Simple Summary
Fish are influenced by their surroundings. We investigated the effect of water temperature ranging from 26 °C to 32 °C on the growth performance and gastric emptying time (GET) of commonly cultured African catfish Clarias gariepinus fingerlings. Water temperatures between 26 °C and 32 °C were satisfactory for the growth of African catfish fingerlings, with GET observed between 10 and 16 h. Our experiment provides baseline data of the effects of water temperature on the culture of catfish.
Abstract
The present study was carried out to analyse the effect of water temperature on two components: (1) growth performance, and (2) gastric emptying time (GET) of African catfish Clarias gariepinus fingerlings. After 70 days, it was observed that experimental temperatures had no significant effects on the growth performance parameters, except for food conversion ratio (FCR) and food conversion efficiency (FCE). GET observation through X-radiography denoted that the shortest GET (10 h) was observed in fish reared at 32 °C and the longest GET (16 h) was observed in fish reared at 26 °C. The rapid digestion rate coincides with the FCR and FCE obtained in this study. Considering the limited scope of our study, more extensive studies on the impact of water temperature on other fish physiological parameters should be pursued. A better understanding of this research topic would be beneficial for the growth of African catfish fingerling aquaculture.
1. Introduction
African catfish Clarias gariepinus, classified under Clarias spp., is among the most cultured freshwater finfish species in the world [1]. In major countries of production, this fish has a steadily developing market value since the demands for fish to cater to population needs are constantly increasing [2]. This introduced freshwater fish species is also favoured by fish farmers due to its rapid growth rate [3], high tolerance to water quality [4], and huge diversification of culture environment, from extensive traditional ponds to intensive recirculating aquaculture system (RAS) tanks [2].
Physical factors such as temperature are of great importance in the life cycle of poikilothermic species [5]. This abiotic master factor could control fish growth [6] as well as their overall physiological activity [7,8]. In general, fish growth increases with increasing water temperature up to a certain point but declines abruptly once the critical limit has been reached [9]; this is due to the increase in energy cost to maintain their metabolism [10]. The optimal growth of fish often lies within their preferred water temperature [11].
Over the past decades, the best temperature for rearing African catfish has been well established; around 30 °C [12]. However, no information on the impact on specific physiological components such as gastric emptying time (GET) has been recorded. Determination of GET is critical based on the assumption that the duration of time for which food items are entirely removed from the stomach is equal to the rate of food completely digested [13]. This physiological component is crucial to estimate the fish feeding rates, energy budgets, and daily rations [10,14,15].
The aim of this study was to determine the effects of water temperature on both the growth performance and GET of African catfish. In this study, temperature selection was based on the fundamental thermal niche (FTN) of species, whereby the “minus three and plus one” formula [16] was applied to the suggested temperature for the optimal growth of African catfish (~30 °C) [12]. By diving deeper into this study, it will surely bring profitable results, meeting with the ever-increasing demands for fish and fish products [2,17] through the most effective aquaculture techniques.
2. Materials and Methods
2.1. Fish Collection and Experimental Design
Fingerlings of C. gariepinus (n = 200, body weight = 15.0 ± 2.0 g) were collected from a local aquarium fish supplier in Bangi, Selangor, Malaysia and transported to the Marine Science laboratory of Universiti Kebangsaan Malaysia (UKM), Bangi, Selangor, Malaysia, where the experiment was conducted. After a week of acclimatization in controlled water temperature (26 °C), 180 individuals were indiscriminately divided into 12 recirculating aquaculture system (RAS) aquaria (size dimension and volume: 67 cm × 40 cm × 35 cm, 94 L; stocking density: 15 fish/tank, 0.02 kg/m3) equipped with their own submersible thermostat heaters (DoPhin 200 W, Penang, Malaysia). The fish were exposed to four experimental temperatures, 26 °C, 28 °C, 30 °C, and 32 °C, in triplicate by initiating a change of 1 °C/day.
At the achieved temperature, fish were once again acclimatized for two days before the initial measurement of fish body weight (Wi). Prior to taking measurements, fish were anesthetized with α-methyl quinoline (TransmoreR; Nika Trading, Puchong, Malaysia) following the method adopted by De et al. [18]. The 70-day experiment started immediately following the initial body weight measurement, between February and April 2020. Fish were fed twice daily (09:00 am and 16:00 pm) at the rate of 2% of mean body wet weight of fish. The dietary feeding rate of fish was adjusted weekly in accordance with the weight of growing fish. Uneaten pellets were collected for the calculation of feed utilization parameters. Other conditional factors such as water level (75% of the total volume of aquaria), pH level (pH 7.1), salinity (0.5 ppt), and photo-regimen (12 h light:12 h dark) were maintained throughout the study in order to preserve the integrity of data obtained in this study.
