Long-Term Determinants of the Seroprevalence of the Hepatitis E Virus in Wild Boar (Sus scrofa)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Animal Sampling
2.3. Risk Factors
2.3.1. Individual Factors
2.3.2. Environmental Factors
2.3.3. Populational Factors
2.3.4. Stochastic Factors
2.4. Serological Analyses
2.5. Statistical Analysis
3. Results
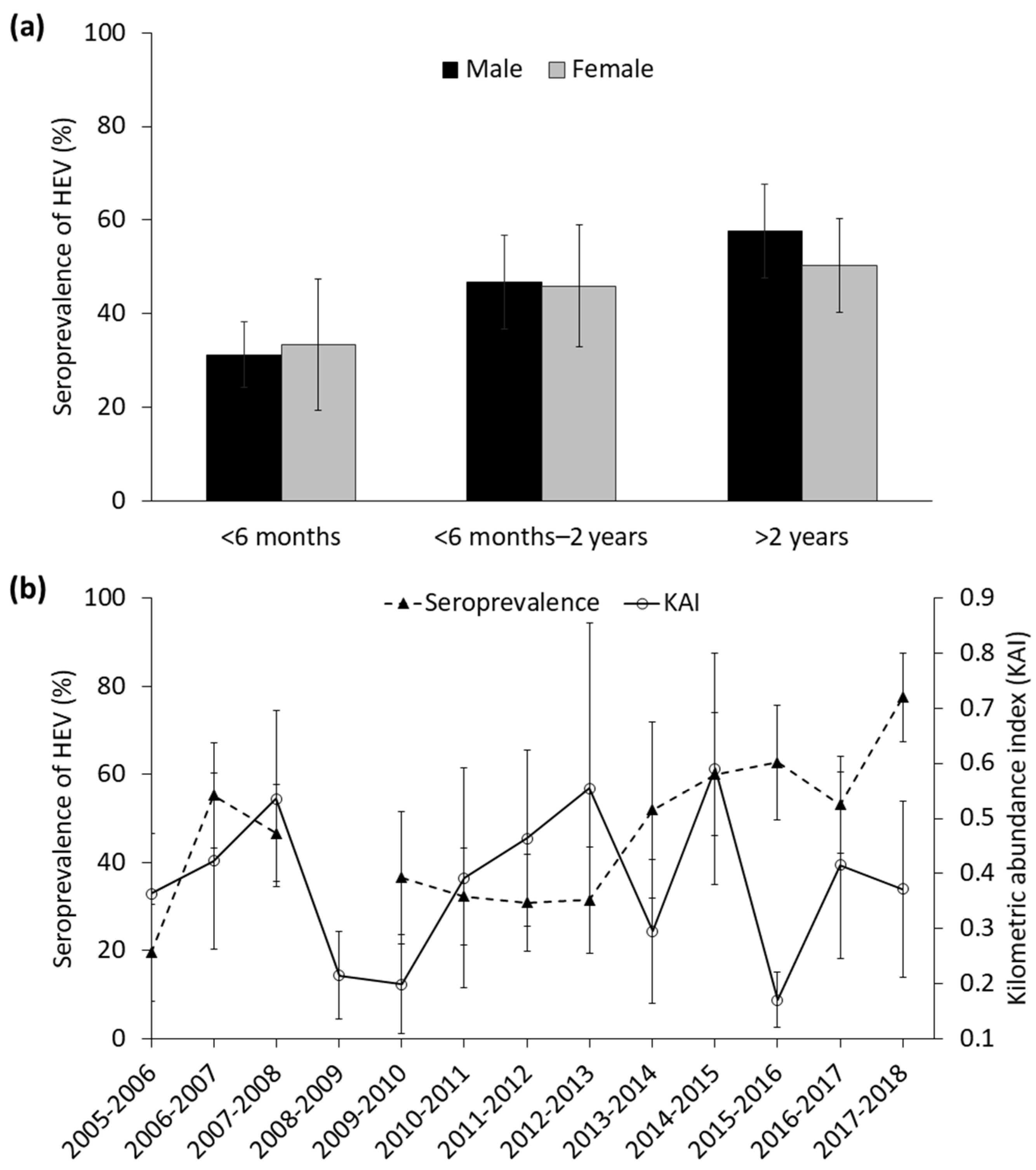
3.1. General Results
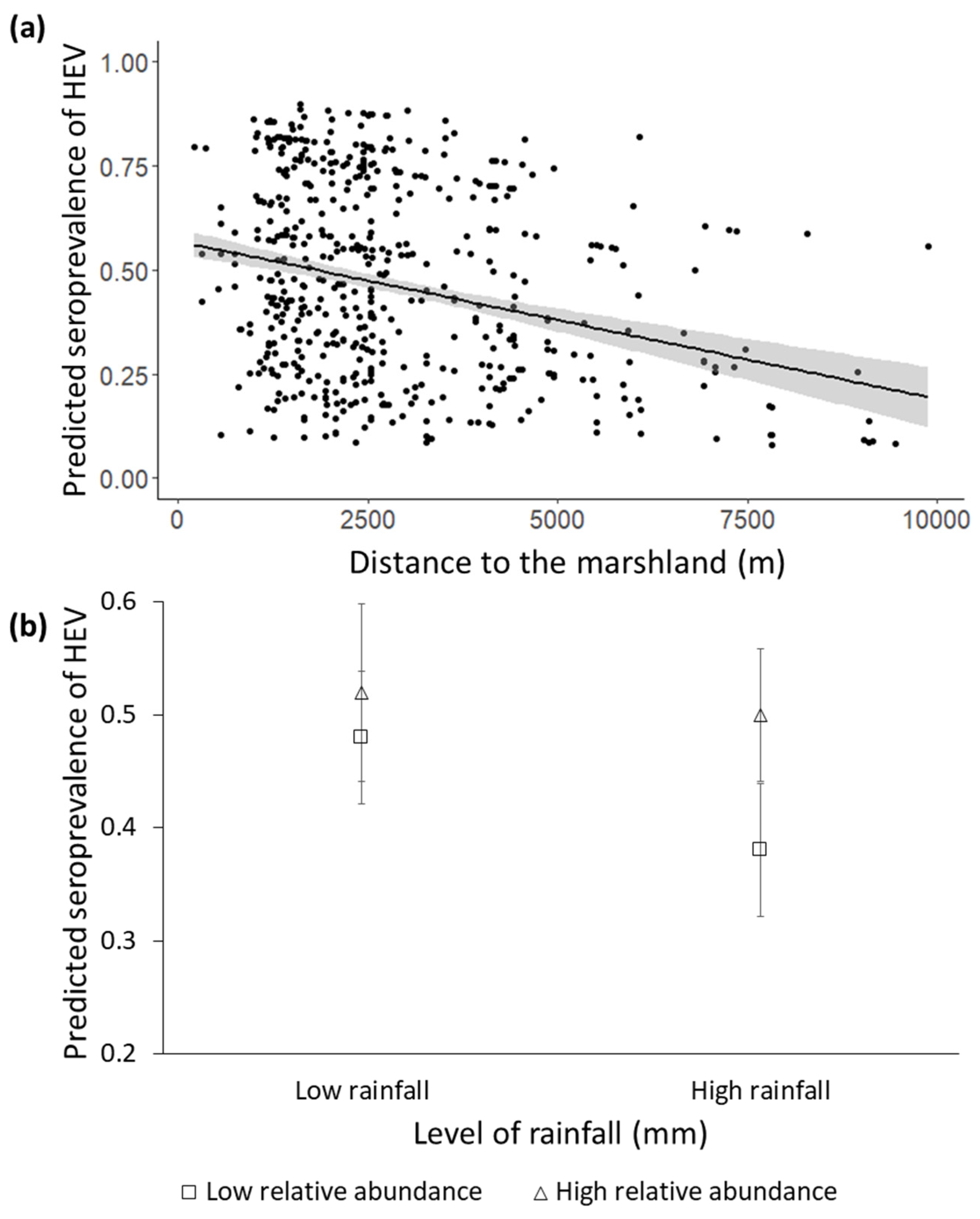
3.2. Factors Determining the Seroprevalence of HEV
3.3. Self-Correlations and Cross-Correlations
4. Discussion
4.1. General Patterns in the Seroprevalence of HEV
4.2. Factors Determining Seroprevalence of HEV
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Emerson, S.U.; Purcell, R.H. Hepatitis E virus. Rev. Med. Virol. 2003, 13, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Avellon, A.; Baylis, S.A.; Ciccaglione, A.R.; Couturier, E.; de Sousa, R.; Epštein, J.; Ethelberg, S.; Faber, M.; Fehér, Á.; et al. Hepatitis E virus: Assessment of the epidemiological situation in humans in Europe, 2014/15. J. Clin. Virol. 2016, 82, 9–16. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Hepatitis Report, 2017; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Kenney, S.P.; Meng, X.-J. Hepatitis E Virus: Animal models and zoonosis. Annu. Rev. Anim. Biosci. 2019, 7, 427–448. [Google Scholar] [CrossRef] [PubMed]
- de Deus, N.; Peralta, B.; Pina, S.; Allepuz, A.; Mateu, E.; Vidal, D.; Ruiz-Fons, F.; Martín, M.; Gortázar, C.; Segalés, J. Epidemiological study of hepatitis E virus infection in European wild boars (Sus scrofa) in Spain. Vet. Microbiol. 2008, 129, 163–170. [Google Scholar] [CrossRef]
- Boadella, M.; Ruiz-Fons, J.F.; Vicente, J.; Martín, M.; Segalés, J.; Gortazar, C. Seroprevalence evolution of selected pathogens in iberian wild boar. Transbound. Emerg. Dis. 2012, 59, 395–404. [Google Scholar] [CrossRef]
- Zheng, Y.; Ge, S.; Zhang, J.; Guo, Q.; Ng, M.H.; Wang, F.; Xia, N.; Jiang, Q. Swine as a principal reservoir of hepatitis E Virus that infects humans in Eastern China. J. Infect. Dis. 2006, 193, 1643–1649. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Frias, M.; Martinez-Peinado, A.; Risalde, M.A.; Rodriguez-Cano, D.; Camacho, A.; García-Bocanegra, I.; Cuenca-Lopez, F.; Gomez-Villamandos, J.C.; Rivero, A. Familial Hepatitis E outbreak linked to wild boar meat consumption. Zoonoses Public Health 2017, 64, 561–565. [Google Scholar] [CrossRef]
