Nanomaterials and Essential Oils as Candidates for Developing Novel Treatment Options for Bovine Mastitis
Abstract
Simple Summary
Abstract
1. Introduction
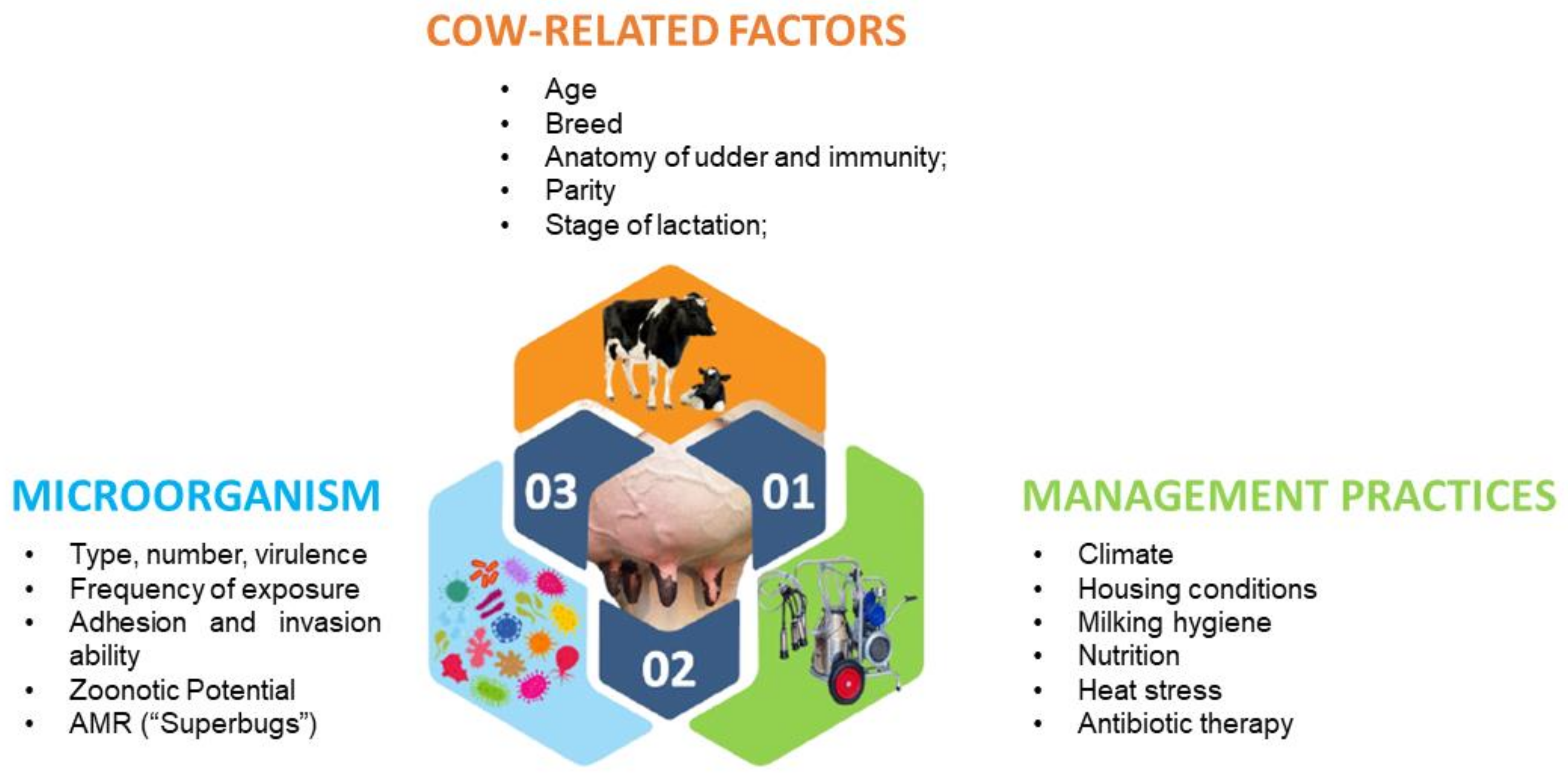
2. Farm Model with “Low Antibiotic Consumption”
3. New Approaches for the Development of Bovine Mastitis Products—Nanomaterials and Essential Oils
3.1. Essential Oils and Vegetal Extracts
3.2. Metallic Nanoparticles
4. Challenges
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Overview Report of the Directorate-General for Health and Food Safety on a Series of Audits Carried Out in 2016 in Order to Evaluate Member State Controls and Use of Indicators to Ensure the Welfare of Cattle on Dairy Farms. 2017. Available online: https://ec.europa.eu/food/audits-analysis/overview_reports (accessed on 1 March 2021).
