Simple Summary
Ovine anaplasmosis is an emerging disease in Europe, able to cause relevant economic losses. This disease has great significance in tropical and subtropical areas; however, climate change has facilitated the wider spread of ovine anaplasmosis throughout Europe during the last few decades. Related to the first ovine anaplasmosis outbreak that occurred in Spain in 2014, an epidemiological study was carried out in the affected area. The results show a high presence of Anaplasma ovis in the analysed flocks, indicating the high transmissibility of the bacteria.
Abstract
Ovine anaplasmosis is a vector-borne disease caused by Anaplasma ovis and mainly transmitted through tick bites. In Spain, the first outbreak of ovine anaplasmosis occurred in 2014. An epidemiological study in fifty-one farms was carried out associated with this outbreak in the affected geographical area. An epidemiological questionnaire was performed. In addition, whole blood samples were taken for molecular analysis in 47 of these farms to determine the prevalence of infection of Anaplasma ovis. A. ovis was present in 44 out of 47 PCR-analysed farms (93.6%). However, only 40.4% of the studied farms showed severe clinical signs. The clinical signs affected mainly young animals, which showed severe anaemia, weakness, anorexia, cachexia and epiphora. The early culling of young animals was more frequently reported by severely affected farms than the analysed farms without clinical signs (71.4% vs. 12.5%, p < 0.001). The geographical area where the farm is located seems to be relevant for the presence of clinical signs of the disease. Ovine anaplasmosis is an emerging disease in Europe that spreads rapidly through tick bites and is capable of causing significant economic losses when it spreads in a naive area and causes an epidemic.
1. Introduction
Ovine anaplasmosis is an emerging disease in Europe, caused by the bacteria Anaplasma ovis, that is transmitted through tick bites. This disease has great significance in developing countries in tropical and subtropical areas. During the last few decades, climate change has facilitated the wider dissemination of ovine anaplasmosis, which is being spread very quickly throughout Europe. In recent years, it has been reported in many different European countries, such as Bulgaria, France, Germany, Greece, Hungary, Italy, Portugal, Romania and Turkey [1,2,3]. In Spain, A. ovis infection was reported for the first time in roe deer (Capreolus capreolus) [4]. The first disease outbreak in sheep associated with severe clinical signs due to A. ovis has only been recently described by our research team [5,6]. The acute phase of the disease is characterised by nonspecific clinical signs such as weakness, depression, a marked loss of body condition, fever and progressive anaemia [5,7]. In lambs, the disease has been observed in the abattoir, with jaundiced carcasses that are condemned from entering the human food chain [6]. Therefore, the impacts of A. ovis are not only on animal welfare, rural livelihoods and farm sustainability but might also result in significant wastage of food intended for human consumption.
Bacteria of the Anaplasma genus belong to the order of the Rickettsiales and are obligate intracellular agents, causing vector-borne diseases in mammals. A. ovis, A. marginale and A. centrale infect erythrocytes in ruminants. A. ovis is a specific pathogen of sheep, although it can also be found in other species. A. phagocytophilum affects a large number of mammalian species, including sheep, causing human granulocytic anaplasmosis [8]. Anaplasmosis is currently considered an emerging zoonosis, and the number of cases in humans has recently increased significantly, mainly associated with A. phagocytophilum. There is a strong suspicion that anaplasmosis caused by A. ovis could also be a zoonosis [9].
Anaplasma genus bacteria are transmitted by mechanical and biological vectors, mainly ticks. Some studies have shown that up to 19 species of ticks can transmit the disease [10], depending on geographical location and seasonality. In Spain, the presence of Anaplasma bacteria in ticks of the Rhipicephalus, Ixodes, Hyaloma, Dermacentor and Amblyomma genera has been demonstrated [11]. In the salivary glands of ticks, bacteria replicate, increasing their infective capacity [12]. In other Anaplasma species, infectivity is achieved when there are 106 microorganisms in ticks’ salivary glands [13]. This dose-dependent biological transmission has greater efficacy and, therefore, greater relevance than the mechanic. Within the erythrocyte, the bacterium replicates by binary fission to form up to eight individual organisms within a simple vacuole [14]. Anaplasma leaves the erythrocyte using a mechanism that has not been well defined, apparently non-lytic, to infect new erythrocytes. The destruction of erythrocytes and the consequent anaemia are not, however, immediate, with noticeable changes around 30–40 days post-infection [5]. The severe haemolytic anaemia associated with this disease is a consequence of the immune response caused by anaplasmosis infection [15].