- Faber, M.; Askar, M.; Stark, K. Case-control study on risk factors for acute hepatitis E in Germany, 2012 to 2014. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef]
- Van der Poel, W.H. Food and environmental routes of Hepatitis E virus transmission. Curr. Opin. Virol. 2014, 4, 91–96. [Google Scholar] [CrossRef]
- Rusiñol, M.; Fernandez-Cassi, X.; Hundesa, A.; Vieira, C.; Kern, A.; Eriksson, I.; Ziros, P.; Kay, D.; Miagostovich, M.; Vargha, M.; et al. Application of human and animal viral microbial source tracking tools in fresh and marine waters from five different geographical areas. Water Res. 2014, 59, 119–129. [Google Scholar] [CrossRef]
- Kukielka, E.; Rodriguez-Prieto, V.; Vicente, J.; Sánchez-Vizcaíno, J.M. Constant Hepatitis E Virus (HEV) circulation in wild boar and red deer in Spain: An increasing concern source of HEV zoonotic transmission. Transbound. Emerg. Dis. 2016, 63, e360–e368. [Google Scholar] [CrossRef]
- García-Bocanegra, I.; Rivero, A.; Caballero-Gómez, J.; López-López, P.; Cano-Terriza, D.; Frías, M.; Jiménez-Ruiz, S.; Risalde, M.A.; Gómez-Villamandos, J.C.; Rivero-Juarez, A. Hepatitis E virus infection in equines in Spain. Transbound. Emerg. Dis. 2019, 66, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Risalde, M.A.; Rivero-Juárez, A.; Romero-Palomo, F.; Frías, M.; López-López, P.; Cano-Terriza, D.; García-Bocanegra, I.; Jiménez-Ruíz, S.; Camacho, Á.; Machuca, I.; et al. Persistence of hepatitis E virus in the liver of non-viremic naturally infected wild boar. PLoS ONE 2017, 12, e0186858. [Google Scholar] [CrossRef]
- Boadella, M.; Casas, M.; Martín, M.; Vicente, J.; Segalés, J.; de la Fuente, J.; Gortázar, C. Increasing Contact with hepatitis E virus in red deer, Spain. Emerg. Infect. Dis. 2010, 16, 1994–1996. [Google Scholar] [CrossRef]
- Lopez-Lopez, P.; de los Risalde, M.A.; Frias, M.; García-Bocanegra, I.; Brieva, T.; Caballero-Gomez, J.; Camacho, A.; Fernández-Molera, V.; Machuca, I.; Gomez-Villamandos, J.C.; et al. Risk factors associated with hepatitis E virus in pigs from different production systems. Vet. Microbiol. 2018, 224, 88–92. [Google Scholar] [CrossRef]