2.2. Growth Performance and Gastric Emptying Time (GET) Evaluation
Following the initial weight measurement, fish were weighed on a weekly basis. Weight records were used to analyse growth performance in terms of:
- Body weight gain (BWG, g) = (Wf − Wi) × n [19],
- Specific growth rate (SGR, % day−1) = (lnWf − lnWi)/t × 100 [20],
- Relative growth rate (RGR, %) = (Wf − Wi)/Wi × 100 [21],
- Daily growth rate (DGR, % day−1) = (Wf − Wi)/t × 100 [8],
- Survival (%) = n/initial number of fish stocked × 100 [22],
- Food consumption (FC, g day−1) = food consumed in g × t−1 [23],
- Food conversion ratio (FCR) = total feed fed/total weight gained [20],
- Food conversion efficiency (FCE, %) = (Weight gain/Feed intake) × 100 [24]
Initial weight and final weight were indicated as Wi and Wf, the total number of fish survived at the end of the experiment was indicated as n, and time (in days) was demonstrated as t.
Fish were returned to their respective experimental tanks after final weight measurement. Fish were starved for 72 h prior to GET evaluation. Following fish pellet preparation [15], fish were evaluated under a microradiographic unit (M60, Softex, Tokyo, Japan) at predetermined times since feeding; 0, 2, 4, 6, 8, 10, 12, 14, 16, and 18 h. Five fishes were selected from each treatment at each predetermined time. The anesthetizing method of De et al. [18] was also adopted for GET evaluation. Fish guts were photographed to trace the movement of food along the alimentary tract. Analysed fish were marked using the fin clipping method and released back to their respective tanks for recovery.
2.3. Statistical Analysis
Statistical analyses performed in this study followed the methods outlined by Mazumder et al. [23]. All data obtained were analysed with one-way analysis of variance (ANOVA), and significant data (p < 0.05) were used for pairwise multiple comparisons with Tukey’s test using MINITAB version 19 (Minitab LLC, State College, PA, USA). A GET graph was generated using Microcal Origin 6.0 graphic software (OriginLab Corporation, Northampton, MA, USA).
3. Results and Remarks
3.1. Growth Performance of African Catfish (C. Gariepinus)
The present study revealed that the elevation of temperature from 26 to 32 °C did not lead to any significant difference (p > 0.05) in regard to growth parameters such as Wi, Wf, BWG, SGR, RGR, DGR, survival rate, and FC (Table 1). Initial weight (Wi) in all treatments showed no significant difference (p > 0.05), reflecting on the homogeneity in fish weight at the beginning of the experiment. This complements several previous observations which agreed that the optimal temperature for the best growth of African catfish lies between 26 and 32 °C [20,21,25,26].

Table 1.
Growth performance and feed utilization parameters of C. gariepinus acclimated to 26, 28, 30, and 32 °C.
Despite no noticeable significant difference in the aforementioned parameters, variation in rearing temperature did influence both the FCR and FCE of the reared African catfish (p < 0.05, Table 1). Higher group temperatures (30 and 32 °C) displayed the best FCR and FCE. The reduced FCR at higher temperatures indicated that fish utilized feed very well [20], and there was an increase in protease activity and protein digestibility [27,28]. As temperature rose, so did appetite, leading FCE to increase [29]. Similar to our study, numerous other freshwater fish species displayed great improvement in FCR and FCE as temperature increased [29,30,31,32]. However, there were two major concerns that arose from the FCR and FCE acquired in our study; (1) the growth performance parameters observed in our study did not match the feed utilization data obtained (no significant difference in growth between treatments), and (2) the value of FCR obtained in our study was noticeably poorer as compared to FCR values obtained in other studies [20,33,34]. The calculations for both FCR and FCE were complicated by the fact that mortality (induced by cannibalism) occurred in all treatments except 26 °C throughout the experimentation period, resulting in data variability. The wide variation may also be due to the differences in the genetic background of fish stocks as well as variation in the feeding types and rates given to fish during the experimentation.
3.2. Gastric Emptying Time
From this study, we discovered that the GET of African catfish was extremely temperature dependent (Figure 1). The X-radiographic observations showed that as water temperature rose from 26 to 32 °C, the GET of African catfish shortened by an interval of two hours. The fastest emptying time was observed in group 32 °C, whereby fish feed was completely digested by the 10th hour. Group 30 °C exhibited a longer emptying rate, whereby fish feed was completely evacuated from fish guts by the 12th hour. Fish in group 28 °C took approximately 14 h in order for the fish feed to be completely evacuated from the alimentary tract. As compared to other experimental groups, the longest time taken (16 h) for fish feed to be completely evacuated from the digestive tract of fish was noted in group 26 °C.
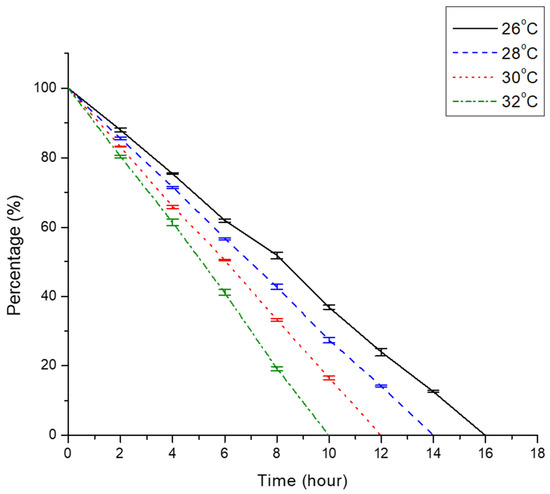
Figure 1.
Percentage of feed retention in fish digestive system at an interval of two hours for each experimental group (26, 28, 30, and 32 °C). Error bars represent the SE of mean percentage values of five fish (n = 5) which were analysed at each time point.
At higher temperatures, the GET became shorter as there was an increase in fish digestive enzyme activity [35,36]. This finding also complements the assertion made by Suja et al. [37], whereby rapid digestion rate coincides with FCE. The time taken for African catfish to completely remove food items from the digestive tract was relatively different compared to other tropical fish species [8,10,23]. These differences were due to the types of feed given throughout the experimental period, feeding regime (e.g., duration and frequency), water temperature, and the fish species studied [23,38].
To summarize, water temperatures ranging from 26 to 32 °C had no significant effect on growth, although they did influence the GET of African catfish. To cover the gaps in this research area, more extensive physiological parameters could be considered. Here, we would also like to acknowledge that despite the substantial growth of African catfish farming today, parties involved in the culturing of this fish need effective containment and eradication plans in place to prevent escapees from harming the surrounding environment.
Author Contributions
Conceptualization, methodology, software, validation, data curation, visualization: S.M.K. and S.K.D.; formal analysis, investigation, writing—original draft preparation, project administration: S.M.K.; resources, writing—review and editing, supervision: S.K.D.; funding acquisition: S.K.D. and M.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by Ministry of Higher Education Malaysia through the Fundamental Research Grant Scheme (FRGS/1/2017/STG03/UKM/02/5), Long-Term Research Grant Scheme (LRGS/1/2020/UMT/01/1), and UKM-Sime Darby Foundation Chair in Climate Change grant (ZF-2019-003).
Institutional Review Board Statement
All animal holding and experimental protocols used in this study were approved by the Animal Ethics Committee of Universiti Kebangsaan Malaysia (approval code no: FST/2016/SIMON/27-July/763-July-2016-May-2017).
Data Availability Statement
The data presented in this study are available on fair request from the corresponding author.
Acknowledgments
We are grateful to the anonymous reviewers and academic editor of Animals Kenji Saitoh for their very useful comments that greatly improved the earlier version of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture: Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; p. 31. [Google Scholar]
- Cultured Aquatic Species Information Programme: Clarias gariepinus. Available online: http://www.fao.org/fishery/culturedspecies/Clarias_gariepinus/en (accessed on 14 August 2021).
- Adan, R.I.Y. Catfish culture in Southeast Asia. SEAFDEC Asian Aquac. 2000, 22, 16–17. [Google Scholar]
- Schram, E.; Roques, J.A.C.; Abbink, W.; Yokohama, Y.; Spanings, T.; De Vries, P.; Bierman, S.; Van De Vis, H.; Flik, G. The impact of elevated water nitrate concentration on physiology, growth and feed intake of African catfish Clarias gariepinus (Burchell 1822). Aquac. Res. 2012, 45, 1499–1511. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, J.; Ahmad, T.; Chakrabarti, R. Effect of Water Temperature on the Physiological Responses of Asian Catfish Clarias batrachus (Linnaeus 1758). Asian Fish. Sci. 2013, 26, 26–38. [Google Scholar] [CrossRef]
- León, C.J.; Hernández, J.M.; León-Santana, M. The effects of water temperature in aquaculture management. Appl. Econ. 2006, 38, 2159–2168. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Paul, A. Climate change and its influence on freshwater fish diseases. Agric. Clim. Chang. 2017, 1, 333–344. [Google Scholar]
- Das, S.K.; Noor, N.M.; Kai, K.S.; Juan, Q.Z.; Iskandar, N.S.M.; De, M. Effects of temperature on the growth, gastric emptying time, and oxygen consumption rate of mahseer (Tor tambroides) under laboratory conditions. Aquac. Rep. 2018, 12, 20–24. [Google Scholar] [CrossRef]
- Jobling, M. Fish bioenergetics. Oceanogr. Lit. Rev. 1995, 9, 785. [Google Scholar]
- De, M.; Ghaffar, M.A.; Bakar, Y.; Das, S.K. Effect of temperature and diet on growth and gastric emptying time of the hybrid, Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂. Aquac. Rep. 2016, 4, 118–124. [Google Scholar] [CrossRef]
- Jobling, M. Temperature and growth: Modulation of growth rate via temperature change. In Global Warming: Implications for Freshwater and Marine Fish; Wood, C.M., McDonald, D.G., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 225–254. [Google Scholar] [CrossRef]
- De Moor, I.J.; Bruton, M.N. Atlas of Alien and Translocated Indigenous Aquatic Animals in Southern Africa; South African National Scientific Programmes Report No 144; Foundation for Research Development, Council for Scientific and Industrial Research: New Delhi, India, 1988. [Google Scholar]
- Seyhan, K.; Grove, D.J. A new approach in modelling gastric emptying in fish. Turk. J. Vet. Anim. Sci. 2003, 27, 1043–1047. [Google Scholar]
- Kawaguchi, Y.; Miyasaka, H.; Genkai-Kato, M.; Taniguchi, Y.; Nakano, S. Seasonal change in the gastric evacuation rate of rainbow trout feeding on natural prey. J. Fish Biol. 2007, 71, 1873–1878. [Google Scholar] [CrossRef]
- Das, S.K.; Ghaffar, M.A.; Bakar, Y.; Brito, M.F.; Mastura, S.S.; Temple, S.E. X-radiographic observations of food passage and nutrient absorption along the alimentary tract of archerfish, Toxotes jaculatrix. Bull. Mar. Sci. 2014, 90, 903–919. [Google Scholar] [CrossRef]
- He, Y.; Wu, X.; Zhu, Y.; Li, H.; Li, X.; Yang, D. Effect of rearing temperature on growth and thermal tolerance of Schizothorax (Racoma) kozlovi larvae and juveniles. J. Therm. Biol. 2014, 46, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Dauda, A.B.; Dasuki, A.; Bichi, A.H. Analysis of constraints to aquaculture development in Sudano-Sahelian region of Nigeria. Trop. Subtrop. Agroecosyst. 2015, 18, 189–193. [Google Scholar]
- De, M.; Ghaffar, M.A.; Bakar, Y.; Cob, Z.C.; Das, S.K. Optimum temperature for the growth form of tiger grouper (Epinephelus fuscoguttatus ♀) × giant grouper (E. Lanceolatus ♂) hybrid. Sains Malays. 2016, 45, 541–549. [Google Scholar]
- Azaza, M.S.; Dhraïef, M.N.; Kraïem, M.M. Effects of water temperature on growth and sex ratio of juvenile Nile tilapia Oreo-chromis niloticus (Linnaeus) reared in geothermal waters in southern Tunisia. J. Therm. Biol. 2008, 33, 98–105. [Google Scholar] [CrossRef]
- Ogunji, J.O.; Awoke, J. Effect of environmental regulated water temperature variations on survival, growth performance and haematology of African catfish, Clarias gariepinus. Our Nat. 2017, 15, 26–33. [Google Scholar] [CrossRef][Green Version]
- Abdulraheem, I.; Otubusin, S.O.; Agbebi, O.T.; Olowofeso, O.; Alegbeleye, W.O.; Abdul, W.O.; Adeyemi, K.; Ashley-Dejo, S.S.; Nathaniel, B. The Growth Response of Clarias gariepinus Hatchlings to Different Dry Feeds. J. Agric. Sci. 2012, 4, 10. [Google Scholar] [CrossRef][Green Version]
- Islam, M.A.; Uddin, M.H.; Uddin, M.J.; Shahjahan, M. Temperature changes influenced the growth performance and physio-logical functions of Thai pangas Pangasianodon hypophthalmus. Aquac. Rep. 2019, 13, 1–7. [Google Scholar]
- Mazumder, S.K.; Ghaffar, M.A.; Das, S.K. Exploring the Suitable Temperature and Diet for Growth and Gastric Emptying Time of Juvenile Malabar Blood Snapper (Lutjanus malabaricus Bloch & Schneider, 1801). Thalass. Int. J. Mar. Sci. 2019, 35, 29–41. [Google Scholar] [CrossRef]
- Basade, Y.; Mohan, M. Effect of feeding frequency on growth performance, feed efficiency and bioenergetics of Golden mahseer early fry. Asian Fish. Sci. 2009, 22, 549–559. [Google Scholar]
- Hogendoorn, H.; Jansen, J.A.J.; Koops, W.J.; Machiels, M.A.M.; Van Ewijk, P.H.; Van Hees, J.P. Growth and production of the African catfish, Clarias lazera (C. & V.): II. Effects of body weight, temperature and feeding level in intensive tank culture. Aquaculture 1983, 34, 265–285. [Google Scholar]
- Britz, P.; Hecht, T. Temperature preferences and optimum temperature for growth of African sharptooth catfish (Clarias gariepinus) larvae and postlarvae. Aquaculture 1987, 63, 205–214. [Google Scholar] [CrossRef]
- Abbink, W.; Garcia, A.B.; Roques, J.A.; Partridge, G.J.; Kloet, K.; Schneider, O. The effect of temperature and pH on the growth and physiological response of juvenile yellowtail kingfish Seriola lalandi in recirculating aquaculture systems. Aquaculture 2012, 330, 130–135. [Google Scholar] [CrossRef]
- Kofuji, P.Y.M.; Akimoto, A.; Hosokawa, H.; Masumoto, T. Seasonal changes in proteolytic enzymes of yellowtail Seriola quin-queradiata (Temminck & Schlegel; Carangidae) fed extruded diets containing different protein and energy levels. Aquac. Res. 2005, 36, 696–703. [Google Scholar]
- Buentell, J.A.; Gatlin, D.M., III; Neill, W.H. Effects of water temperature and dissolved oxygen on daily feed consumption, feed utilization and growth of channel catfish (Ictalurus punctatus). Aquaculture 2000, 182, 339–352. [Google Scholar] [CrossRef]
- Andrews, J.W.; Stickney, R.R. Interactions of Feeding Rates and Environmental Temperature on Growth, Food Conversion, and Body Composition of Channel Catfish. Trans. Am. Fish. Soc. 1972, 101, 94–99. [Google Scholar] [CrossRef]
- Hilge, V. The influence of temperature on the growth of the European catfish (Silurus glanis L.). J. Appl. Ichthyol. 1985, 1, 27–31. [Google Scholar] [CrossRef]
- Pandit, N.; Nakamura, M. Effect of High Temperature on Survival, Growth and Feed Conversion Ratio of Nile Tilapia, Oreochromis niloticus. Our Nat. 2010, 8, 219–224. [Google Scholar] [CrossRef]
- Marimuthu, K.; Umah, R.; Muralikrishnan, S.; Xavier, R.; Kathiresan, S. Effect of different feed application rate on growth, survival and cannibalism of African catfish, Clarias gariepinus fingerlings. Emir. J. Food Agric. 2011, 23, 330–337. [Google Scholar]
- Akinwole, A.; Faturoti, E. Biological performance of African Catfish (Clarias gariepinus) cultured in recirculating system in Ibadan. Aquac. Eng. 2007, 36, 18–23. [Google Scholar] [CrossRef]
- Bowyer, J.N.; Booth, M.A.; Qin, J.G.; D’Antignana, T.; Thomson, M.J.S.; Stone, D.A.J. Temperature and dissolved oxygen in-fluence growth and digestive enzyme activities of yellowtail kingfish Seriola lalandi (Valenciennes, 1833). Aquac. Res. 2014, 45, 2010–2020. [Google Scholar] [CrossRef]
- Golovanova, I.L.; Golovanov, V.K.; Smirnov, A.; Pavlov, D.D. Effect of ambient temperature increase on intestinal mucosa amylolytic activity in freshwater fish. Fish Physiol. Biochem. 2013, 39, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Suja, B.; Phillips, H.; Lochmann, R.; Chen, R. Effect of Temperature on Growth, Feed Utilization, and Immune Status of Channel Catfish in a Recirculating System. N. Am. J. Aquac. 2009, 71, 64–72. [Google Scholar] [CrossRef]
- Fry, G.C.; Milton, D.A. Age, growth and mortality estimates for populations of red snappers Lutjanus erythropterus and L. malabaricus from northern Australia and eastern Indonesia. Fish. Sci. 2009, 75, 1219–1229. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).