- Reshi, A.A.; Husain, I.; Bhat, S.A.; Rehman, M.U.; Razak, R.; Bilal, S.; Mir, M.R. Bovine Mastitis as an Evolving Disease and Its Impact on the Dairy Industry. Int. J. Curr. Res. Rev. 2015, 7, 48–55. [Google Scholar]
- Li, N.; Richoux, R.; Boutinaud, M.; Martin, P.; Gagnaire, V. Role of somatic cells on dairy processes and products: A review. Dairy Sci. Technol. 2014, 94, 517–538. [Google Scholar] [CrossRef] [PubMed]
- Alhussien, M.N.; Dang, A.K. Impact of different seasons on the milk somatic and differential cell counts, milk cortisol and neutrophils functionality of three Indian native breeds of cattle. J. Therm. Biol. 2018, 78, 27–35. [Google Scholar] [CrossRef]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef] [PubMed]
- Dufour, S.; Labrie, J.; Jacques, M. The Mastitis Pathogens Culture Collection. Microbiol. Resour. Announc. 2019, 8, e00133-19. [Google Scholar] [CrossRef] [PubMed]
- Tarazona-Manrique, L.E.; Villate-Hernández, J.R.; Andrade-Becerra, R.J. Bacterial and fungal infectious etiology causing mastitis in dairy cows in the highlands of Boyacá (Colombia). Rev. Fac. Med. Vet. Zootec. 2019, 66, 208–218. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M. Causes, types, etiological agents, prevalence, diagnosis, treatment, prevention, effects on human health and future aspects of bovine mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Bórawski, P.; Pawlewicz, A.; Parzonko, A.; Jayson, K.H.; Holden, L. Factors Shaping Cow’s Milk Production in the EU. Sustainability 2020, 12, 420. [Google Scholar] [CrossRef]
- Fortune Business Insights. Market Research Report “Bovine Mastitis Market Size, Share &COVID-19 Impact Analysis, By Type (Clinical, and Sub-Clinical), By Product (Antibiotics, and Others), By Route of Administration (Intra-Mammary, and Systemic), By Therapy (Lactating Period and Dry Period) and Regional Forecast, 2020–2027. August 2020. Available online: https://www.fortunebusinessinsights.com/bovine-mastitis-market-103482 (accessed on 1 March 2021).
- Hill, E.K.; Li, J. Current and future prospects for nanotechnology in animal production. J. Anim. Sci. Biotechnol. 2017, 8, 26. [Google Scholar] [CrossRef]
- Youssef, F.S.; El-Banna, H.A.; Elzorba, H.Y.; Galal, A.M. Application of some nanoparticles in the field of veterinary medicine. Int. J. Vet. Sci. Med. 2019, 7, 78–93. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Advanced applications of nanotechnology in veterinary medicine. Environ. Sci. Pollut. Res. 2020, 27, 19073–19086. [Google Scholar] [CrossRef]
- Cerbu, C.; Kah, M.; White, J.; Astete, C.; Sabliov, C. Fate of Biodegradable Engineered Nanoparticles Used in Veterinary Medicine as Delivery Systems from a One Health Perspective. Molecules 2021, 26, 523. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.; Liu, L.; Hou, Y.; Li, L. Nanotechnology in agriculture, livestock, and aquaculture in China. A review. Agron. Sustain. Dev. 2015, 35, 369–400. [Google Scholar] [CrossRef]
- Gopi, M.; Pearlin, B.; Kumar, R.D.; Shanmathy, M.; Prabakar, G. Role of Nanoparticles in Animal and Poultry Nutrition: Modes of Action and Applications in Formulating Feed Additives and Food Processing. Int. J. Pharmacol. 2017, 13, 724–731. [Google Scholar] [CrossRef]
- Konkol, D.; Wojnarowski, K. The Use of Nanominerals in Animal Nutrition as a Way to Improve the Composition and Quality of Animal Products. J. Chem. 2018, 2018, 5927058. [Google Scholar] [CrossRef]
- Fesseha, H.; Degu, T.; Getachew, Y. Nanotechnology and its Application in Animal Production: A Review. Vet. Med. Open J. 2020, 5, 43–50. [Google Scholar] [CrossRef]
- ESVAC (European Medicines Agency). European Database of Sales of Veterinary Antimicrobial Agents. Available online: https://esvacbi.ema.europa.eu/analytics/saw.dll?PortalPages (accessed on 10 May 2021).