- Jiménez De Oya, N.; De Blas, I.; Blázquez, A.B.; Martín-Acebes, M.A.; Halaihel, N.; Gironés, O.; Saiz, J.C.; Escribano-Romero, E. Widespread distribution of hepatitis e virus in Spanish pig herds. BMC Res. Notes 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Juarez, A.; Risalde, M.A.; Frias, M.; García-Bocanegra, I.; Lopez-Lopez, P.; Cano-Terriza, D.; Camacho, A.; Jimenez-Ruiz, S.; Gomez-Villamandos, J.C.; Rivero, A. Prevalence of hepatitis E virus infection in wild boars from Spain: A possible seasonal pattern? BMC Vet. Res. 2018, 14, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Juarez, A.; Dashti, A.; López-López, P.; Muadica, A.S.; Risalde, M.D.L.A.; Risalde, M.D.L.A.; Köster, P.C.; Machuca, I.; Bailo, B.; De Mingo, M.H.; et al. Protist enteroparasites in wild boar (Sus scrofa ferus) and black Iberian pig (Sus scrofa domesticus) in southern Spain: A protective effect on hepatitis e acquisition? Parasites Vectors 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Seminati, C.; Mateu, E.; Peralta, B.; de Deus, N.; Martin, M. Distribution of hepatitis E virus infection and its prevalence in pigs on commercial farms in Spain. Vet. J. 2008, 175, 130–132. [Google Scholar] [CrossRef]
- Acevedo, P.; Vicente, J.; Höfle, U.; Cassinello, J.; Ruiz-Fons, F.; Gortazar, C. Estimation of European wild boar relative abundance and aggregation: A novel method in epidemiological risk assessment. Epidemiol. Infect. 2007, 135, 519–527. [Google Scholar] [CrossRef]
- Larska, M.; Krzysiak, M.K.; Jabłoński, A.; Kesik, J.; Bednarski, M.; Rola, J. Hepatitis E Virus Antibody Prevalence in Wildlife in Poland. Zoonoses Public Health 2015, 62, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Schielke, A.; Sachs, K.; Lierz, M.; Appel, B.; Jansen, A.; Johne, R. Detection of hepatitis E virus in wild boars of rural and urban regions in Germany and whole genome characterization of an endemic strain. Virol. J. 2009, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Forgách, P.; Nowotny, N.; Erdélyi, K.; Boncz, A.; Zentai, J.; Szűcs, G.; Reuter, G.; Bakonyi, T. Detection of Hepatitis E virus in samples of animal origin collected in Hungary. Vet. Microbiol. 2010, 143, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Ruiz, S.; García-Bocanegra, I.; Acevedo, P.; Espunyes, J.; Triguero-Ocaña, R.; Cano-Terriza, D.; Torres-Sánchez, M.J.; Vicente, J.; Risalde, M.A. A survey of shared pathogens at the domestic-wild ruminants’ interface in Doñana National Park (Spain). Transbound. Emerg. Dis 2021, 1–9. [Google Scholar] [CrossRef]
- Barroso, P.; García-Bocanegra, I.; Acevedo, P.; Palencia, P.; Carro, F.; Jiménez-Ruiz, S.; Almería, S.; Dubey, J.P.; Cano-Terriza, D.; Vicente, J. Long-term determinants of the seroprevalence of toxoplasma gondii in a wild ungulate community. Animals 2020, 10, 2349. [Google Scholar] [CrossRef]