The diagnostic technique used before the development of molecular techniques was the examination of whole blood in the form of a smear, in which vacuoles were observed in an optical microscope inside the erythrocytes [16]. However, this technique is now obsolete, and, currently, the most common diagnostic method is the polymerase chain reaction (PCR), since it is capable of detecting infections with a minimum of 0.0001% of infected erythrocytes. In addition, PCR-amplified products can be sequenced to differentiate the species of Anaplasma. Currently, the msp4 gene, encoding a major membrane protein, is used to differentiate the different Anaplasma species [17]. Serological tests are limited to competition Enzyme-Linked Immunosorbent Assay cELISA methods that are designed to detect the MSP5 protein, which is present in all Anaplasma species. Currently, there is only one commercial kit for A. marginale that has demonstrated successful detection of A. ovis infection in sheep [18].
Anaplasma ovis is silently transmitted, not always causing obvious clinical signs. Furthermore, if left untreated, infected sheep remain carriers for life, favouring the spread of the infection. The present study was carried out in the geographic region where the first anaplasmosis outbreak occurred in Spain, at the time of occurrence. The main objective was to determine the collective prevalence of the infection in the region as well as to detect possible farm management factors that could have influenced the appearance of clinical signs.
2. Materials and Methods
A severe outbreak of ovine anaplasmosis affecting a wide geographical area occurred for the first time in Spain in the Matarraña region (Aragón, Spain). Young animals around one year of age were the most severely affected, and the main clinical signs were severe anaemia, anorexia, depression and chronic weight loss. To determine the presence of Anaplasma ovis in the flocks of the area, samples were taken according to the size of the flocks and pools of 5 blood samples were made to detect the presence of A. ovis by means of qPCR.
The care and use of animals were performed in accordance with the Spanish Policy for Animal Protection RD53/2013, which meets the European Union Directive 2010/63 on the protection of animals used for experimental and other scientific purposes.
2.1. Epidemiological Questionnaire and Selected Farms
Fifty-one sheep farms in the Matarraña region voluntarily participated in the epidemiological survey performed from March to November 2015. An epidemiological questionnaire was distributed to each farmer, asking questions related to breed, flock size, reproductive management, grazing areas, feeding, health plans, diseases present in the flock, observation of anaplasmosis compatible symptoms, presence of vectors, etc. In addition, blood samples with anticoagulant (EDTA) were collected from the jugular vein through a vacutainer system and frozen at minus 20 °C before being sent to the laboratory for molecular analysis.
A classification of the farms in three levels was performed according to the epidemiological survey, focused on the presence/absence of animals with compatible clinical signs and the use of antibiotics to treat them. Farms of type I were those without anaplasmosis-compatible clinical signs. Farms of type II had animals with compatible clinical signs, but no antibiotics had been administered. Finally, farms of type III had animals with compatible clinical signs that had been treated with antibiotics. In Figure 1, the number of farms sampled by municipalities within the Matarraña region is shown.
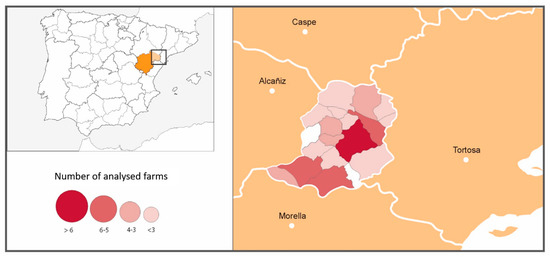
Figure 1.
Number of farms sampled by municipalities within the Matarraña region.
In the Matarraña region, despite its small size (926.06 km2), there are two well-differentiated natural areas. The northern region, Bajo Matarraña, is characterised by a continental climate with open spaces with small elevations. The main crops are olive trees (Olea Europea), almond trees (Prunus dulcis) and rainfed cereal, interspersed with forests of Aleppo pine (Pinus halepensis). Meanwhile, the southern region, Alto Matarraña, has a higher altitude (700–1396 m), and the climate is more humid, with extensive forest formations of black pine (Pinus nigra) and Scots pine (Pinus sylvestris) [19] (Figure 2).
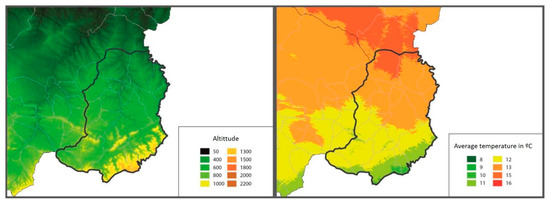
Figure 2.
Maps of the Matarraña region, where the altitude (left) and average temperature in °C (right) are shown (http://www.opengis.uab.es/wms/aragon/. Access 15 May 2021).