- ESVAC: Vision, Strategy and Objectives 2016–2020 Surveillance of Veterinary Antimicrobial Consumption. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/european-surveillance-veterinary-antimicrobial-consumption-esvac-vision-strategy-2016-2020_en.pd (accessed on 10 May 2021).
- ECDC; EFSA; EMA. ECDC, EFSA and EMA Joint Scientific Opinion on a list of outcome indicators as regards surveillance of antimicrobial resistance and antimicrobial consumption in humans and food-producing animals. EFSA J. 2017, 15, e05017. [Google Scholar] [CrossRef]
- Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018. Trends from 2010 to 2018—Tenth ESVAC Report. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2018-trends-2010-2018-tenth-esvac-report_en.pdf (accessed on 10 May 2021).
- Kimera, Z.I.; Mshana, S.E.; Rweyemamu, M.M.; Mboera, L.E.G.; Matee, M.I.N. Antimicrobial use and resistance in food-producing animals and the environment: An African perspective. Antimicrob. Resist. Infect. Control. 2020, 9, 37. [Google Scholar] [CrossRef]
- Aiassa, V.; Barnes, A.I.; Smania, A.M.; Albesa, I. Sublethal ciprofloxacin treatment leads to resistance via antioxidant systems in Proteus mirabilis. FEMS Microbiol. Lett. 2012, 327, 25–32. [Google Scholar] [CrossRef]
- Jørgensen, K.M.; Wassermann, T.; Jensen, P.Ø.; Hengzuang, W.; Molin, S.; Høiby, N.; Ciofu, O. Sublethal Ciprofloxacin Treatment Leads to Rapid Development of High-Level Ciprofloxacin Resistance during Long-Term Experimental Evolution of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, G.M.; Fromberg, A.; Ng, Y.; Gram, L. Sublethal Concentrations of Antibiotics Cause Shift to Anaerobic Metabolism in Listeria monocytogenes and Induce Phenotypes Linked to Antibiotic Tolerance. Front. Microbiol. 2016, 7, 1091. [Google Scholar] [CrossRef]
- Li, J.; Phulpoto, I.A.; Zhang, G.; Yu, Z. Acceleration of emergence of E. coli antibiotic resistance in a simulated sublethal concentration of copper and tetracycline co-contaminated environment. AMB Express 2021, 11, 14. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.; Joshi, L. Biocide Use in the Antimicrobial Era: A Review. Molecules 2021, 26, 2276. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial-Consumption-in-the-EU-Annual-Epidemiological-Report. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Antimicrobial-consumption-in-the-EU-Annual-Epidemiological-Report-2019.pdf (accessed on 10 May 2021).
- In the Red Zone Antimicrobial Resistance: Lessons from Romania. Available online: https://epha.org/wp-content/uploads/2017/06/In-the-red-zone-EPHA.pdf (accessed on 10 May 2021).
- Torumkuney, D.; Nica, M.; Nistor, I.; Vatcheva-Dobrevska, R.; Petrović, V.; Stoica, A.; Hanicar, B.; Antic, D.; Morrissey, I. Results from the Survey of Antibiotic Resistance (SOAR) 2014–16 in Bulgaria, Romania, Serbia and Croatia. J. Antimicrob. Chemother. 2018, 73, v2–v13. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Nolte, O. Antimicrobial resistance in the 21st century: A multifaceted challenge. Protein Pept. Lett. 2014, 21, 330–335. [Google Scholar] [CrossRef]
- Barriere, S.L. Clinical, economic and societal impact of antibiotic resistance. Expert Opin. Pharmacother. 2015, 16, 151–153. [Google Scholar] [CrossRef]
- Friedman, N.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef]
- Naylor, N.R.; Atun, R.; Zhu, N.; Kulasabanathan, K.; Silva, S.; Chatterjee, A.; Knight, G.M.; Robotham, J.V. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control. 2018, 7, 58. [Google Scholar] [CrossRef]
- Nathan, C. Resisting antimicrobial resistance. Nat. Rev. Genet. 2020, 18, 259–260. [Google Scholar] [CrossRef]
- Addressing Antimicrobial Resistance: Progress in the Animal Sector, But This Health Threat Remains a Challenge for the EU. Special Report by European Court of Auditors. 2019. Available online: https://www.stopamr.eu/wp-content/uploads/2019/11/ECA-AMR-report.pdf (accessed on 8 March 2021).