- Caballero-Gómez, J.; Jiménez-Ruiz, S.; Lopez-Lopez, P.; Vicente, J.; Risalde, M.A.; Cano-Terriza, D.; Frias, M.; Barasona, J.A.; Rivero, A.; García-Bocanegra, I.; et al. Emergent subtype of hepatitis E virus genotype 3 in wild boar in Spain. Transbound. Emerg. Dis. 2019, 66, 1803–1808. [Google Scholar] [CrossRef]
- Ruiz-Fons, F.; Segalés, J.; Gortázar, C. A review of viral diseases of the European wild boar: Effects of population dynamics and reservoir rôle. Vet. J. 2008, 176, 158–169. [Google Scholar] [CrossRef]
- Rutjes, S.A.; Lodder-Verschoor, F.; Lodder, W.J.; van der Giessen, J.; Reesink, H.; Bouwknegt, M.; de Roda Husman, A.M. Seroprevalence and molecular detection of hepatitis E virus in wild boar and red deer in The Netherlands. J. Virol. Methods 2010, 168, 197–206. [Google Scholar] [CrossRef]
- Barroso, P.; Acevedo, P.; Vicente, J. The importance of long-term studies on wildlife diseases and their interfaces with humans and domestic animals: A review. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef]
- Laguna, E.; Barasona, J.A.; Triguero-Ocaña, R.; Mulero-Pázmány, M.; Negro, J.J.; Vicente, J.; Acevedo, P. The relevance of host overcrowding in wildlife epidemiology: A new spatially explicit aggregation index. Ecol. Indic. 2018, 84, 695–700. [Google Scholar] [CrossRef]
- Triguero-Ocaña, R.; Barasona, J.A.; Carro, F.; Soriguer, R.C.; Vicente, J.; Acevedo, P. Spatio-temporal trends in the frequency of interspecific interactions between domestic and wild ungulates from Mediterranean Spain. PLoS ONE 2019, 14, e0211216. [Google Scholar] [CrossRef]
- Barasona, J.A.; Latham, M.C.; Acevedo, P.; Armenteros, J.A.; Latham, A.D.M.; Gortazar, C.; Carro, F.; Soriguer, R.C.; Vicente, J. Spatiotemporal interactions between wild boar and cattle: Implications for cross-species disease transmission. Vet. Res. 2014, 45, 122. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J. Hepatitis E virus: Animal reservoirs and zoonotic risk. Vet. Microbiol. 2010, 140, 256–265. [Google Scholar] [CrossRef]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef]
- MAPA. Real Decreto 223/1988, de 14 de Marzo, Sobre Protección de Los Animales Utilizados para Experimentación y Otros Fines Científicos; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1988; pp. 8509–8512.
