3.1. Experiment 1
There were no significant differences in any measure of live performance when cattle were provided supplemental methionine (
Table 4). There was a tendency for methionine supplementation to increase the average daily gain (ADG) during the final 28 d (
p = 0.09), however, this trend was not observed for the duration of the feeding period, and ADG was numerically lower in all treatment groups when compared to controls. Previously reported results of methionine supplementation in finishing beef cattle are conflicted. Hussein and Berger [
12] failed to elicit a significant response in performance when cattle were supplemented with increasing levels of a ruminally protected lysine-methionine blend. However, [
5] reported a significant improvement in ADG and an additional 10 kg of final live weight when steers were supplied with 4 g/(head d) of supplemental methionine. Based on currently reported results and inconsistency in previously conducted studies, the lack of performance response could be due to maximized methionine utilization in the energy-rich diet provided or may be because the experimental diets were not limiting in methionine. In a study evaluating the effect of post-ruminal amino acid supply on intake of lactating dairy cows, [
13] found that methionine abomasally infused at 140% of its calculated intestinally absorbable requirement significantly reduced DMI and had negative impacts on measures of performance. Conversely, the observed response in the current study could be due to lysine and methionine levels being lower than required for a measurable impact, as levels fed in the present study were lower than reported in some previous studies [
1,
3].
Hot carcass weight was unaffected by the inclusion of supplemental methionine (
p = 0.61), which agrees with the similar final BW observed across all treatments (
Table 5). Although carcass weights were similar, there was a linear increase in LM area for increasing levels of methionine (
p = 0.04). This equated to a 9% increase in LM area for steers supplemented with 12 g/(head d) of ruminally protected methionine when compared to controls. Additionally, methionine supplementation tended to reduce calculated yield grade (
p = 0.08), which was likely a function of the improved LM area. All other carcass characteristics were similar among treatments. Previous work at the same institution showed increased HCW when cattle were supplemented with 12 g/(head d) of encapsulated lysine and 4 g/(head ·d) of encapsulated methionine, and similar improvements when supplemented with methionine alone [
5]. Though some studies have reported impacts on carcass characteristics when cattle are fed elevated bypass methionine, results are conflicting, and others have reported similar carcass characteristics in supplemented and unsupplemented animals [
12].
Though days of postmortem aging improved tenderness as measured by WBSF, the addition of supplemental methionine showed little impact on this metric (
Table 6). No differences in shear force were observed between treatments in steaks aged 7, 14, or 21 d (
p ≥ 0.27). Following 28 d of postmortem aging, steaks from supplemented cattle tended to have decreased estimation of tenderness as measured by WBSF with increasing levels of methionine (
p = 0.09). This response agrees with previous work presented by [
14] where similar reductions in tenderness were observed in the LM of lambs supplemented with methionine. Furthermore, evidence shows when cattle are supplemented with both ruminally protected lysine and methionine, tenderness decreases [
5]. Still, [
15] reported that steaks with a shear force ≤ 4.3 kg were considered acceptable to consumers 86% of the time, and [
16] reported consumer acceptance 100% of the time when values were less than 3.9 kg of shear force. Although a linear increase in shear force was observed after 28-d of postmortem aging in the current study, all steaks fell well below the thresholds established in previous studies.
3.2. Experiment 2
No significant treatment × day interactions were observed for gene expression (
p ≥ 0.10), so the following section will focus on the main effects of methionine supplementation and day (
Table 7). There was a significant day effect for all genes of interest (
p ≤ 0.02). The abundance of eIF4EBP1 mRNA decreased after d 0. The abundance of AKT, IGF-I, MHC-I, MHC-IIA, and MHC-IIX mRNA were greatest on d 42 and 56, and the RAGA mRNA abundance was greatest on d 56 compared to d 0. The observed day effect was expected due to the normally observed accretion of protein as animals approach physiological maturity. The increase in skeletal muscle mass as animals progress along the growth curve is due to increased mTOR signaling resulting in the augmented transcription and translation [
17]. Though no significant treatment differences were observed, there was a tendency for reduced mRNA expression of AKT (
p = 0.08) and increased MHC-I expression (
p = 0.10) in the LM of methionine supplemented steers. Decreased expression of AKT could lead to a downregulation of mTORC1 which could inhibit downstream signaling for protein synthesis [
18]. However, an increase in MHC-I gene expression would suggest a shift in muscle fiber types.
There were no significant treatment × day interactions (
p ≥ 0.14) for protein expression as measured by Western blot (
Table 8). Similar to gene expression results, there was a significant day effect for all proteins evaluated (
p ≤ 0.04). Protein abundance of AKT was greatest on d 28, though abundance measured at all other time points was significantly lower than measured at d 0 (
p ≤ 0.05). Activated protein kinase alpha (AMPKα) protein abundance was greatest at d 0 and 56 and were significantly depressed (
p < 0.05) at d 28 and 42. Protein abundance of eIF4EBP1 was greatest on d 14 and 28, and pAMPKα abundance showed its greatest expression at d 42. Both Raptor and RAGA expression was greatest on d 42 and saw a general trend upward over time. Methionine supplementation did not significantly affect protein expression as measured by Western blot but tended to reduce the expression of Raptor when compared to 0MET (
p = 0.08). In neonatal pigs, feeding a diet with balanced levels of amino acids led to increased levels of RAGA-mTOR interaction [
19]. Level of phosphorylation of AKT, eIF4EBP1, RAGA, and Raptor were not measured; however, the functionality of these proteins due to methionine supplementation would be elucidated thusly. Activation of mTOR signaling pathway proteins by phosphorylation is necessary for subsequent effects on growth [
20,
21]. In bovine mammary cells, the addition of lysine and methionine resulted in increased levels of phosphorylated mTOR [
6]. The abundance of both MHC-I and total MHC-II proteins in LM biopsy samples were greatest on d 56 (
p < 0.05), but no treatment effect was observed.
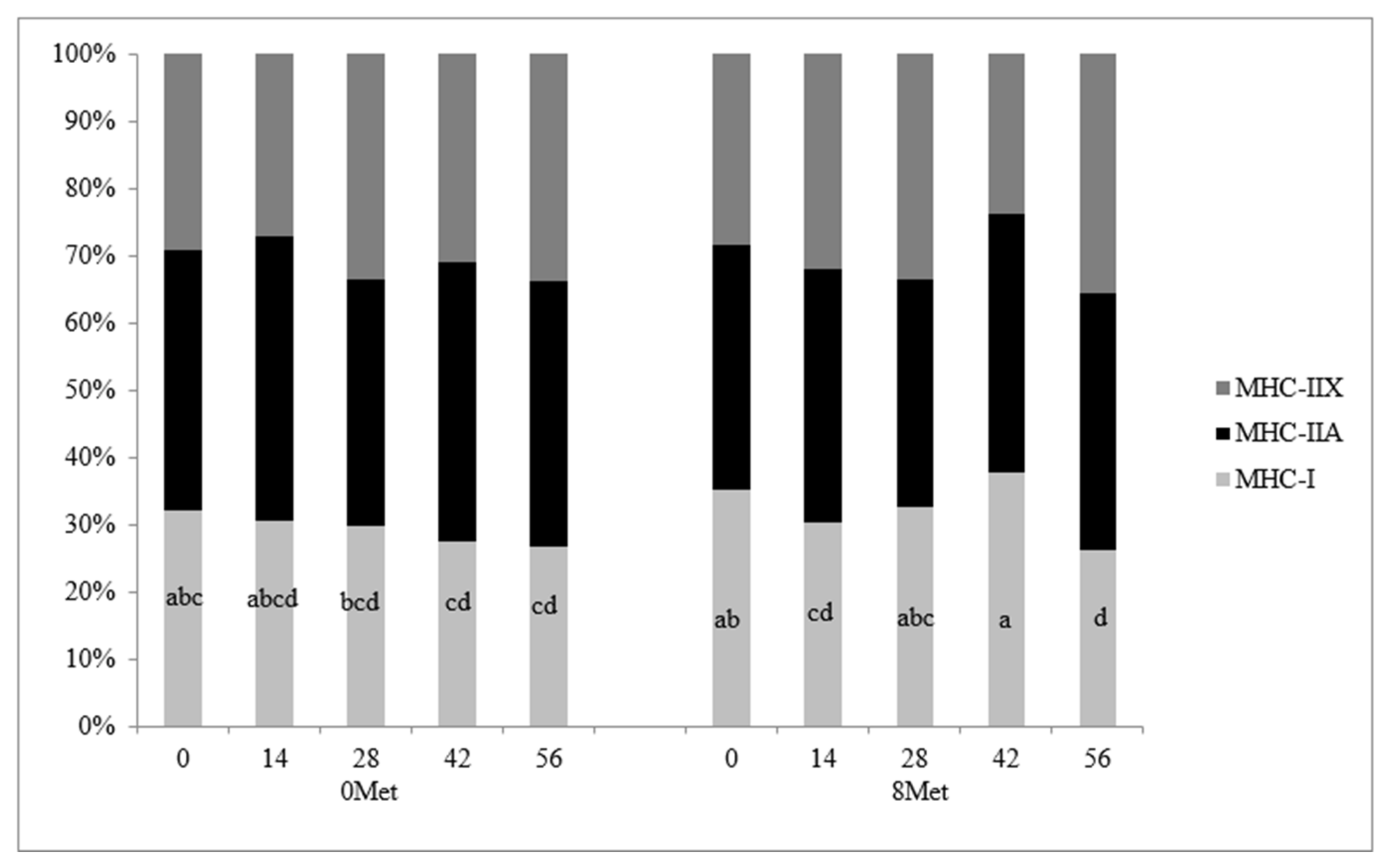
The distribution of skeletal muscle fiber types is presented in
Figure 1. A significant treatment × day interaction (
p = 0.03) was observed for MHC-I fibers, as this fiber type was reduced in 0MET steers and maintained its abundance over time in cattle supplemented with encapsulated methionine. This reflects the tendency for increased MHC-I gene expression in 8MET steers. This response in cattle supplemented with both methionine and lysine is similar to results found by [
5], where the combination of the two supplemented amino acids caused an increase in cross-sectional area of the muscle fibers but no change in the proportion of MHC-I fibers when compared to cattle supplemented with lysine only. No differences due to treatment, day, or the interaction thereof were detected in MHC-IIA fibers (
p > 0.16). A significant day effect was observed for MHC-IIX fibers (
p = 0.03), with the proportion of MHC-IIX fibers increasing from d 0 to 56, which agrees with [
22]. A significant day effect (
p < 0.01) was also observed for the cross-sectional area of all three fiber types (
Table 9 and
Figure 2). The cross-sectional area was significantly greater in all fiber types at d 56 when compared to the area measured at d 0 (
p < 0.05). This data mimics the findings of [
22], who concluded that fiber cross-sectional area increases as animals age.