- Amann, S.; Neef, K.; Kohl, S. Antimicrobial resistance (AMR). Eur. J. Hosp. Pharm. 2019, 26, 175–177. [Google Scholar] [CrossRef]
- Davies, R.; Wales, A. Antimicrobial Resistance on Farms: A Review Including Biosecurity and the Potential Role of Disinfectants in Resistance Selection. Compr. Rev. Food Sci. Food Saf. 2019, 18, 753–774. [Google Scholar] [CrossRef] [PubMed]
- Blake, O.; Glaser, M.; Bertolini, L.; te Brömmelstroet, M. How policies become best practices: A case study of best practice making in an EU knowledge sharing project. Eur. Plan. Stud. 2020, 1–21. [Google Scholar] [CrossRef]
- Deruelle, T. A tribute to the foot soldiers: European health agencies in the fight against antimicrobial resistance. Health Econ. Policy Law 2021, 16, 23–37. [Google Scholar] [CrossRef]
- Sinha, M.K.; Thombare, N.N.; Mondal, B. Subclinical Mastitis in Dairy Animals: Incidence, Economics, and Predisposing Factors. Sci. World J. 2014, 2014, 523984. [Google Scholar] [CrossRef] [PubMed]
- Krömker, V.; Leimbach, S. Mastitis treatment-Reduction in antibiotic usage in dairy cows. Reprod. Domest. Anim. 2017, 52, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Manimaran, A.; Kumaresan, A.; Jeyakumar, S.; Sreela, L.; Mooventhan, P.; Sivaram, M. Mastitis effects on reproductive performance in dairy cattle: A review. Trop. Anim. Health Prod. 2017, 49, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Getaneh, A.M.; Gebremedhin, E.Z. Meta-analysis of the prevalence of mastitis and associated risk factors in dairy cattle in Ethiopia. Trop. Anim. Health Prod. 2017, 49, 697–705. [Google Scholar] [CrossRef]
- Derakhshani, H.; Fehr, K.B.; Sepehri, S.; Francoz, D.; De Buck, J.; Barkema, H.W.; Plaizier, J.C.; Khafipour, E. Invited review: Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. J. Dairy Sci. 2018, 101, 10605–10625. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, A.-M.; Liski, E.; Pyörälä, S.; Taponen, S. Pathogen-specific production losses in bovine mastitis. J. Dairy Sci. 2018, 101, 9493–9504. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Imran, M. Diagnosis of bovine mastitis: From laboratory to farm. Trop. Anim. Health Prod. 2018, 50, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Koop, G.; Lam, T.; Van Der Lans, I.; Vernooij, J.; Hogeveen, H. Farm-level risk factors for bovine mastitis in Dutch automatic milking dairy herds. J. Dairy Sci. 2019, 102, 4522–4535. [Google Scholar] [CrossRef]
- Gomes, F.; Saavedra, M.J.; Henriques, M. Bovine mastitis disease/pathogenicity: Evidence of the potential role of microbial biofilms. Pathog. Dis. 2016, 74, 74. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Cerempei, A.; Muresan, E.I.; Cimpoeşu, N.; Carp-Cărare, C.; Rimbu, C.M. Dyeing and antibacterial properties of aqueous extracts from quince (Cydonia oblonga) leaves. Ind. Crop. Prod. 2016, 94, 216–225. [Google Scholar] [CrossRef]
- Cristina, R.; Cristina, H.; Petruța, A.; Eleonora, G.; Cătălin, C.-C.; Carmen, C.; Viorel, F.; Mariana, G.; Gabriel, D.; Anca, M. The Antibacterial Activity and Synergies between Morusin and Some Antibiotics against MRSA Strains—Preliminary Study. Sci. Pap. Vet. Med. Iasi Rom. 2017, 61, 39–47. [Google Scholar]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Miron, A.; Aelenei, P.; Trifan, A.; Rimbu, C.M.; Horhogea, C.; Luca, S.; Neagu, A.; Wolfram, E.; Aprotosoaie, A. Plant-derived products as antibiotic enhancers and antibiotic-resistance modifying agents. In Proceedings of the 67th International Congress and Annual Meeting of the Society for Medicinal Plant and Natural Product Research (GA), Innsbruck, Austria, 1–5 September 2019. [Google Scholar]
- Aelenei, P.; Luca, S.V.; Horhogea, C.E.; Rimbu, C.M.; Dimitriu, G.; Macovei, I.; Silion, M.; Aprotosoaie, A.C.; Miron, A. Morus alba leaf extract: Metabolite profiling and interactions with antibiotics against Staphylococcus spp. including MRSA. Phytochem. Lett. 2019, 31, 217–224. [Google Scholar] [CrossRef]