2.2. Sample Size
From each flock, a sample pooling including five animals younger than five years, who were randomly selected, was performed as a strategy to detect the presence of A. ovis in each farm. Thus, in type I farms, it was decided to sample 30 animals analysed in 6 pools of five sheep. A prevalence lower than 10% was assumed to indicate a flock free from infection, with a 95% confidence interval. In type II farms, five animals analysed in one pool were sampled. In these farms, a very high prevalence of A. ovis was expected; then, those animals with compatible clinical signs were sampled. In this type of farm, previous studies have revealed that the expected prevalence, detected by PCR of “pools” of 5 samples, was greater than 90%. Again, a 95% confidence interval was assumed. Finally, in type III farms, 15 animals were sampled in three pools of five animals. Unlike the previous case, the prevalence detected in previous studies in this type of farm was lower due to the antibiotic action. As there was a high probability that the disease was present on the farm, it was decided to consider a flock free of A. ovis with a prevalence lower than 20%. Assuming a 95% confidence interval, the target population for sampling, in this case, included all animals younger than five years since the treatment received might have masked the clinical signs, preventing the selection of sick animals.
2.3. Haematological Analysis
Samples of blood with anticoagulant (EDTA) were collected from the jugular vein through a vacutainer system from fifty-two clinically affected animals randomly selected from affected farms. These animals were selected as they presented symptoms such as anorexia or pale mucous membranes. Haematology was performed using an automatic haematological counter Vet-ABC (DIVASA-FARMAVIC S.A., Barcelona, Spain). Measured parameters were leukocytes, erythrocytes, haemoglobin, haematocrit, platelets, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC). In addition, a microscopic evaluation of blood smears stained with 10% Giemsa was performed for the detection of Anaplasma organisms infecting erythrocytes.
2.4. PCR Analysis
The commercial kit, MagMAX™ Pathogen RNA/DNA (Thermo Fisher Scientific, Waltham, MA, USA), with an automated magnetic particle processor (KingFisher Flex System, Thermo Fisher Scientific), was used for nucleic acid extraction according to the manufacturer’s instructions. Amplification was carried out in a 7500 fast Real-Time PCR machine (Applied Biosystems, Waltham, MA, USA), and results were analysed with the respective software (7500 software v2.3).
A total of 864 blood samples were taken from 45 sheep farms (Table 1), although finally, only 111 pools were analysed by means of qPCR because as soon as a pool tested positive in one of the studied farms, analysis of the remaining samples from that flock was stopped. In six of the analysed flocks, blood samples could not be taken due to logistical reasons.

Table 1.
Number of farms and animals sampled in each type of sheep farm analysed.
The specific detection of Anaplasma ovis was carried out by using the commercial kit EXOone Anaplasma ovis (EXOPOL S.L.) and following the manufacturer’s instructions. This qPCR assay has an analytical sensitivity of 50 copies of genomic equivalent/reaction and includes a quantified synthetic positive control. The assay targets the single copy MSP4 gene that is reported to allow a specific differentiation of Anaplasma ovis from the highly related Anaplasma marginale [20]. Additionally, an endogenous control was also included in all of the assays in order to avoid false-negative results. The bacterial load was expressed using the quantification cycle (Cq), which is the cycle number where the PCR amplification curve intersects the threshold line [21]. The Cq value can be used to quantify or to determine the presence/absence of the target sequence.
2.5. Statistical Analysis
All the information obtained in the epidemiological survey and in the different analytical tests was integrated into the same statistical matrix and processed with the statistical package SPSS 20.0 (SPSS Inc., Chicago, IL, USA) in order to determine the statistical relationship between the presence of A. ovis and clinical signs, as well as its possible risk factors. The farm was used as a study unit. The disease study was carried out by categorising the flocks according to clinical signs into three types: flocks without clinical signs of anaplasmosis, flocks with mild signs (less than 1% of young animals affected) and flocks with severe signs (more than 5% of infected young animals). In addition, flocks were classified according to the presence of A. ovis in the PCR-analysed samples as free or infected flocks. The study of the variables included in the survey, and the criteria for determining infection, were also analysed using non-parametric Chi-square tests. For those cases in which it was possible (2 × 2 tables), the relative risk was calculated. The values corresponding to numerical variables, such as counts obtained from haematologies, censuses, percentages of deviation, etc., were also analysed by ANOVA tests when the normality tests were passed or by non-parametric tests in cases that lacked normal distribution. The non-parametric tests used were Mann–Whitney for the presence of the etiological agent and Kruskall–Wallis for clinical criteria. Likewise, a logistic regression was carried out with the analysed data; however, due to the low number of sampled farms, no significant results were obtained. In all cases, results with p < 0.05 were assumed to be significant.