No treatment × day interactions (
p ≥ 0.16) were observed for nuclei and satellite cell density (
Table 10). A tendency for a day effect on nuclei density was observed (
p = 0.10). In the methionine supplemented group, an increase in myonuclei was observed (
p = 0.05), meaning increased satellite cell incorporation into the muscle fiber [
11,
23]. This difference was not observed in previous work [
5]. A significant day effect (
p < 0.01) was observed for myonuclei, Myf5 positive satellite cells, PAX7 positive satellite cells, and dual expressing satellite cells. Over time, the density of myonuclei increased, with a 1.5-fold increase at d 56 when compared to d 0 (
Figure 3). This was expected, as cattle rely on the incorporation of nuclei into the myofiber to sustain muscle growth [
11]. The density of both Myf5 positive and dual expressing cells decreased from d 0 to d 56, while the density of PAX7 positive cells increased during this time. Supplementing steers with 8 g/(head d) of methionine significantly increased myonuclei density (
p = 0.05). Methionine also tended to reduce the density of Myf5 expressing cells (
p = 0.10) while decreasing the density of PAX7 positive satellite cells (
p = 0.09). The increased density of myofibrillar nuclei in methionine supplemented cattle could explain the improved LM area that was observed in Exp. 1, as this incorporation into the cell is necessary for muscle hypertrophy to follow. It could be proposed that longissimus biopsy samples for Exp. 2 were collected before the increase in skeletal muscle fiber cross-sectional area could be observed, as Exp. 1 animals were supplemented for 111 or 139 d. Myf5 is considered to be involved in the regulation of the proliferation of satellite cells [
24]. The observed tendency for increased density of PAX7 expressing satellite cells in the 8MET treatment suggested methionine supplementation increased proliferation and subsequent differentiation of satellite cells [
24,
25,
26].
This theory of increased satellite cell fusion into the muscle fiber was supported by the increased density of myonuclei in methionine supplemented cattle. The decreased expression of dual positive satellite cells on d 42 and d 56 for both treatments suggested an increase in the fusion of satellite cells into the muscle fiber. The increase PAX7 positive satellite cells seen at d 42 and 56 could result in increased expression of dual positive cells, as PAX7 positive satellite cells can produce dual positive daughter satellite cells [
27]. The combination of increased satellite cell fusion and proliferation are indicators of potential muscle hypertrophy, as satellite cells tend to fuse into the muscle fiber proceeding and concurrently with muscle protein synthesis [
28]. This is supported by the increase in density of nuclei not associated with satellite cells that have appeared to fuse into the existing muscle fiber to support post-natal skeletal muscle hypertrophy.