- Ginovyan, M.; Trchounian, A. Novel approach to combat antibiotic resistance: Evaluation of some Armenian herb crude extracts for their antibiotic modulatory and antiviral properties. J. Appl. Microbiol. 2019, 127, 472–480. [Google Scholar] [CrossRef]
- Mahavy, C.E.; Duez, P.; ElJaziri, M.; Rasamiravaka, T. African Plant-Based Natural Products with Antivirulence Activities to the Rescue of Antibiotics. Antibiotics 2020, 9, 830. [Google Scholar] [CrossRef]
- Gahamanyi, N.; Song, D.-G.; Cha, K.H.; Yoon, K.-Y.; Mboera, L.E.; Matee, M.I.; Mutangana, D.; Amachawadi, R.G.; Komba, E.V.; Pan, C.-H. Susceptibility of Campylobacter Strains to Selected Natural Products and Frontline Antibiotics. Antibiotics 2020, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Cunsolo, V.; Schicchi, R.; Chiaramonte, M.; Inguglia, L.; Arizza, V.; Cusimano, M.; Schillaci, D.; Di Francesco, A.; Saletti, R.; lo Celso, F.; et al. Identification of New Antimicrobial Peptides from Mediterranean Medical Plant Charybdis pancration (Steinh.) Speta. Antibiotics 2020, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Aelenei, P.; Rimbu, C.M.; Horhogea, C.E.; Lobiuc, A.; Neagu, A.-N.; Dunca, S.I.; Motrescu, I.; Dimitriu, G.; Aprotosoaie, A.C.; Miron, A. Prenylated phenolics as promising candidates for combination antibacterial therapy: Morusin and kuwanon G. Saudi Pharm. J. 2020, 28, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Wafa, W.M.; Ahmed, R.H.; Ramadan, M.A.-H. Synergistic effects of pomegranate and rosemary extracts in combination with antibiotics against antibiotic resistance and biofilm formation of Pseudomonas aeruginosa. Braz. J. Microbiol. 2020, 51, 1079–1092. [Google Scholar] [CrossRef]
- Casciaro, B.; Mangiardi, L.; Cappiello, F.; Romeo, I.; Loffredo, M.R.; Iazzetti, A.; Calcaterra, A.; Goggiamani, A.; Ghirga, F.; Mangoni, M.L.; et al. Naturally-Occurring Alkaloids of Plant Origin as Potential Antimicrobials against Antibiotic-Resistant Infections. Molecules 2020, 25, 3619. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef]
- Kou, J.; Xin, T.Y.; McCarron, P.; Gupta, G.; Dureja, H.; Satija, S.; Mehta, M.; Bakshi, H.A.; Tambuwala, M.M.; Collet, T.; et al. Going Beyond Antibiotics: Natural Plant Extracts as an Emergent Strategy to Combat Biofilm-Associated Infections. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Sakarikou, C.; Kostoglou, D.; Simões, M.; Giaouris, E. Exploitation of plant extracts and phytochemicals against resistant Salmonella spp. in biofilms. Food Res. Int. 2020, 128, 108806. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Yin, G.; Wang, J.; Wang, P.; Chen, Z.-Y.; Wang, T.; Ren, G. Antimicrobial activities of Asian ginseng, American ginseng, and notoginseng. Phytother. Res. 2020, 34, 1226–1236. [Google Scholar] [CrossRef]
- Bouari, C.; Nadăs, G.; Chirilă, F.; Răpuntean, S.; Cătoi, C.; Tăbăran, F.A.; Gal, A.; Taulescu, M.; Fit, N. Prevalence and Antimicrobial Susceptibility Profiles of Pathogens Isolated from Bovine Mastitis Milk in Transylvania. Rom. Bull. Univ. Agric. Sci. Vet. 2016, 73, 329–333. [Google Scholar] [CrossRef][Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, R.I.; Oprean, R.; Benedec, D.; Hanganu, D.; Duma, M.; Oniga, I.; Vlase, L. LC-MS Analysis, Antioxidant and Antimicrobial Activities for Five Species of Mentha Cultivated in Romania. Dig. J. Nanomater. Biostruct. 2014, 9, 559–566. [Google Scholar]
- Bobis, O.; Dezmirean, D.S.; Tomos, L.; Chirila, F.; Marghitas, L.A. Influence of phytochemical profile on antibacterial activity of different medicinal plants against gram-positive and gram-negative bacteria. Appl. Biochem. Microbiol. 2015, 51, 113–118. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Pașca, C.; Mărghitaș, L.; Dezmirean, D.; Bobiș, O.; Bonta, V.; Chirilă, F.; Matei, I.; Fiț, N. Medicinal Plants Based Products Tested on Pathogens Isolated from Mastitis Milk. Molecules 2017, 22, 1473. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safety—Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef] [PubMed]
- Olaru, D.G.; Olaru, A.; Kassem, G.H.; Popescu-Driga, M.V.; Pinosanu, L.R.; Dumitrascu, D.I.; Popescu, E.L.; Hermann, D.M.; Popa-Wagner, A. Toxicity and Health Impact of Nanoparticles. Basic Biology and Clinical Perspective. Rom. J. Morphol. Embryol. 2019, 60, 787–792. [Google Scholar] [PubMed]
- Ahmed, T.; Shahid, M.; Noman, M.; Bilal Khan Niazi, M.; Zubair, M.; Almatroudi, A.; Khurshid, M.; Tariq, F.; Mumtaz, R.; Li, B. Bioprospecting a native silver-resistant Bacillus safensis strain for green synthesis and subsequent antibacterial and anticancer activities of silver nanoparticles. J. Adv. Res. 2020, 24, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Zhu, H.; Du, M.; Fox, L.; Zhu, M.-J. Bactericidal effects of Cinnamon cassia oil against bovine mastitis bacterial pathogens. Food Control 2016, 66, 291–299. [Google Scholar] [CrossRef]
- Taga, I.; Lan, C.Q.; Altosaar, I. Plant Essential Oils and Mastitis Disease: Their Potential Inhibitory Effects on Pro-inflammatory Cytokine Production in Response to Bacteria Related Inflammation. Nat. Prod. Commun. 2012, 7, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Nardoni, S.; Ebani, V.V.; D’Ascenzi, C.; Pistelli, L.; Mancianti, F. Sensitivity of Entomopathogenic Fungi and Bacteria to Plants Secondary Metabolites, for an Alternative Control of Rhipicephalus (Boophilus) microplus in Cattle. Front. Pharmacol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Abboud, M.; El Rammouz, R.; Jammal, B.; Sleiman, M. In Vitro and In Vivo Antimicrobial Activity of Two Essential Oils Thymus Vulgaris and Lavandula Angustifolia against Bovine Staphylococcus and Streptococcus Mastitis Pathogen. Middle East J. Agric. Res. 2015, 4, 975–983. [Google Scholar]
- Ksouri, S.; Djebir, S.; Hadef, Y.; Benakhla, A. Survey of Bovine Mycotic Mastitis in Different Mammary Gland Statuses in Two North-Eastern Regions of Algeria. Mycopathologia 2015, 179, 327–331. [Google Scholar] [CrossRef]
- Cerioli, M.F.; Moliva, M.V.; Cariddi, L.N.; Reinoso, E.B. Effect of the Essential Oil of Minthostachys verticillata (Griseb.) Epling and Limonene on Biofilm Production in Pathogens Causing Bovine Mastitis. Front. Vet. Sci. 2018, 5, 146. [Google Scholar] [CrossRef]
- Grzesiak, B.; Kołodziej, B.; Głowacka, A.; Krukowski, H. The Effect of Some Natural Essential Oils Against Bovine Mastitis Caused by Prototheca zopfii Isolates In Vitro. Mycopathologia 2018, 183, 541–550. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, G.S.; Micheletti, A.C.; Padilha, R.G.; de Souza de Paula, J.; Alves, F.M.; Leal, C.R.B.; Garcez, F.R.; Garcez, W.S.; Yoshida, N.C. Antimicrobial Potential of Essential Oils from Cerrado Plants against Multidrug–Resistant Foodborne Microorganisms. Molecules 2020, 25, 3296. [Google Scholar] [CrossRef]
- Báez-Magaña, M.; Ochoa-Zarzosa, A.; Alva-Murillo, N.; Salgado-Garciglia, R.; López-Meza, J.E. Lipid-Rich Extract from Mexican Avocado Seed (Persea americana var. drymifolia) Reduces Staphylococcus aureus Internalization and Regulates Innate Immune Response in Bovine Mammary Epithelial Cells. J. Immunol. Res. 2019, 2019, 7083491. [Google Scholar] [CrossRef] [PubMed]
- Procópio, T.F.; Moura, M.C.; Bento, E.F.L.; Soares, T.; Coelho, L.C.B.B.; Bezerra, R.P.; Mota, R.A.; Porto, A.L.F.; Paiva, P.M.G.; Napoleão, T.H. Looking for alternative treatments for bovine and caprine mastitis: Evaluation of the potential of Calliandra surinamensis leaf pinnulae lectin (CasuL), both alone and in combination with antibiotics. Microbiol. Open 2019, 8, e869. [Google Scholar] [CrossRef]
- Gomes, F.; Rodrigues, M.E.; Martins, N.; Ferreira, I.C.; Henriques, M. Phenolic Plant Extracts Versus Penicillin G: In Vitro Susceptibility of Staphylococcus aureus Isolated from Bovine Mastitis. Pharmaceuticals 2019, 12, 128. [Google Scholar] [CrossRef]
- Kher, M.N.; Sheth, N.R.; Bhatt, V.D. In Vitro Antibacterial Evaluation of Terminalia chebula as an Alternative of Antibiotics against Bovine Subclinical Mastitis. Anim. Biotechnol. 2019, 30, 151–158. [Google Scholar] [CrossRef]
- Mordmuang, A.; Brouillette, E.; Voravuthikunchai, S.P.; Malouin, F. Evaluation of a Rhodomyrtus tomentosa ethanolic extract for its therapeutic potential on Staphylococcus aureus infections using in vitro and in vivo models of mastitis. Vet. Res. 2019, 50, 49. [Google Scholar] [CrossRef]
- Sobrinho Santos, E.M.; Almeida, A.C.; Santos, H.O.; Cangussu, A.R.; Costa, K.S.; Alves, J.N.; Bertucci Barbosa, L.C.; Aguiar, R.W.S. Mechanism of Brassica oleracea performance in bovine infectious mastitis by bioinformatic analysis. Microb. Pathog. 2019, 129, 19–29. [Google Scholar] [CrossRef]
- Ksouri, S.; Djebir, S.; Bentorki, A.A.; Gouri, A.; Hadef, Y.; Benakhla, A. Antifungal activity of essential oils extract from Origanum floribundum Munby, Rosmarinus officinalis L. and Thymus ciliatus Desf. against Candida albicans isolated from bovine clinical mastitis. J. Mycol. Méd. 2017, 27, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.-W.; Cha, C.-N.; Lee, S.-M.; Kim, M.-J.; Park, J.-Y.; Yoo, C.-Y.; Son, S.-E.; Kim, S.; Lee, H.-J. Therapeutic effect of oregano essential oil on subclinical bovine mastitis caused by Staphylococcus aureus and Escherichia coli. Korean J. Vet. Res. 2015, 55, 253–257. [Google Scholar] [CrossRef]
- Fratini, F.; Casella, S.; Leonardi, M.; Pisseri, F.; Ebani, V.V.; Pistelli, L.; Pistelli, L. Antibacterial activity of essential oils, their blends and mixtures of their main constituents against some strains supporting livestock mastitis. Fitoterapia 2014, 96, 1–7. [Google Scholar] [CrossRef]
- Rapuntean, S.; Bolfă, P.F.; Taulescu, M.; Catoi, C. In Vitro Evaluation of the Antimicrobial Properties of Some Plant Essential Oils against Clinical Isolates of Prototheca Spp. Rom. Biotechnol. Lett. 2011, 16, 6146–6152. [Google Scholar]
- Lopes, T.S.; Fontoura, P.S.; Oliveira, A.; Rizzo, F.A.; Silveira, S.; Streck, A.F. Use of plant extracts and essential oils in the control of bovine mastitis. Res. Vet. Sci. 2020, 131, 186–193. [Google Scholar] [CrossRef]
- Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Gołębiewski, M. Silver and Copper Nanoparticles—An Alternative in Future Mastitis Treatment and Prevention? Int. J. Mol. Sci. 2019, 20, 1672. [Google Scholar] [CrossRef] [PubMed]
- Akintelu, S.A.; Bo, Y.; Folorunso, A.S. A Review on Synthesis, Optimization, Mechanism, Characterization, and Antibacterial Application of Silver Nanoparticles Synthesized from Plants. J. Chem. 2020, 2020, 3189043. [Google Scholar] [CrossRef]
- Fei Guo, C.; Sun, T.; Cao, F.; Liu, Q.; Ren, Z. Metallic nanostructures for light trapping in energy-harvesting devices. Light Sci. Appl. 2014, 3, e161. [Google Scholar] [CrossRef]
- Ghasemzadeh, F.; Esmaeili Shayan, M. Nanotechnology in the Service of Solar Energy Systems. In Nanotechnology and the Environment; IntechOpen: London, UK, 2020. [Google Scholar]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K. Potential applications of engineered nanoparticles in medicine and biology: An update. JBIC J. Biol. Inorg. Chem. 2018, 23, 1185–1204. [Google Scholar] [CrossRef]