3. Results
3.1. Epidemiological Survey
Twenty-one of the 51 farms surveyed during this study reported currently or previously having animals with clinical signs of severe anaplasmosis (21/51: 41.2%). One hundred per cent of the affected farmers had or previously had animals with weakness, anorexia and cachexia, and 95.2% (20/21) of these farmers reported having seen animals with epiphora among those affected. Other symptoms such as lameness, haematuria/haemoglobinuria or abortions were seen in a much lower proportion. In Table 2, it can be seen that weakness, anorexia and cachexia were clinical signs clearly associated with mild or severe anaplasmosis. However, epiphora and haematuria were only present in severe anaplasmosis cases, haematuria being observed only in 4.8% of animals affected by severe anaplasmosis. Lameness does not seem to be a symptom of anaplasmosis, and the number of abortions increased with the severity of the disease.

Table 2.
Percentage of farms with mild or severe anaplasmosis that reported have seen the following clinical signs in the animals—weakness, anorexia, cachexia, epiphora, haematuria, lameness and abortion—in comparison with those without anaplasmosis.
In addition, 71.4% (15/21) of the severely affected farms had a higher incidence of culling young (<3 years old) animals than farms without clinical signs (3/24, 12.5%; p < 0.001), but these levels were similar to farms that showed mild clinical signs of anaplasmosis (3/6; 50.0%; p = 0.367). The flocks with severe anaplasmosis had a 16.7-times (3.58–77.68) greater risk of culling young animals than those with no clinical signs of anaplasmosis.
In the studied area, three different sheep breeds were reared, Rasa Aragonesa, Ojinegra de Teruel and Maellana, all of them local breeds that are very well adapted to the type of grazing and climate of the area. The study results do not show significant differences in the presence of anaplasmosis clinical signs between the different breeds. In Table 3, the presence of clinical anaplasmosis is analysed in relation to the breed.

Table 3.
Relationship between clinical signs of anaplasmosis and the breed reared on the farm.
Although clinical signs of anaplasmosis were observed throughout the whole Matarraña region, severe clinical signs were seen much more frequently in farms located in the north of the province (21/36 north vs. 0/15 south p < 0.001) (Figure 3, Table 4). The risk of having severe anaplasmosis in the north was 2.0-times (2.86–1.40) higher than in the south of the region.
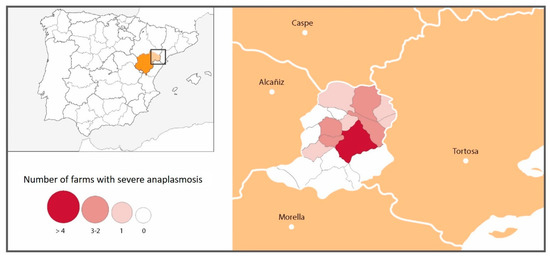
Figure 3.
Number and location of farms with severe anaplasmosis.

Table 4.
Analysed farms with severe anaplasmosis clinical signs in the Matarraña region.
Likewise, the average altitude at which those farms that suffered severe anaplasmosis were located was 128 m lower than those showing the absence of symptoms (652 m in flocks without anaplasmosis vs. 623 m in mild anaplasmosis and 524 m in those flocks with severe anaplasmosis) (p = 0.056).
The presence of ticks in the entire area was very relevant. During the visits to the farms, sporadic samples of ticks found on the animals were collected, and in all cases, the identified species were Rhipicephalus turanicus (Dr. Estrada-Peña’s identification). Ninety-two per cent of the farmers surveyed (46/50) saw ticks on their animals, and 78.0% (39/50) observed horseflies, spring being the time at which the highest numbers were observed. Furthermore, 36.0% (18/50) said that the number of ticks had increased in recent years. The relationship between seeing an increase in vectors and the presentation of severe anaplasmosis was significant (11/18 vs. 10/32, p = 0.040). Finally, the risk was 3.5-times (1.03–11.56) higher in the group of farms that reported an increase in the number of ticks. Table 5 shows the increased presence of ticks in the last few years reported by the farmers and the absence of treatment for ticks in those farms without anaplasmosis or with mild or severe clinical signs of the disease.

Table 5.
Increase in the presence of ticks observed and absence of treatments against ticks in those farms that showed severe or mild anaplasmosis or absence of clinical signs.
3.2. Haematological Analysis
Forty-six out of 52 analysed sheep with clear anaplasmosis clinical signs (88.5%) showed severe normochromic normocytic anaemia. In addition, six animals showed leukopenia (11.5%) and only one (1.9%) presented leukocytosis (Table 6).

Table 6.
Average values of the haematological analysis performed in sick animals. Measured parameters were leucocytes, erythrocytes, haemoglobin, haematocrit, platelets, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC).
3.3. Anaplasma ovis Detection by qPCR
Forty-five out of the 51 farms visited were finally analysed using molecular techniques (qPCR). Forty-one of the 45 analysed farms showed at least one blood pool positive for Anaplasma ovis (91.1%); in 36 of these farms, all the pools tested positive (87.8%). In addition, twenty-one (87.5%) out of twenty-four flocks that showed clinical signs had positive PCR results, as well as 19 of the 20 without clinical signs (95.0%) (Table 7).

Table 7.
Anaplasma ovis detection by PCR in the analysed farms in relation to the presence of clinical signs.
4. Discussion
Ovine anaplasmosis caused by Anaplasma ovis is a disease transmitted by vectors, mainly ticks, that is widespread in tropical and subtropical areas. This disease is frequently diagnosed in countries such as Brazil, Mexico, Iran and India, with a prevalence more significant than 50%, followed by others such as Morocco and Tunisia, with a prevalence between 20% and 50%, or China, with results ranging between 9.4% and 65.3% [22,23]. However, in recent years, the number of references for this disease in Europe has increased exponentially; the disease has been described in different European countries [1,3,24,25], indicating the rapid spread of A. ovis throughout the continent. In Spain, A. ovis infection was detected in roe deer in 2008 [4] and sheep in 2013 [26]. However, the first disease outbreak was recently described by our research team [5,6]. Epidemiological studies assessing the distribution of A. ovis across Europe are lacking, and no epidemiological studies have been developed in Spain so far. In the present study, A. ovis was present in 41 out of 45 PCR-analysed farms, which means a collective prevalence in the small ruminant farms of the Matarraña region of 91.1%. However, three of the flocks with clinical signs of anaplasmosis were PCR-negative. This could be due to the fact that farmers experiencing a severe outbreak of the disease treated the animals with antibiotics, mainly oxytetracycline and doxycycline. Although it has not yet been reported in the ovine literature, based on the authors’ experience extrapolated from the use of these antibiotics in other species, treatment with antibiotics, especially doxycycline, destroys the bacteria, causing sick animals to become A. ovis PCR-negative.
The main biological vectors of anaplasmosis are ticks, mainly of the genera Rhipicephalus (R. bursa and R. turanicus), Dermacentor (D. silvarum, D. marginatus and D. andersoni), Ixodes and Ambliyomma [10,11,27,28,29]. In our study, the only tick species found in all the samples taken was Rhipicephalus turanicus. Moreover, 92.0% of the surveyed farmers reported detecting the presence of ticks on their animals, especially in spring, and 36.0% said that the number of ticks had increased in recent years. It was observed that the relationship between seeing an increase in vectors and the presentation of severe anaplasmosis in the farm was significant (p < 0.05), with a 3.77-times more risk of suffering anaplasmosis among farmers who detected an increase in the number of ticks. However, this assessment could have a certain degree of subjectivity since most farmers knew that ticks are the primary vector of this disease. It is interesting to highlight that 45.0% of the farmers that reported severe anaplasmosis did not use treatments against ticks. Climate change and the increase in global temperatures have favoured the survival of ticks in the environment, contributing to the spread of A. ovis as it occurs in other species, including humans. In an environment suitable for ticks, the rise in temperatures increases their survival and their period of activity, which, together with the coexistence of wild and domestic ruminants, provides a wide range of reservoirs and hosts, favouring the high prevalence of the disease [4,30].
Although the collective prevalence of A. ovis infection was 91.1%, only 40.4% of the studied farms showed severe anaplasmosis clinical signs, which affected mainly young animals. As has been reported in the literature, once the animals overcome the infection, if they have not been treated with antibiotics, they remain carriers for years, giving A. ovis PCR-positive results but showing no signs of disease [5,7]. Sick sheep showed severe anaemia, weakness, anorexia, cachexia and epiphora. Haematuria, being common in other haemoparasitosis, is not frequent in ovine anaplasmosis because it is a chronic process [2]. In our study, only 4.8% of the animals affected by severe anaplasmosis presented haematuria. In addition, the early culling of cachectic young animals was repeatedly reported by affected farmers (71.4%). Other authors also described this, highlighting the severe normocytic normochromic anaemia as the main symptom of this disease [5,7,31,32]. In agreement with these data, the present study found that 88.5% of the affected animals showed severe normochromic normocytic anaemia, with average values of erythrocytes of 6.7 (threshold values: 9–14 × 106/mm3) and 22.7% of haematocrit (28–40%). It has been described that Anaplasma bacteria parasitise erythrocytes using a mechanism that is not well-defined but apparently non-lytic and that the destruction of erythrocytes and the consequent anaemia are a result of the immune response to anaplasma in the body [15].
While clinical signs of anaplasmosis were observed throughout the Matarraña region, severe clinical signs were seen much more frequently in farms located in the north of the province, with the risk of suffering severe anaplasmosis being 2.36-times higher in the north than in the south of the region (21/36 north vs. 0/15 south). Torina et al., 2008 [19] reported different A. marginale, A. ovis and A. phagocytophilum prevalences in the eastern and western sectors of the island of Sicily. These significant differences were related to differences in the management of the animals, to the diverse populations of wild reservoirs and especially to the variances between the most favourable habitats for the different species of ticks [19]. The Matarraña region has high mountain goat (Capra pirenaica hispanica) and roe deer populations (Capreolus capreolus), which could act as a reservoir for the disease. During the period of our study, 47 mountain goats were tested by means of qPCR in this area, and 19 of these (40.4%) tested positive for A. ovis (De la Fuente, personal communication). In addition, in the Matarraña region, there are two well-differentiated natural areas: the north, characterised by a continental climate with open spaces with small elevations, and the south, with a higher altitude and more humid climate [19]. Thus, differences were found in the presence of severe anaplasmosis according to the altitude at which the farms were located. Mountain goats and roe deer were initially only present in the more mountainous areas of the south. However, over the years, they have spread to the entire Matarraña region. Although A. ovis was present on practically all farms (91.1%), the northern farms were more severely affected. This could be because the disease entered silently from wild animals in the south; over time, it has spread throughout the region, with the greatest prevalence of clinical signs currently in the northern area due to the A. ovis infection-naïve population. Corona (2004) reported that anaplasmosis enzootic stability or endemic equilibrium occurs in warm climates where ticks are abundant throughout the year [33]. This implies the presence of a high percentage of infected animals, with the rare occurrence of clinical disease. As noted by the author, this relationship is maintained due to two factors: the passive immunity provided by colostrum and the early infection of the animals. This could explain the low rate of severe anaplasmosis in the South region of Matarraña.
Finally, the influence of breed on the severity of anaplasmosis in sheep has also been reported in several studies [31,34,35]. However, in our study, no significant difference in the presence of severe anaplasmosis was found across breeds.
5. Conclusions
Ovine anaplasmosis is an emerging disease in Europe that spreads rapidly through tick bites and is able of causing significant economic losses when it enters a naive area, causing an epidemic. However, there is a lack of epidemiological studies assessing the presence of Anaplasma ovis on small ruminant flocks. With the results obtained in the present study, it can be concluded that Anaplasma ovis rapidly spreads among all flocks in the affected area when management, climatic and environmental conditions are prone to the development of ticks, its primary vector. The data obtained in the present study also seem to indicate that as the disease becomes endemic in the area, the presence of clinical signs of the disease decreases. These results suggest that the detection of A. ovis by molecular techniques is insufficient to diagnose clinical anaplasmosis. Confirmation of the disease by clinical signs is needed, especially confirmation of a severe normocytic normochromic anaemia, the most diagnostic symptom of the disease.
Author Contributions
Conceptualisation, D.L. and J.M.G.; Data curation, M.L. and J.M.G.; Formal analysis, J.M.G.; Funding acquisition, L.M.F.; Investigation, M.L., D.L., L.M.F., M.E.M., N.L., C.J., S.V.-S., A.Á.B., C.B. and M.R.d.A.; Methodology, L.M.F., M.R.d.A. and D.L.; Project administration, D.L.; Resources, L.M.F.; Software, J.M.G.; Validation, L.M.F., D.L.; Writing—original draft, D.L.; Writing—review and editing, L.M.F., S.V.-S., M.L., J.M.G., A.Á.B. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the I Ethics Committee of the University of Zaragoza (protocol code P154/14, 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors would like to especially acknowledge the help and collaboration of Agustín Estrada-Peña in the identification of the ticks found. In addition, we would like to acknowledge the veterinarians and the farmers involved in the study.
Conflicts of Interest
Exopol, S.L. is a private laboratory with which the University of Zaragoza has a collaboration agreement and through which analyses are carried out at no cost.
References
- Hornok, S.; Elek, V.; de la Fuente, J.; Naranjo, V.; Farkas, R.; Majoros, G.; Foldvari, G. First serological and molecular evidence on the endemicity of Anaplasma ovis and A. marginale in Hungary. Vet. Mic. 2007, 122, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Renneker, S.; Abdo, J.; Salih, D.E.A.; Karagenç, T.; Bilgiç, H.; Torina, A.; Seitzer, U. Can Anaplasma ovis in small ruminants be neglected any longer? Transbound. Emerg. Dis. 2013, 60, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S. Haemoparasites in small ruminants in European countries: Challenges and clinical relevance. Small Rum. Res. 2016, 142, 22–27. [Google Scholar] [CrossRef]
- De la Fuente, J.; Ruiz-Fons, F.; Naranjo, V.; Torina, A.; Rodríguez, O.; Gortázar, C. Evidence of Anaplasma infections in European roe deer (Capreolus capreolus) from Southern Spain. Res. Vet. Sci. 2008, 84, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C.; Benito, A.; Arnal, J.L.; Ortín, A.; Gómez, M.; López, A.; Villanueva-Saz, S.; Lacasta, D. Anaplasma ovis in sheep: Experimental infection, vertical transmission and colostral immunity. Small Rum. Res. 2019, 178, 7–14. [Google Scholar] [CrossRef]
- Lacasta, D.; Ferrer, L.M.; Sanz, S.; Labanda, R.; González, J.M.; Benito, A.A.; Ruiz, H.; Rodríguez-Largo, A.; Ramos, J.J. Anaplasmosis Outbreak in lambs: First Report Causing Carcass Condemnation. Animals 2020, 10, 1851. [Google Scholar] [CrossRef]
- Yasini, S.P.; Khaki, Z.; Rahbari, S.; Kazemi, B.; Amoli, J.S.; Gharabaghi, A.; Jalali, S.M. Hematologic and clinical aspects of experimental ovine anaplasmosis caused by Anaplasma ovis in Iran. Iran J. Parasitol. 2012, 7, 91–98. [Google Scholar]
- Rar, V.; Golovljova, I. Anaplasma, Ehrlichia, and “Candidatus neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 2011, 11, 1842–1861. [Google Scholar] [CrossRef]
- Chochlakis, D.; Ioannou, I.; Tselentis, Y.; Psaroulaki, A. Human Anaplasmosis and Anaplasma ovis variant. Emerg. Infect. Dis. 2010, 16, 1031–1032. [Google Scholar] [CrossRef]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Garcia-Garcia, J.C. Anaplasma marginale (Rickettsiales: An¬aplasmataceae): Recent advances in defining host-pathogen adaptations of a tick-borne Rickettsia. Parasitology 2004, 129, 285–300. [Google Scholar] [CrossRef]
- Estrada-Peña, A. Orden Ixodida: Las garrapatas. IDE@-SEA (2015). 2015, pp. 1–15. Available online: http://sea-entomologia.org (accessed on 15 January 2021).
- Kocan, K.M.; Stiller, D.; Goff, W.L.; Claypool, P.L.; Edwards, W.; Ewing, S.A.; McGuire, T.C.; Hair, J.A.; Barron, S.J. Development of Anaplasma marginale in male Dermacentor andersoni transferred from infected to susceptible cattle. Am. J. Vet. Res. 1992, 5, 499–507. [Google Scholar]
- Brayton, K.A. Transmisión de Anaplasma marginale por garrapatas. Rev. Mex. Ciencias Pecuarias 2012, 3 (Suppl. 1), 41–50. [Google Scholar]
- Ristic, M.; Watrach, A.M. Anaplasmosis. VI. Studies and hypothesis concerning the cycle of development of the causative agent. Am. Vet. Res. 1963, 24, 267–277. [Google Scholar]
- Brown, W.C. Adaptive immunity to Anaplasma pathogens and immune dysregulation: Implications for bacterial persistence. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 241–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eriks, I.S.; Palmer, G.H.; McGuire, T.C.; Barbet, A.F. Detection and quantification of Anaplasma marginale in carrier by using a nucleic acid probe. Clin. Microbiol. 1989, 27, 279–284. [Google Scholar] [CrossRef]
- De la Fuente, J.; Garcia-Garcia, J.C.; Blouin, E.; Saliki, J.T.; Kocan, K.M. Infection of tick cells and bovine erythrocytes with one genotype of the intracellular Anaplasma marginale excludes infection with other genotypes. Clin. Diagn. Lab. Immunol. 2002, 9, 658–668. [Google Scholar] [CrossRef]
- Scoles, G.A.; Goff, W.L.; Lysyk, T.J.; Lewis, G.S.; Knowles, D.P. Validation of an Anaplasma marginale cELISA for use in the diagnosis of A. ovis infections in domestic sheep and Anaplasma spp. in wild ungulates. Vet. Microbiol. 2008, 130, 184–190. [Google Scholar] [CrossRef]
- Benavente Serrano, J.A.; Thomson Listerra, T. Comarca del Matarraña, Comarcalización de Aragón, Gobierno de Aragón. 2003. Available online: http://bibliotecavirtual.aragon.es (accessed on 15 February 2021).
- Torina, A.; Agnone, A.; Blanda, V.; Alongi, A.; D’Agostino, R.; Caracappa, S.; Marino, A.; Di Marco, V.; De la Fuente, J. Development and validation of two PCR tests for the detection of and differentiation between Anaplasma ovis and Anaplasma marginale. Ticks Tick Borne Dis. 2012, 3, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Wittwer, C.T. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Niu, Q.; Brayton, K.A.; Luo, J.; Liu, G.; Yin, H.; Liu, Z. Identification of Anaplasma ovis appendage-associated protein (AAAP) for development of an indirect ELISA and its application. Parasites Vectors 2017, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Urán, J.; Cardona, J.A. Prevalencia de Anaplasma spp. En el ámbito mundial: Revisión sistemática 1978-2018. Hechos Microbiol. 2019, 10, 30–38. [Google Scholar]
- Giadinis, N.; Chochlakis, D.; Tselentis, Y.; Petridou, E.; Karatzias, H.; Psaroulaki, A. Acute and chronic anaplasmosis due to Anaplasma ovis infection in dairy goats. In Proceedings of the 6th International Meeting on Rickettsiae and Rickettsial Diseases, Crete, Greece, 5–7 June 2011. [Google Scholar]
- Alessandra, T.; Santo, C. Tick-borne diseases in sheep and goats: Clinical and diagnostic aspects. Small Rumin. Res. 2012, 106, S6–S11. [Google Scholar] [CrossRef]
- Barandika, J.F.; Oporto, B.; Ros-García, A.; Povedano, I.; Hurtado, A.; García-Pérez, A.L. Infección por anaplasmas y piroplasmas en un rebaño ovino de raza Latxa a lo largo del periodo de pastoreo en monte. J. Soc. Esp. Ovinot. Caprinot. 2013, 507–513, 18–20. [Google Scholar]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Coetzee, J.F.; Ewing, S. The natural history of Anaplasma marginale. Vet. Paras. 2010, 167, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, A.; Grenda, S. Bacteria of the genus Anaplasma–characteristics of Anaplasma and their vectors: A review. Vet. Med. 2008, 53, 573–584. [Google Scholar] [CrossRef]
- Stuen, S. Haemoparasites–challenging and wasting infections in small ruminants. A review. Animals 2020, 10, 2179. [Google Scholar] [CrossRef]
- Bouchard, C.; Dibernardo, A.; Koffi, J.; Wood, H.; Leighton, P.A.; Lindsay, L.R. An Increased risk of tick-borne diseases with climate and environmental changes. Commun. Dis. Rep. CDR Rev. 2019, 45, 83–89. [Google Scholar] [CrossRef]
- Ciani, E.; Alloggio, I.; Petazzi, F.; Pieragostini, E. Looking for prognosticators in ovine anaplasmosis: Discriminant analysis of clinical and haematological parameters in lambs belonging to differently susceptible breeds experimentally infected with Anaplasma ovis. Acta Vet. Scan. 2013, 55, 71. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Gallois, M.; Fontugne, M.; Allain, E.; Denoual, M.; Moutailler, S.; Devillers, E.; Zientara, S.; Memmi, M.; Chauvin, A.; et al. Epidemiology and genetic diversity of Anaplasma ovis in goats in Corsica, France. Parasites Vectors 2019, 12, 3. [Google Scholar] [CrossRef]
- Corona, B.; Rodríguez, M.; Martínez, S. Anaplasmosis bovina. Rev. Electr. Vet. 2005, 6, 1–27. [Google Scholar]
- Hurtado, A.; Barandika, J.F.; Oporto, B.; Minguijón, E.; Povedano, I.; García-Pérez, A.L. Ticks and Tick-borne Diseases Risks of suffering tick-borne diseases in sheep translocated to a tick infested area: A laboratory approach for the investigation of an outbreak. Ticks Tick Borne Dis. 2015, 6, 31–37. [Google Scholar] [CrossRef]
- Pieragostini, E.; Ciani, E.; Rubino, G.; Petazzi, F. Tolerance to Tick-Borne Diseases in Sheep: Highlights of a Twenty-Year Experience in a Mediterranean Environment. Health Manag. Diff. Appr. Solut. 2011, 451–476. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).