- EEC. Council Directive 86/609/EEC of 24 November 1986 on the Approximation of Laws, Regulations and Administrative Provisions of the Member States Regarding the Protection of Animals Used for Experimental and Other Scientific Purposes; Publication Office of the EU: Luxembourg, 1986; pp. 1–29. [Google Scholar]
- ASAB. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2012, 83, 301–309. [Google Scholar] [CrossRef]
- Arenas-Montes, A.; García-Bocanegra, I.; Paniagua, J.; Franco, J.J.; Miró, F.; Fernández-Morente, M.; Carbonero, A.; Arenas, A. Blood sampling by puncture in the cavernous sinus from hunted wild boar. Eur. J. Wildl. Res. 2013, 59, 299–303. [Google Scholar] [CrossRef]
- Saenz de Buruaga, M.; Lucio-Calero, A.; Purroy, F.J. Reconocimiento de Sexo y Edad en Especies Cinegéticas, 1st ed.; Edilesa: Madrid, Spain, 2001; ISBN 9788480123716. [Google Scholar]
- Barroso, P.; Barasona, J.A.; Acevedo, P.; Palencia, P.; Carro, F.; Negro, J.J.; Torres, M.J.; Gortázar, C.; Soriguer, R.C.; Vicente, J. Long-term determinants of tuberculosis in the ungulate host Community of Doñana National Park. Pathogens 2020, 9, 445. [Google Scholar] [CrossRef]
- Triguero-Ocaña, R.; Martínez-López, B.; Vicente, J.; Barasona, J.A.; Martínez-Guijosa, J.; Acevedo, P. Dynamic network of interactions in the wildlife-livestock interface in mediterranean spain: An epidemiological point of view. Pathogens 2020, 9, 120. [Google Scholar] [CrossRef]
- REDIAM Consejería de Medio Ambiente y Ordenación del Territorio, Andalucía, España: Red de Información Ambiental de Andalucía, REDIAM. Available online: http://www.juntadeandalucia.es/medioambiente/site/rediam (accessed on 8 July 2014).
- Neumann, S.; Hackl, S.S.; Piepenschneider, M.; Vina-Rodriguez, A.; Dremsek, P.; Ulrich, R.G.; Groschup, M.H.; Eiden, M. Serologic and molecular survey of Hepatitis E Virus in German deer populations. Source J. Wildl. Dis. Wildl. Dis. Assoc. 2016, 52, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhang, L.; Gong, L.; Lv, J.; Feng, Y.; Liu, J.; Song, L.; Xu, Q.; Jiang, M.; Xu, A. Hepatitis E Virus in Yellow Cattle, Shandong, Eastern China. Emerg. Infect. Dis. 2016, 22, 2211–2212. [Google Scholar] [CrossRef]
- Huang, F.; Li, Y.; Yu, W.; Jing, S.; Wang, J.; Long, F.; He, Z.; Yang, C.; Bi, Y.; Cao, W.; et al. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology 2016, 64, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.P.; Gaillard, J.M.; Bideau, E. Kilometric index as biological indicator for monitoring forest roe deer populations. Acta Theriol. 1991, 36, 315–328. [Google Scholar] [CrossRef]
- Vicente, J.; Barasona, J.A.; Acevedo, P.; Ruiz-Fons, J.F.; Boadella, M.; Diez-Delgado, I.; Beltran-Beck, B.; González-Barrio, D.; Queirós, J.; Montoro, V.; et al. Temporal trend of tuberculosis in wild ungulates from Mediterranean Spain. Transbound. Emerg. Dis. 2013, 60, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Barnaud, E.; Rogée, S.; Garry, P.; Rose, N.; Pavio, N. Thermal inactivation of infectious Hepatitis E Virus in experimentally contaminated food. Appl. Environ. Microbiol. 2012, 78, 5153–5159. [Google Scholar] [CrossRef] [PubMed]