- Pöttler, M.; Cicha, I.; Unterweger, H.; Janko, C.; Friedrich, R.P.; Alexiou, C. Nanoparticles for regenerative medicine. Nanomedicine 2019, 14, 1929–1933. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Falchi, L.; Khalil, W.; Hassan, M.; Marei, W.F. Perspectives of nanotechnology in male fertility and sperm function. Int. J. Vet. Sci. Med. 2018, 6, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.M.; Rhoads, C.E.; Mustapha, P.A.; Tardif, S.; Parrish, J.J.; Willard, S.T.; Ryan, P.L. Treatment of boar sperm with nanoparticles for improved fertility. Theriogenology 2019, 137, 75–81. [Google Scholar] [CrossRef]
- Neculai-Valeanu, A.-S.; Ariton, A. Game-Changing Approaches in Sperm Sex-Sorting: Microfluidics and Nanotechnology. Animals 2021, 11, 1182. [Google Scholar] [CrossRef]
- Popa, V.I.; Căpraru, A.-M.; Grama, S.; Măluţan, T. Nanoparticles Based on Modified Lignins with Biocide Properties. Cellul. Chem. Technol. 2011, 45, 221–226. [Google Scholar]
- Liang, W.; Yu, A.; Wang, G.; Zheng, F.; Jia, J.; Xu, H. Chitosan-based nanoparticles of avermectin to control pine wood nematodes. Int. J. Biol. Macromol. 2018, 112, 258–263. [Google Scholar] [CrossRef]
- Zikeli, F.; Vinciguerra, V.; D’Annibale, A.; Capitani, D.; Romagnoli, M.; Mugnozza, G.S. Preparation of Lignin Nanoparticles from Wood Waste for Wood Surface Treatment. Nanomaterials 2019, 9, 281. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Truşcă, R.-D.; Ilie, C.-I.; Oprea, O.-C.; Andronescu, E. Innovative Antimicrobial Chitosan/ZnO/Ag NPs/Citronella Essential Oil Nanocomposite—Potential Coating for Grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef]
- Tan, C.; McClements, D. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef]
- Alirezalu, K.; Yaghoubi, M.; Poorsharif, L.; Aminnia, S.; Kahve, H.; Pateiro, M.; Lorenzo, J.; Munekata, P. Antimicrobial Polyamide-Alginate Casing Incorporated with Nisin and ε-Polylysine Nanoparticles Combined with Plant Extract for Inactivation of Selected Bacteria in Nitrite-Free Frankfurter-Type Sausage. Foods 2021, 10, 1003. [Google Scholar] [CrossRef]
- Medina, J.; Calabi-Floody, M.; Aponte, H.; Santander, C.; Paneque, M.; Meier, S.; Panettieri, M.; Cornejo, P.; Borie, F.; Knicker, H. Utilization of Inorganic Nanoparticles and Biochar as Additives of Agricultural Waste Composting: Effects of End-Products on Plant Growth, C and Nutrient Stock in Soils from a Mediterranean Region. Agronomy 2021, 11, 767. [Google Scholar] [CrossRef]
- Semida, W.; Abdelkhalik, A.; Mohamed, G.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.; Ali, E. Foliar Application of Zinc Oxide Nanoparticles Promotes Drought Stress Tolerance in Eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Picchi, V.; Gobbi, S.; Fattizzo, M.; Zefelippo, M.; Faoro, F. Chitosan Nanoparticles Loaded with N-Acetyl Cysteine to Mitigate Ozone and Other Possible Oxidative Stresses in Durum Wheat. Plants 2021, 10, 691. [Google Scholar] [CrossRef]
- García-Sánchez, S.; Gala, M.; Žoldák, G. Nanoimpact in Plants: Lessons from the Transcriptome. Plants 2021, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Khalofah, A.; Kilany, M.; Migdadi, H. Phytostimulatory Influence of Comamonas testosteroni and Silver Nanoparticles on Linum usitatissimum L. under Salinity Stress. Plants 2021, 10, 790. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, H.; Abiri, R.; Sanusi, R.; Shaharuddin, N.; Noor, A.; Ab Shukor, N.; Abdul-Hamid, H.; Ahmad, S. Toward a Better Understanding of Metal Nanoparticles, a Novel Strategy from Eucalyptus Plants. Plants 2021, 10, 929. [Google Scholar] [CrossRef]
- Iravani, S. Methods for Preparation of Metal Nanoparticles. In Metal Nanoparticles; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 53. [Google Scholar] [CrossRef]
- Neculai-Văleanu, S.; Ariton, A.-M.; Matei, A.-C.; Mădescu, B.-M.; Davidescu, M.-A.; Poroșnicu, I.; Creangă, Ș. Green Synthesis of Silver Nanoparticles Using Curcuma Longa Plant Extract and Their Possible Applications. In Proceedings of the International Scientific Congress “Life Sciences, A Challenge for The Future”, Iași, