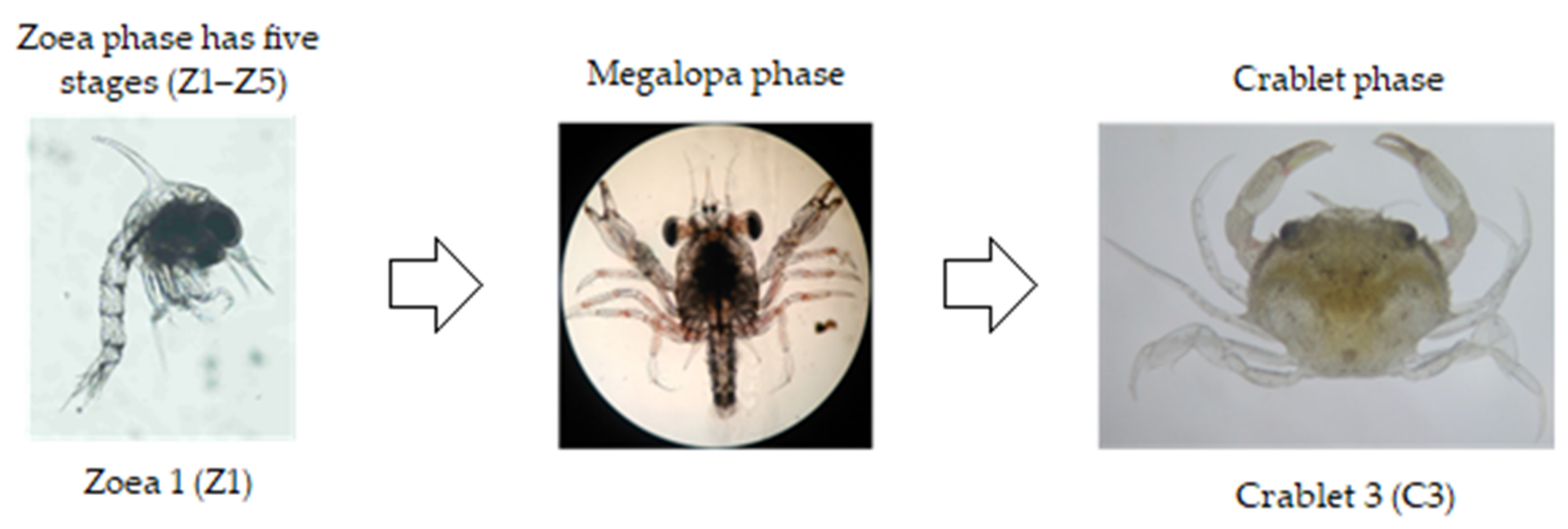
2. Mud Crab Nursery
3. Mud Crab Nursery Techniques
3.1. Nursery Area
3.2. Rearing Techniques
| Initial Stage of Mud Crab (Scylla spp.) | Treatment | Stocking Density | Rearing Medium | Type of Shelter | Feeding | Rearing Duration (Days) | SR (%) | SGR-CW (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| M to C1 (S. serrata) | Feeding regimes: (a) Artemia nauplii, (b) boosted Artemia, (c) dried shrimp (Acetes spp.), (d) dried mud worm (Marphysa spp.) | 10 ind/L | Bowl of 5 L volume (filled 3 L culture water) | none | (a) (b) (c) (d) (a,b) (a,c) (a,d) (b,c) (b,d) (c,d) | Until M metamorphosed to crabs or died | 38.9 46.7 12.8 6.7 49.4 40.0 57.8 45.6 41.7 6.1 | [16] | |
| Z5 and M (mix) to C2–3 (S. serrata) | Feeding regimes | 133 ind/m2 | Aquarium | Paranet pieces | Artemia nauplii only Artemia nauplii + PL (post larvae) shrimp feed Artemia nauplii + dried shrimp Artemia nauplii + shrimp feed | 14 | 26.66 31.66 20.00 11.66 | [19] | |
| M to C1 (Scylla spp.) | Salinity 24–30 ppt Temperature 25–30 °C pH 7.5–8.5 Water depth 60–80 cm | 30 ind/m2 | 20 m2 (4 × 5 m) net cages | Bunched black nets and coconut fronds | Phytoplankton and zooplankton grown with organic and inorganic fertilisers | 30 | 30–50 | [22] | |
| M to crablet stages (S. serrata) | Stocking density | 10 ind/m2 20 ind/m2 30 ind/m2 | Net cages | Dried coconut fronds | Macerated brown mussel meat (Modiolus metcalfei) or fish (20–30% of biomass) | 30 | 53.33 48.30 50.00 | [27] | |
| M to crablet stages (S. serrata) | Salinity 26 ppt Salinity 32 ppt | 1000 ind/m3 | Concrete tanks and net cages | PVC pipe cuttings, black nets, seaweed Gracilariopsis bailinae | Artemia nauplii, adult artemia, trash fish, green mussel or Acetes | 18 | 40.10 26.20 | [38] | |
| M to C1 (S. serrata) | Feeding regimes | 12 ind/L | Tall conical-based plastic | None | 100% MBD 100% Artemia 75% MBD:25% Artemia 50% MBD:50% Artemia 25% MBD:75% Artemia | Until M metamorphosed to C1or died | ±6.5 ±5.0 ±5.0 ±3.5 ±8.5 | [39] | |
| M to C1 (S. serrata) | Feeding regimes | Individually | Round flat-bottomed plastic | None | 100% MBD 100% Artemia | Until M metamorphosed to C1 or died | 90 90 | [39] | |
| M to C1 (S. serrata) | Feeding regimes: Microbound diets (MBD) | Individually | Flat-bottomed circular aquaria (250 mL) | None | MBD (prepared using dried rotifers) MBD (prepared using Artemia meal) MBD (prepared using squid meal) MBD (prepared using fish meal) Live Artemia | Until M metamorphosed to C1 or died | 46.67 46.67 60.00 60.00 80.00 | [40] | |
| M to C1 (S. serrata) | Feeding regimes: MBD prepared using squid meal (containing different levels of dietary cholesterol) | Individually | Flat-bottomed aquaria (250 mL) | None | MBD + 0.14% cholesterol level MBD + 0.20% cholesterol level MBD + 0.40% cholesterol level MBD + 0.80% cholesterol level MBD + 1.00% cholesterol level Live Artemia | Until M metamorphosed to C1or died | 26.70 60.00 53.30 73.30 46.70 53.30 | [41] | |
| M to C1 (S. serrata) | Feeding regimes: MBD (containing various levels of supplemental dietary lecithin and cholesterol) | Individually | Flat-bottomed aquaria (250 mL) | None | MBD (containing lecithin 5.5 g.kg−1 + cholesterol 0.3 kg−1) MBD (containing lecithin 5.2 g.kg−1 + cholesterol 7.9 kg−1) MBD (containing lecithin 23.1 g.kg−1 + cholesterol 0.1 kg−1) MBD (containing lecithin 27.8 g.kg−1 + cholesterol 10.7 kg−1) MBD (containing lecithin 39.7 g.kg−1 + cholesterol 0.6 kg−1) MBD (containing lecithin 44.1 g.kg−1 + cholesterol 8.8 kg−1) Live Artemia | Until M metamorphosed to C1 or died | 20 27 33 53 60 60 60 | [42] | |
| M to C1 (S. serrata) | Feeding regimes: MBD (containing various fish oil:corn oil ratios) | Individually | Flat-bottomed circular aquaria (250 mL) | None | MBD (fish oil:corn oil = 0:1) MBD (fish oil:corn oil = 1:2) MBD (fish oil:corn oil = 2:1) MBD (fish oil:corn oil = 3:1) MBD (fish oil:corn oil = 1:0) MBD (fish oil:corn oil = 1:1) Live Artemia | Until M metamorphosed to C1 or died | 35 55 60 65 65 70 60 | [43] | |
| M to crablet stages (S. paramamosain) | Temp. 20 °C Temp. 24 °C Temp. 28 °C Temp. 32 °C Temp. 36 °C Temp. Ambient (27–30 °C) | Individually | Plastic cup with diameter of 6–9 cm | None | Frozen Artemia nauplii, frozen adult Artemia and artificial feed | 45 | 0 86.67 96.67 80.00 0 93.33 | 0 3.74 4.50 3.95 0 4.38 | [24] |
| M to C1 (S. paramamosain) | Feeding regimes | Individually | Plastics beakers (0.5 L) | None | Live Acetes (LA) Minced shrimp meat (MSM) Locally formulated feed (LFF) Commercial feed (CF) LA + MSM LA + LFF LA + CF | Until M metamorphosed to C1 or died | 85–100 | [44] | |
| M to C1 (S. paramamosain) | Feeding regimes | Communal (250 ind./hole | Earthen dugout holes 60 × 60 × 20 cm (length × width × depth) | Live Acetes (LA) Minced shrimp meat (MSM) Locally formulated feed (LFF) Commercial feed (CF) LA + MSM LA + LFF LA + CF | 10 | 47.9–87.5 | [44] | ||
| C1 to several crablet stages (S. paramamosain) | Stocking density | 110 ind/m2 175 ind/m2 230 ind/m2 | PVC containers | Sand substrate | Artemia biomass and chopped peeled shrimp | 15 | 71.3 61.7 57.5 | [28] | |
| C1 to several crablet stages (S. paramamosain) | Shelter | 110 ind/m2 | Cement tank | Clay brick Without clay brick | Peeled shrimp | 17 | 25.3 13.5 | [28] | |
| C1 to several crablet stages (S. paramamosain) | Shelter types | 100 ind/m2 | Composite tank | Bricks Clamshell Without shelter | Peeled shrimp | 21 | 40 41 30 | [28] | |
| Crablets of 7–10 days old (±C3) (S. olivacea) | Rearing medium | 50 ind/m2 | Fibre tank Hapa net | Paranet pieces | Acetes spp. | 21 | 21.33 37.33 | [31] | |
| Z5 and M (mix) to crablet (day 7) (S. olivacea) | Rearing medium | 5000 ind/tank 1500 ind/tank 1360 ind/tank | Circular fiberglass tank Rectangular cement tank Circular cement tank | None | Enriched Artemia nauplii | 13 | 40.14 34.65 22.67 | [32] | |
| Z5 and M to C3 (S. tranquebarica) | Feeding regimes | Communally | Fiberglass tanks (vol. ± 4 tons) | Paranet pieces | Live Artemia nauplii + shrimp meat Live Artemia nauplii and artificial feed (shrimp post-larvae feed) | 14 | 16.46 5.72 | [20] | |
| Z4–Z5 to C1 (S. olivacea) | Feeding regimes: substitution of nauplii Artemia (NA) with microdiet (MD) | 12 ind./L | Conical fiberglass tank (filled with 150 L of seawater) | NA 100% NA 75% + MD 25% NA 50% + MD 50% NA 25% + MD 75% MD 100% | 15 | 2.42 4.22 5.61 4.89 2.1 | [45] |
3.2.1. Seed Stocking Strategies
3.2.2. Age of Megalopa
3.2.3. Transportation of Megalopa Stage
3.2.4. Feeding Strategies
4. Water Quality
| Parameters | Optimum Range/Value | Sampling Frequency |
|---|---|---|
| Dissolved oxygen (DO) | >5 ppm (mud crabs are tolerant of low oxygen levels) [60] | Twice a day |
| pH | 7.5–8.5(<0.5 daily variation) [59] | Twice a day |
| Temperature | 28–30 °C [24,30,66,68] | Daily |
| Salinity | 20–25 ppt [30,33,61,62,63,64] | Daily |
| Total ammonia nitrogen (TAN) | <3 ppm (crablets have a tolerance to high ammonia) [60] | Weekly |
| Un-ionised ammonia (NH3) | <0.01 ppm [59] | Weekly |
| Nitrite (NO2) | <10 ppm at salinity >15 ppt; <5 ppm at salinity <15 ppt [60] | Weekly |
| Alkalinity | >80 ppm (ideally 120 ppm) [60] | Weekly |
| Hardness | >2000 ppm [60] | Weekly |
| Hydrogensulphide | <0.1 ppm [60] | Weekly |
| Turbidity | 20–30 cm [60] | Daily |
5. Harvest and Transportation of Crablets
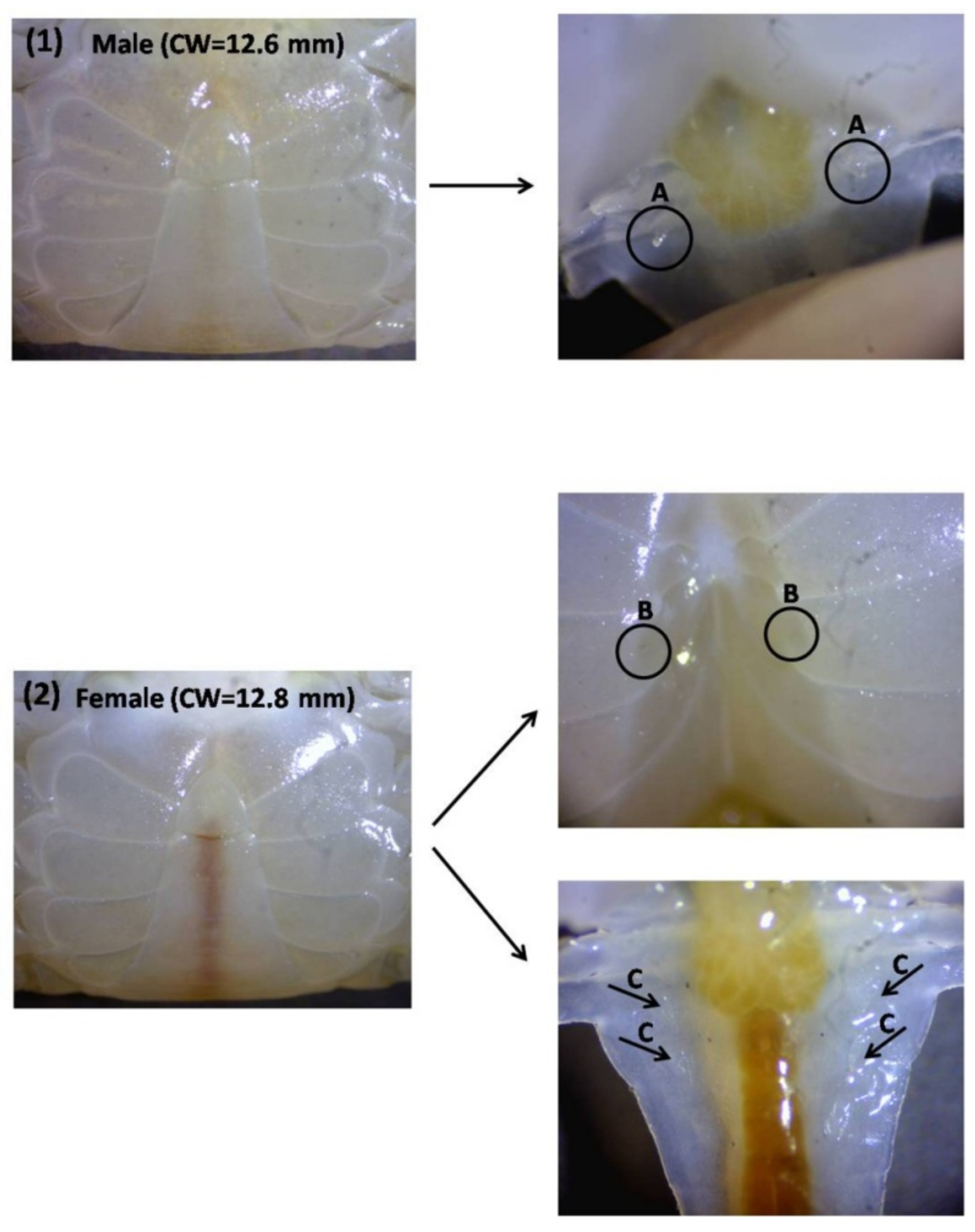
6. Sex Differentiation in the Crablet Stage
7. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sathiadhas, R.; Najmudeen, T. Economic evaluation of mud crab farming under different production systems in India. Aquac. Econ. Manag. 2004, 8, 99–110. [Google Scholar] [CrossRef]
- Waiho, K.; Fazhan, H.; Quinitio, E.T.; Baylon, J.C.; Fujaya, Y.; Azmie, G.; Wu, Q.; Shi, X.; Ikhwanuddin, M.; Ma, H. Larval rearing of mud crab (Scylla): What lies ahead. Aquaculture 2018, 493, 37–50. [Google Scholar] [CrossRef]
- Gunarto; Syafaat, M.N.; Herlinah; Parenrengi, A.; Mustafa, A. Biological Aspects and Seed Production Technique of Mud Crabs Scylla spp.; Amafrad Press: Jakarta, Indonesian, 2016; p. 62. [Google Scholar]
- Fazhan, H.; Waiho, K.; Norfaizza, W.I.W.; Megat, F.H.; Ikhwanuddin, M. Inter-species mating among mud crab genus Scylla in captivity. Aquaculture 2017, 471, 49–54. [Google Scholar] [CrossRef]
- Fazhan, H.; Waiho, K.; Wee, H.B.; Surzanne, M.A.; Ma, H.; Ikhwanuddin, M. Predicting the sacculinid Sacculina beauforti infection status of the orange mud crab Scylla olivacea by discriminant analysis. Aquaculture 2018, 491, 128–134. [Google Scholar] [CrossRef]
- Ikhwanuddin, M.; Lan, S.S.; Abdul Hamid, N.; Fatiha, Z.S.N.; Azra, M.N.; Siti Aisah, A.; Abol-Munafi, A.B. The embryonic development of orange mud crab, Scylla olivacea (Herbst, 1796) held in captivity. Iran. J. Fish. Sci. 2015, 14, 885–895. [Google Scholar]
- Ikhwanuddin, M.; Ghazali, A.; Nahar, S.F.; Wee, W.; Azra, M.N.; Abol-Munafi, A.B. Testis maturation stages of mud crab (Scylla olivacea) broodstock on different diets. Sains Malays. 2018, 47, 427–432. [Google Scholar] [CrossRef]
- Triño, A.T.; Rodriguez, E.M. Mud crab (Scylla serrata) culture in tidal flats with existing mangroves. In Mangrove-Friendly Aquaculture: Proceedings of the Workshop on Mangrove Friendly Aquacultureorganized by the SEAFDEC Aquaculture Department, Iloilo City, Philippines, 11–15 January 1999; Garcia, L.M.B., Castaños, M.T., Surtida, M.B., Eds.; Aquaculture Department, Southeast Asian Fisheries Development Center: Tigbauan, Philippines, 2000; pp. 171–176. [Google Scholar]
- Khatun, M.M.; Kamal, D.; Ikejima, K.; Yi, Y. Comparisons of growth and economic performance among monosex and mixed-sex culture of red mud crab (Scylla olivacea Herbst, 1796) in bamboo pens in the tidal flats of mangrove forests, Bangladesh. Aquac. Res. 2009, 40, 473–485. [Google Scholar] [CrossRef]
- Widodo, A.F.; Sulaeman; Jompa, H. Grow out of mud crab, Scylla serrata in mangrove area using silvo-fishery system. In Proceedings of the Seminar Nasional Limnologi V, Bogor, Indonesian, 1 December 2010; pp. 750–759. [Google Scholar]
- Pillai, S.L.; Rajapackiam, S.; Sunderarajan, D. Mud crab Scylla tranquebarica culture in earthen pond at Tuticorin. Mar. Biol. Ass. India. 2002, 44, 245–248. [Google Scholar]
- Syafaat, M.N.; Gunarto. The grow-out cultures of hatchery-produced mud crab Scylla tranquebarica (fabricius, 1798) cultured at different pond locations. Media Akuakultur 2018, 13, 21–30. [Google Scholar] [CrossRef]
- Herlinah; Sulaeman; Widodo, A.F.; Gunarto. Application of box system as effort to increase of mud crab (Scylla serrata) grow out production in concrete pond. In Proceedings of the Seminar Nasional Tahunan VIII Hasil Penelitian Perikanan dan Kelautan, Gajah Mada University, Yogyakarta, Indonesian, October 2011; p. 8. [Google Scholar]
- Syafaat, M.N.; Gunarto; Sahabuddin. Water quality condition of mud crab, Scylla paramamosain culture using recirculating aquaculture system at different stocking density. In Proceedings of the Forum Inovasi Teknologi Akuakultur 2015, Jakarta, Indonesian, October 2015; pp. 781–788. [Google Scholar]
- Islam, M.L.; Siddiky, M.N.M.; Yahya, K. Age and size at sexual maturity of same age group of male green mud crab (Scylla paramamosain). J. Entomol. Zool. 2018, 6, 366–371. [Google Scholar]
- Williams, G.R.; Wood, J.; Dalliston, B.; Shelley, C.C.; Kuo, C.M. Mud crab (Scylla serrata) megalopa larvae exhibit high survival rates on Artemia-based diets. In Mud Crab Aquaculture and Biology, ACIAR Proceeding; Keenan, C.P., Blackshaw, A., Eds.; ACIAR: Canberra, Australia, 1999; pp. 131–140. [Google Scholar]
- Hamasaki, K.; Obata, Y.; Dan, S.; Kitada, S. A review of seed production and stock enhancement for commercially important portunid crabs in Japan. Aquac. Int. 2010, 19, 217–235. [Google Scholar] [CrossRef]
- Kulasekarapandian, S.; Panigrahi, A. Biology and fishery of mud crabs. In Anonim. Training Manual on Mud Crab Breeding and Culture; Central Institute of Brackishwater Aquaculture: Chennai, India, 2009; pp. 1–9. [Google Scholar]
- Syafaat, M.N.; Gunarto; Usman. Rearing of mud crab (Scylla serrata) megalopa to crablet stages with different additional feed types. In Proceedings of the Forum Inovasi Teknologi Akuakultur 2016, Jakarta, Indonesian, 31 August 2016; pp. 209–214. [Google Scholar]
- Syafaat, M.N.; Gunarto; Sulaeman; Herlinah; Ma, H.; Ikhwanuddin, M. Effects of different feeding regimes on larvae and crablets of purple mud crab, Scylla tranquebarica (Fabricius, 1798). Aquac. Rep. 2019, 15, 100231. [Google Scholar] [CrossRef]
- Mann, D.; Paterson, B. Status of crab seed production and grow-out in Queensland. In Mud Crab Aquaculture in Australia and Southest Asia, Proceedings of the ACIAR Crab Aquaculture Scoping Study and Workshop, Bribie Island, Australia, 28–29 April 2003; Allan, G., Fielder, D., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 2003; pp. 36–41. [Google Scholar]
- Onn, K.K. Current practices in juvenile mud crab rearing. Aquac. Asia Pac. 2013, 9, 44–46. [Google Scholar]
- Quinitio, E.T.; Parado-Estepa, F.D.; Rodriguez, E. Seed production of mud crab Scylla spp. Aquac. Asia 2002, 7, 29–31. [Google Scholar]
- Syafaat, M.N.; Mohammad, S.; Azra, M.N.; Ma, H.; Abol-Munafi, A.B.; Ikhwanuddin, M. Effect of water temperature on survival, growth and molting cycle during early crablet instar of mud crab, Scylla paramamosain (Estampador, 1950). Thalass. Int. J. Mar. Sci. 2020, 36, 543–551. [Google Scholar] [CrossRef]
- Syafaat, M.N. Effect of Water Temperature on Growth Performance, Moulting Cycle, Survival Rate and Sex Ratio of Mud Crab, Scylla Paramamosain during Nursery Phase. Master’s Thesis, Universiti Malaysia Terengganu, Terennganu, Malaysia, 2019. [Google Scholar]
- Hassan, A.; Hai, T.N.; Chatterji, A.; Sukumaran, N. Preliminary study on the feeding regime of laboratory reared mud crab larva, Scylla serrata (Forsskal, 1775). World Appl. Sci. J. 2011, 14, 1651–1654. [Google Scholar]
- Rodriguez, E.M.; Quinitio, E.T.; Parado-Estepa, F.D.; Millamena, O.M. Culture of Scylla serrata megalops in brackishwater ponds. Asian Fish. Sci. 2001, 14, 185–189. [Google Scholar]
- Ut, V.N.; Le Vay, L.; Nghia, T.T.; Hanh, T.T.H. Development of nursery culture techniques for the mud crab Scylla paramamosain (Estampador). Aquac. Res. 2007, 38, 1563–1568. [Google Scholar] [CrossRef]
- Ye, H.; Tao, Y.; Wang, G.; Lin, Q.; Chen, X.; Li, S. Experimental nursery culture of the mud crab Scylla paramamosain (Estampador) in China. Aquac. Int. 2010, 19, 313–321. [Google Scholar] [CrossRef]
- Ruscoe, I.M.; Shelley, C.C.; Williams, G.R. The combined effects of temperature and salinity on growth and survival of juvenile mud crabs (Scylla serrata Forskål). Aquaculture 2004, 238, 239–247. [Google Scholar] [CrossRef]
- Syafaat, M.N.; Gunarto; Sulaeman; Herlinah. Nursery of mud crab (Scylla olivacea Herbst, 1796) hatchery production seed in different containers. In Proceedings of the National Symposium of Marine and Fisheries IV, Hasanuddin University, Makassar, Indonesian, 9 May 2017; pp. 808–816. [Google Scholar]
- Gunarto; Nurbaya; Zakaria, M. Culture of Scylla olivacea zoea 5 and megalopa in different kind of tanks. In Proceedings of the Konferensi Akuakultur Indonesia 2013, Semarang, Indonesian, December 2013; pp. 28–36. [Google Scholar]
- Gunarto; Parenrengi, A. Crablet of mangrove crab, Scylla olivacea rearing at the different salinity regimes. J. Aquac. Res. Dev. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Mirera, O.; Moksnes, P.-O. Cannibalistic interactions of juvenile mud crabs Scylla serrata: The effect of shelter and crab size. Afr. J. Mar. Sci. 2013, 35, 545–553. [Google Scholar] [CrossRef]
- Gunarto. Effect of using the sea weed, Gracillaria spp. in mud crab juvenile’s nursery, Scylla olivacea in captivity. In Proceedings of the Seminar Nasional Perikanan Indonesia, State College of Fisheries, Jakarta, Indonesian, 2012; pp. 76–82. [Google Scholar]
- Lee, D.O.’C.; Wickins, J.F. Crustacean Farming; John Wiley & Sons, Inc.: New York, NY, USA, 1992. [Google Scholar]
- Macintosh, D.J.; Goncalves, F.; Soares, A.M.V.M.; Moser, S.M.; Paphavisit, N. Transport mechanisms of crab megalopae in mangrove ecosystems, with special reference to a mangrove estuary in Ranong, Thailand. In Mud Crab Aquaculture and Biology, ACIAR Proceeding; Keenan, C.P., Blackshaw, A., Eds.; ACIAR: Canberra, Australia, 1999; pp. 178–186. [Google Scholar]
- Quinitio, E.T.; Parado-Estepa, F.D.; Millamena, O.M.; Rodriguez, E.; Borlongan, E. Seed production of mud crab Scylla serrata juveniles. Asian Fish. Sci. 2001, 14, 161–174. [Google Scholar]
- Genodepa, J.; Zeng, C.; Southgate, P.C. Preliminary assessment of a microbound diet as an Artemia replacement for mud crab, Scylla serrata, megalopa. Aquaculture 2004, 236, 497–509. [Google Scholar] [CrossRef]
- Holme, M.-H.; Zeng, C.; Southgate, P.C. Use of microbound diets for larval culture of the mud crab, Scylla serrata. Aquaculture 2006, 257, 482–490. [Google Scholar] [CrossRef]
- Holme, M.-H.; Zeng, C.; Southgate, P.C. The effects of supplemental dietary cholesterol on growth, development and survival of mud crab, Scylla serrata, megalopa fed semi-purified diets. Aquaculture 2006, 261, 1328–1334. [Google Scholar] [CrossRef]
- Holme, M.-H.; Southgate, P.; Zeng, C. Assessment of dietary lecithin and cholesterol requirements of mud crab, Scylla serrata, megalopa using semi-purified microbound diets. Aquac. Nutr. 2007, 13, 413–423. [Google Scholar] [CrossRef]
- Holme, M.-H.; Southgate, P.C.; Zeng, C. Survival, development and growth response of mud crab, Scylla serrata, megalopae fed semi-purified diets containing various fish oil:corn oil ratios. Aquaculture 2007, 269, 427–435. [Google Scholar] [CrossRef]
- Ong, Q.M.; Fotedar, R.; Ho, T.T.T. Selection of locally available diets for rearing Scylla paramamosain megalopa to first crablet stage. Aquaculture 2020, 525, 735319. [Google Scholar] [CrossRef]
- Usman, U.; Kamaruddin, K.; Laining, A. Substitusi penggunaan nauplius Artemia dengan pakan mikro dalam pemeliharaan larva kepiting bakau, Scylla olivacea. J. Ris. Akuakultur 2018, 13, 29–38. [Google Scholar] [CrossRef]
- Antony, J.; Balasubramanian, C.P.; Balamurugan, J.; Sandeep, K.P.; Biju, I.F.; Vijayan, K.K. Optimisation of nursery rearing for megalopa of giant mud crab Scylla serrata (Forskal, 1775). Indian J. Fish. 2019, 66, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Quinitio, E.T.; Parado-Estepa, F.D. Transport of Scylla serrata megalopae at various densities and durations. Aquaculture 2000, 185, 63–71. [Google Scholar] [CrossRef]
- Heasman, M.; Fielder, D. Laboratory spawning and mass rearing of the mangrove crab, Scylla serrata (Forskal), from first zoea to first crab stage. Aquaculture 1983, 34, 303–316. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Q.; Wang, G.; Li, S.; Ye, H. Feeding in the megalopae of the mud crab (Scylla paramamosain): Mechanisms, plasticity, role of chelipeds and effect of prey density. Mar. Freshw. Behav. Physiol. 2013, 46, 321–336. [Google Scholar] [CrossRef]
- Serrano, A.E. Changes in gut evacuation time of larval mud crab, Scylla serrata (Crustacea:Portunidae) fed artificial plankton or live food. AACL Bioflux 2012, 5, 240–248. [Google Scholar]
- Serrano, A.E., Jr.; Traifalgar, R.F. Ontogeny and induction of digestive enzymes in Scylla serrata larvae fed live or artificial feeds or their combination. AACL Bioflux 2012, 5, 101–111. [Google Scholar]
- Gunarto, G.; Syafaat, M.N.; Herlinah, H.; Sulaeman, S.; Muliani, M. The effects of an artificial commercial feed supplementation on larval rearing and crablet production of mud crab Scylla tranquebarica. Indones. Aquac. J. 2018, 13, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Permadi, S.; Juwana, S. Production of crab seed Scylla paramamosain fed with Artemia nauplii and formulated diet containing spirulina and digezym. Oceanol. Limnol. Indones. 2015, 41, 181–189. (In Indonesian) [Google Scholar]
- Quinitio, E.T.; Estepa-Parado, F.; Alava, V. Development of hatchery techniques for the mud crab Scylla serrata (Forskal): Comparison of feeding schemes. In Mud Crab Aquaculture and Biology, ACIAR Proceeding; Keenan, C.P., Blackshaw, A., Eds.; ACIAR: Canberra, Australia, 1999; pp. 125–130. [Google Scholar]
- Taufik, M.; Bachok, Z.; Azra, M.N.; Ikhwanuddin, M. Effects of various microalgae on fatty acid composition and survival rate of the blue swimming crab Portunus pelagicus larvae. Indian J. Mar. Sci. 2016, 45, 1512–1521. [Google Scholar]
- Hamasaki, K.; Suprayudi, M.A.; Takeuchi, T. Effects of dietary N-3HUFA on larval morphogenesis and metamorphosis to megalops in the seed production of the mud crab, Scylla serrata (Brachyura: Portunidae). Suisanzoshoku 2002, 50, 333–340. [Google Scholar]
- Gunarto; Herlinah/. Level of crablet production in mangrove crab Scylla paramamosain with feeding enrichment using HUFA and vitamin C on larvae stages. J. IlmuTeknol. Kelaut. Trop. 2015, 7, 511–520. [Google Scholar]
- Rodriguez, E.M.; Parado-Estepa, F.D.; Quinitio, E.T. Extension of nursery culture of Scylla serrata (Forsskål) juveniles in net cages and ponds. Aquac. Res. 2007, 38, 1588–1592. [Google Scholar] [CrossRef]
- Ganesh, K.; Raj, Y.C.T.S.; Perumal, S.; Srinivasan, P.; Sethuramalingam, A. Breeding, larval rearing and farming of mangrove crab, Scylla serrata (Forskal, 1775). In Advances in Marine and Brackishwater Aquaculture; Springer Science and Business Media LLC: Berlin, Germany, 2015; pp. 163–172. [Google Scholar]
- Shelley, C.; Lovatelli, A. Mud Crab Aquaculture—A Practical Manual. Fisheries and Aquaculture Technical Paper No.567; FAO: Rome, Italy, 2011; p. 78. [Google Scholar]
- Nurdiani, R.; Zeng, C. Effects of temperature and salinity on the survival and development of mud crab, Scylla serrata (Forsskål), larvae. Aquac. Res. 2007, 38, 1529–1538. [Google Scholar] [CrossRef]
- Baylon, J.C. Effects of salinity and temperature on survival and development of larvae and juveniles of the mud crab, Scylla serrata (Crustacea: Decapoda: Portunidae). J. World Aquac. Soc. 2010, 41, 858–873. [Google Scholar] [CrossRef]
- Baylon, J.C. Survival and development of larvae and juveniles of the mud crab [Scylla olivacea Forskal (Crustacea: Decapoda: Portunidae)] at various temperatures and salinities. Philipp. Agric. Sci. 2011, 94, 195–204. [Google Scholar]
- Baylon, J.C. The combined effects of salinity and temperature on the survival and development of zoea, megalopa and crab instar larvae of mud crab, Scylla tranquebarica (Fabricius 1798). Asian Fish. Sci. 2013, 26, 14–25. [Google Scholar] [CrossRef]
- Azra, M.N.; Ikhwanuddin, M. Larval culture and rearing techniques of commercially important crab, Portunus pelagicus (Linnaeus, 1758): Present status and future prospects. Songklanakarin J. Sci. Technol. 2015, 37, 135–145. [Google Scholar]
- Gong, J.; Yu, K.; Shu, L.; Ye, H.; Li, S.; Zeng, C. Evaluating the effects of temperature, salinity, starvation and autotomy on molting success, molting interval and expression of ecdysone receptor in early juvenile mud crabs, Scylla paramamosain. J. Exp. Mar. Biol. Ecol. 2015, 464, 11–17. [Google Scholar] [CrossRef]
- Azra, M.N.; Ikhwanuddin, M.; Abol-Munafi, A.B. Behavioural data on instar crab movement at different thermal acclimation. Data Brief 2019, 22, 998–1002. [Google Scholar] [CrossRef]
- Syafaat, M.; Azra, M.; Mohamad, F.; Che-Ismail, C.; Amin-Safwan, A.; Asmat-Ullah, M.; Syahnon, M.; Ghazali, A.; Abol-Munafi, A.B.; Ma, H.; et al. Thermal tolerance and physiological changes in mud crab, Scylla paramamosain crablet at different water temperatures. Animals 2021, 11, 1146. [Google Scholar] [CrossRef]
- Wu, H.J.; Sun, L.B.; Li, C.B.; Li, Z.Z.; Zhang, Z.; Wen, X.B.; Hu, Z.; Zhang, Y.L.; Li, S.K. Enhancement of the immune response and protection against Vibrio parahaemolyticus by indigenous probiotic Bacillus strains in mud crab (Scylla paramamosain). Fish. Shellfish. Immunol. 2014, 41, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Yamin, M.; Sulaeman. Transportation of mud crab crablet (Scylla paramamosain) with dry system. In Proceedings of the Forum Inovasi Teknologi Akuakultur 2011, Jakarta, Indonesian, December 2011; pp. 1297–1302. [Google Scholar]
- Sulaeman; Yamin, M.; Parenrengi, A. Transport of mud crab (Scylla paramamosain) crablets at different packing densities. J. Riset Akuakultur 2008, 3, 99–104. [Google Scholar]
- Keenan, C.P.; Davie, P.; Mann, D. A revision of the genus Scylla De Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bull. Zool. 1998, 46, 217–245. [Google Scholar]
- Fazhan, H.; Waiho, K.; Fujaya, Y.; Rukminasari, N.; Ma, H.; Ikhwanuddin, M. Sexual dimorphism in mud crabs: A tale of three sympatric Scylla species. PeerJ 2021, 9, e10936. [Google Scholar] [CrossRef] [PubMed]
- Heasman, M.P. Aspects of the General Biology and Fishery of the Mud Crab Scylla Serrata (Forskal) in Moreton Bay, Queensland. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2016. [Google Scholar]
- Venugopal, G.; Rasvi, S.S.H.; Babu, S.P.P.; Reddy, P.R.; Mohan, K.M.; Rao, P.S.; Patnaik, R.R. Performance evaluation of mud crab Scylla serrata (Forskal, 1775) in monoculture, monosex culture and polyculture. J. Mar. Biol. Assoc. 2012, 54, 5–8. [Google Scholar] [CrossRef]
| Crablet Stage | Moulting Interval (Days) ± SD | Carapace Width (mm) ± SD | Carapace Length (mm) ± SD |
|---|---|---|---|
| C1 to C2 | 4.67 ± 0.50 | 3.41 ± 0.10 (C1) 4.80 ± 0.34 (C2) | 3.03 ± 0.10 (C1) 3.71 ± 0.32 (C2) |
| C2 to C3 | 5.88 ± 0.99 | 6.00 ± 0.25 (C3) | 4.55 ± 0.30 (C3) |
| C3 to C4 | 7.34 ± 1.03 | 7.83 ± 0.61 (C4) | 5.69 ± 0.40 (C4) |
| C4 to C5 | 9.33 ± 2.18 | 9.56 ± 0.73 (C5) | 6.78 ± 0.46 (C5) |
| C5 to C6 | 11.95 ± 2.14 | 12.08 ± 1.07 (C6) | 8.49 ± 0.69 (C6) |
| Life Stage of Mud Crabs (Days/Sizes) | Crablet (D20) S. paramamosain | Crablet (With Width Carapace Less Than 1 cm) S. paramamosain | Crablet (D37) S. tranquebarica |
|---|---|---|---|
| Methods | Wet | Dry (without water) | Wet |
| Medium | Plastic bags (filled with oxygen) | Plastic bags (filled with oxygen) | Styrofoam box (40 × 50 cm) |
| Density | 50, 100, 150 ind/pack | 200 and 300 crablets | 700 ind/box |
| Shelter | A nylon net (20 × 40 cm) | Wet cloth along with seaweed (Gracillaria spp.) | Paranet |
| Duration (h) | 5 | 5 | 6 |
| Survival rate (%) | 88–97 | 98–99 | >95 |
| Reference | [71] | [70] | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syafaat, M.N.; Azra, M.N.; Waiho, K.; Fazhan, H.; Abol-Munafi, A.B.; Ishak, S.D.; Syahnon, M.; Ghazali, A.; Ma, H.; Ikhwanuddin, M. A Review of the Nursery Culture of Mud Crabs, Genus Scylla: Current Progress and Future Directions. Animals 2021, 11, 2034. https://doi.org/10.3390/ani11072034
Syafaat MN, Azra MN, Waiho K, Fazhan H, Abol-Munafi AB, Ishak SD, Syahnon M, Ghazali A, Ma H, Ikhwanuddin M. A Review of the Nursery Culture of Mud Crabs, Genus Scylla: Current Progress and Future Directions. Animals. 2021; 11(7):2034. https://doi.org/10.3390/ani11072034
Chicago/Turabian StyleSyafaat, Muhammad Nur, Mohamad Nor Azra, Khor Waiho, Hanafiah Fazhan, Ambok Bolong Abol-Munafi, Sairatul Dahlianis Ishak, Mohammad Syahnon, Azmie Ghazali, Hongyu Ma, and Mhd Ikhwanuddin. 2021. "A Review of the Nursery Culture of Mud Crabs, Genus Scylla: Current Progress and Future Directions" Animals 11, no. 7: 2034. https://doi.org/10.3390/ani11072034
APA StyleSyafaat, M. N., Azra, M. N., Waiho, K., Fazhan, H., Abol-Munafi, A. B., Ishak, S. D., Syahnon, M., Ghazali, A., Ma, H., & Ikhwanuddin, M. (2021). A Review of the Nursery Culture of Mud Crabs, Genus Scylla: Current Progress and Future Directions. Animals, 11(7), 2034. https://doi.org/10.3390/ani11072034