- EBD-CSIC. Equipo de Seguimiento del Espacio Nacional de Doñana. Available online: http://icts.ebd.csic.es/datos-meteorologicos (accessed on 5 July 2019).
- Ivanova, A.; Tefanova, V.; Reshetnjak, I.; Kuznetsova, T.; Geller, J.; Lundkvist, Å.; Janson, M.; Neare, K.; Velström, K.; Jokelainen, P.; et al. Hepatitis E Virus in domestic pigs, wild boars, pig farm workers, and hunters in Estonia. Food Environ. Virol. 2015, 7, 403–412. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. GLM and GAM for count data. In Mixed Effects Models and Extensions in Ecology with R; Springer: Berlin/Heidelberg, Germany, 2009; Volume 21, pp. 209–243. ISBN 9780387874579. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- George, D.; Mallery, P. IBM SPSS for Windows Statistics 19 Step by Step: A Simple Guidance and Reference, 12th ed.; Pearson: Boston, MA, USA, 2012; ISBN 9780205985517. [Google Scholar]
- Martinelli, N.; Pavoni, E.; Filogari, D.; Ferrari, N.; Chiari, M.; Canelli, E.; Lombardi, G. Hepatitis E Virus in Wild Boar in the Central Northern Part of Italy. Transbound. Emerg. Dis. 2015, 62, 217–222. [Google Scholar] [CrossRef]
- Thiry, D.; Mauroy, A.; Saegerman, C.; Licoppe, A.; Fett, T.; Thomas, I.; Brochier, B.; Thiry, E.; Linden, A. Belgian Wildlife as Potential Zoonotic Reservoir of Hepatitis E Virus. Transbound. Emerg. Dis. 2017, 64, 764–773. [Google Scholar] [CrossRef]
- Wang, H.; Castillo-Contreras, R.; Saguti, F.; López-Olvera, J.R.; Karlsson, M.; Mentaberre, G.; Lindh, M.; Serra-Cobo, J.; Norder, H. Genetically similar hepatitis E virus strains infect both humans and wild boars in the Barcelona area, Spain, and Sweden. Transbound. Emerg. Dis. 2019, 66, 978–985. [Google Scholar] [CrossRef]
- Kozyra, I.; Jabłoński, A.; Bigoraj, E.; Rzeżutka, A. Wild boar as a sylvatic reservoir of hepatitis E Virus in Poland: A cross-sectional population study. Viruses 2020, 12, 1113. [Google Scholar] [CrossRef]
- Montagnaro, S.; De Martinis, C.; Sasso, S.; Ciarcia, R.; Damiano, S.; Auletta, L.; Iovane, V.; Zottola, T.; Pagnini, U. Viral and antibody prevalence of Hepatitis E in European wild boars (Sus scrofa) and hunters at zoonotic risk in the latium region. J. Comp. Pathol. 2015, 153, 1–8. [Google Scholar] [CrossRef]
- Kantala, T.; Heinonen, M.; Oristo, S.; von Bonsdorff, C.-H.; Maunula, L. Hepatitis E Virus in young pigs in Finland and characterization of the isolated partial genomic sequences of genotype 3 HEV. Foodborne Pathog. Dis. 2015, 12, 253–260. [Google Scholar] [CrossRef]
- Pavio, N.; Meng, X.-J.; Renou, C. Zoonotic hepatitis E: Animal reservoirs and emerging risks. Vet. Res. 2010, 41, 46. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernandez Escamez, P.S.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- Salines, M.; Andraud, M.; Rose, N. From the epidemiology of hepatitis E virus (HEV) within the swine reservoir to public health risk mitigation strategies: A comprehensive review. Vet. Res. 2017, 48, 31. [Google Scholar] [CrossRef]
- Gortázar, C.; Torres, M.J.; Vicente, J.; Acevedo, P.; Reglero, M.; de la Fuente, J.; Negro, J.J.; Aznar-Martín, J. Bovine tuberculosis in Doñana Biosphere Reserve: The role of wild ungulates as disease reservoirs in the last Iberian lynx strongholds. PLoS ONE 2008, 3, e2776. [Google Scholar] [CrossRef] [PubMed]
- Palencia, P.; Vicente, J.; Barroso, P.; Barasona, J.Á.; Soriguer, R.C.; Acevedo, P. Estimating day range from camera-trap data: The animals’ behaviour as a key parameter. J. Zool. 2019, 309, 182–190. [Google Scholar] [CrossRef]
- Braza, F.; Alvarez, F. Utilisation de l’habitat et organisation sociale du sanglier (Sus scrofa L.) à Doñana (Sud-Ouest de l’Espagne). Can. J. Zool. 1989, 67, 2047–2051. [Google Scholar] [CrossRef]
- Venero, J.L. Dieta de los grandes fitófagos silvestres del Parque Nacional de Doñana. Acta Vertebr. 1984, 11, 45–69. [Google Scholar]
- Khuroo, M.; Khuroo, M.; Khuroo, N. Transmission of Hepatitis E Virus in developing countries. Viruses 2016, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Barasona, J.A.; Acevedo, P.; Diez-Delgado, I.; Queiros, J.; Carrasco-García, R.; Gortazar, C.; Vicente, J. Tuberculosis-associated death among adult wild boars, Spain, 2009–2014. Emerg. Infect. Dis. 2016, 22, 2178–2180. [Google Scholar] [CrossRef]
- Lu, Y.H.; Qian, H.Z.; Hu, A.Q.; Qin, X.; Jiang, Q.W.; Zheng, Y.J. Seasonal pattern of hepatitis E virus prevalence in swine in two different geographical areas of China. Epidemiol. Infect. 2013, 141, 2403–2409. [Google Scholar] [CrossRef][Green Version]
- Parashar, D.; Khalkar, P.; Arankalle, V.A. Survival of hepatitis A and E viruses in soil samples. Clin. Microbiol. Infect. 2011, 17, E1–E4. [Google Scholar] [CrossRef]
- Ruiz-Fons, F.; Vicente, J.; Vidal, D.; Höfle, U.; Villanúa, D.; Gauss, C.; Segalés, J.; Almería, S.; Montoro, V.; Gortázar, C. Seroprevalence of six reproductive pathogens in European wild boar (Sus scrofa) from Spain: The effect on wild boar female reproductive performance. Theriogenology 2006, 65, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Andraud, M.; Dumarest, M.; Cariolet, R.; Aylaj, B.; Barnaud, E.; Eono, F.; Pavio, N.; Rose, N. Direct contact and environmental contaminations are responsible for HEV transmission in pigs. Vet. Res. 2013, 44, 102. [Google Scholar] [CrossRef] [PubMed]
- Kukielka, E.; Barasona, J.A.; Cowie, C.E.; Drewe, J.A.; Gortazar, C.; Cotarelo, I.; Vicente, J. Spatial and temporal interactions between livestock and wildlife in South Central Spain assessed by camera traps. Prev. Vet. Med. 2013, 112, 213–221. [Google Scholar] [CrossRef]
- Carrasco-Garcia, R.; Barroso, P.; Perez-Olivares, J.; Montoro, V.; Vicente, J. Consumption of big game remains by scavengers: A potential risk as regards disease transmission in Central Spain. Front. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Boadella, M.; Vicente, J.; Ruiz-Fons, F.; de la Fuente, J.; Gortazar, C. Effects of culling Eurasian wild boar on the prevalence of Mycobacterium bovis and Aujeszky’s disease virus. Prev. Vet. Med. 2012, 107, 214–221. [Google Scholar] [CrossRef]
- Vicente, J.; Apollonio, M.; Blanco-Aguiar, J.A.; Borowik, T.; Brivio, F.; Casaer, J.; Croft, S.; Ericsson, G.; Ferroglio, E.; Gavier-Widen, D.; et al. Science-based wildlife disease response. Science. 2019, 364, 943–944. [Google Scholar] [CrossRef]
- Nan, Y.; Wu, C.; Zhao, Q.; Zhou, E.-M. Zoonotic Hepatitis E Virus: An ignored risk for public health. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
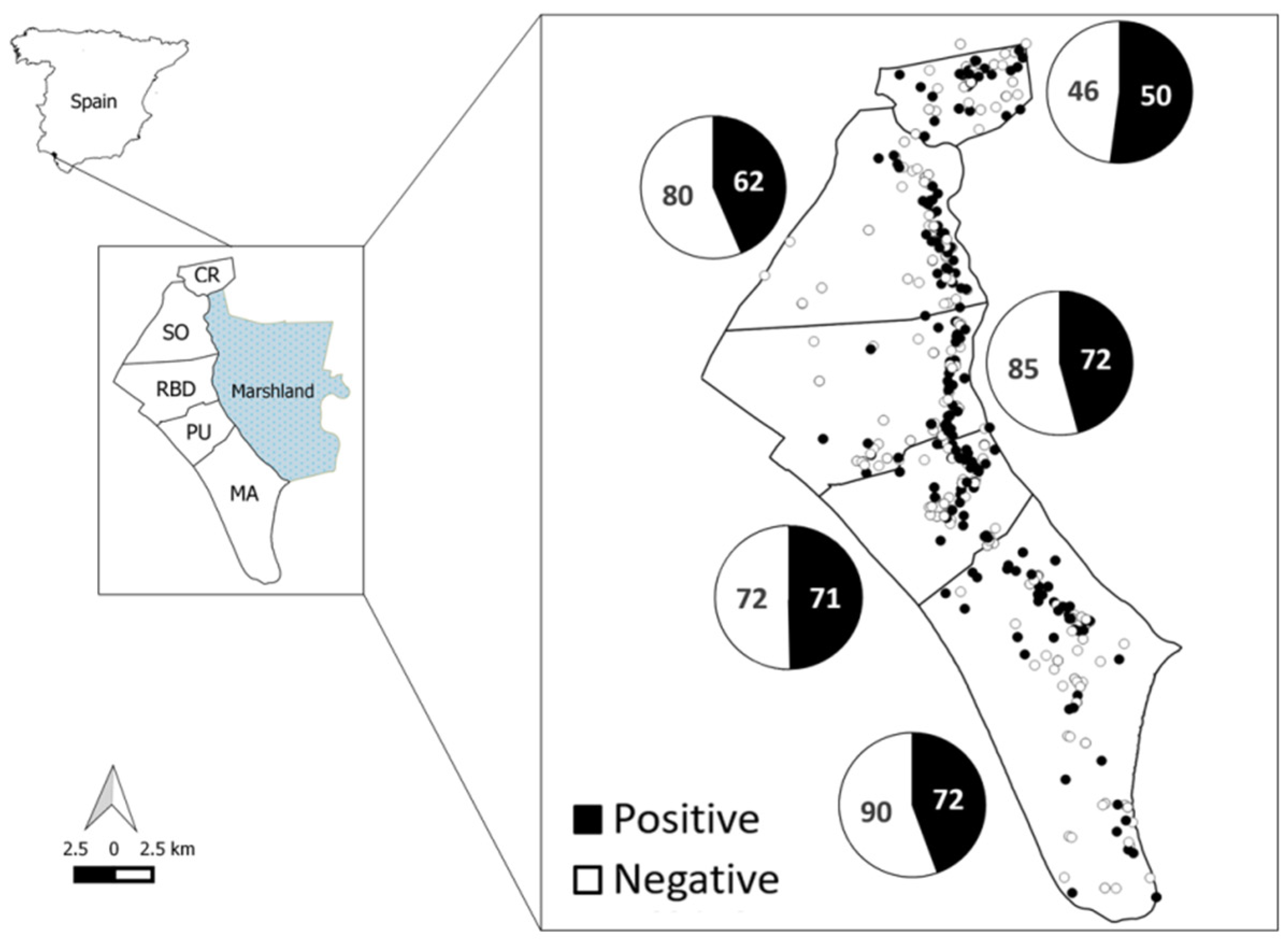
| Season | CR | SO | RBD | PU | MA | Total |
|---|---|---|---|---|---|---|
| Seroprevalence (N) | Seroprevalence (N) | Seroprevalence (N) | Seroprevalence (%) | Seroprevalence (%) | Seroprevalence (%) | |
| 2005–2006 | - | 16.7 (6) | 25 (12) | 12.5 (8) | 20 (25) | 19.6 (51) |
| 2006–2007 | 71.4 (14) | 57.1 (14) | 35.3 (17) | 66.7 (18) | 25 (4) | 55.2 (67) |
| 2007–2008 | - | 19.2 (26) | 42.9 (7) | 69.2 (13) | 56.3 (32) | 46.7 (75) |
| 2008–2009 | - | - | - | - | - | - |
| 2009–2010 | 100 (2) | 50 (4) | 41.2 (17) | - | 2.2 (18) | 36.6 (41) |
| 2010–2011 | 29.4 (17) | 40 (10) | 35 (20) | 25 (12) | 33.3 (9) | 32.4 (68) |
| 2011–2012 | 25 (8) | 31.6 (19) | 26.3 (19) | 54.5 (11) | 18.2 (11) | 30.9 (68) |
| 2012–2013 | 0 (9) | 0 (6) | 38.5 (13) | 42.9 (7) | 47.4 819) | 31.5 (54) |
| 2013–2014 | - | - | 57.1 (7) | 50 (18) | - | 52.0 (25) |
| 2014–2015 | 100 (2) | 81.3 (16) | 75 (8) | 41.2 (17) | 28.6 (7) | 60.0 (50) |
| 2015–2016 | 73.3 (15) | 41.7 (12) | 80 (10) | 33.3 (6) | 75 (8) | 62.7 (51) |
| 2016–2017 | 46.7 (15) | 46.7 (15) | 78.6 (14) | 35 (20) | 66.7 (15) | 53.2 (79) |
| 2017–2018 | 78.6 (14) | 78.6 (14) | 53.8 (13) | 92.3 (13) | 85.7 (14) | 77.5 (71) |
| Total 2005–2018 | 52.1 (96) | 43.7 (142) | 54.1 (157) | 49.7 (143) | 44.4 (162) | 46.7 (700) |
| Variables | F df (x,y) | Estimate ± SD 2 | p |
|---|---|---|---|
| Age class 1 | 5.30 (2, 690) | 1–2 years: 0.46 ± 0.25 >2 years: 0.76 ± 0.21 | <0.01 |
| Distance to marsh | 3.09 (1, 690) | −0.0001 ± 0.0006 | 0.03 |
| Abundance of wild boar (KAI) | 4.07 (1, 690) | −0.85 ± 0.72 | 0.22 |
| Rainfall | 0.01 (1, 690) | −0.002 ± 0.001 | 0.05 |
| Rainfall*abundance of wild boar | 3.74 (1, 690) | 0.002 ± 0.001 | 0.05 |
| Intra-specific seroprevalence | 102.59 (1, 690) | 0.04 ± 0.005 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso, P.; Risalde, M.A.; García-Bocanegra, I.; Acevedo, P.; Barasona, J.Á.; Caballero-Gómez, J.; Jiménez-Ruiz, S.; Rivero-Juárez, A.; Montoro, V.; Vicente, J. Long-Term Determinants of the Seroprevalence of the Hepatitis E Virus in Wild Boar (Sus scrofa). Animals 2021, 11, 1805. https://doi.org/10.3390/ani11061805
Barroso P, Risalde MA, García-Bocanegra I, Acevedo P, Barasona JÁ, Caballero-Gómez J, Jiménez-Ruiz S, Rivero-Juárez A, Montoro V, Vicente J. Long-Term Determinants of the Seroprevalence of the Hepatitis E Virus in Wild Boar (Sus scrofa). Animals. 2021; 11(6):1805. https://doi.org/10.3390/ani11061805
Chicago/Turabian StyleBarroso, Patricia, María A. Risalde, Ignacio García-Bocanegra, Pelayo Acevedo, José Ángel Barasona, Javier Caballero-Gómez, Saúl Jiménez-Ruiz, Antonio Rivero-Juárez, Vidal Montoro, and Joaquín Vicente. 2021. "Long-Term Determinants of the Seroprevalence of the Hepatitis E Virus in Wild Boar (Sus scrofa)" Animals 11, no. 6: 1805. https://doi.org/10.3390/ani11061805
APA StyleBarroso, P., Risalde, M. A., García-Bocanegra, I., Acevedo, P., Barasona, J. Á., Caballero-Gómez, J., Jiménez-Ruiz, S., Rivero-Juárez, A., Montoro, V., & Vicente, J. (2021). Long-Term Determinants of the Seroprevalence of the Hepatitis E Virus in Wild Boar (Sus scrofa). Animals, 11(6), 1805. https://doi.org/10.3390/ani11061805










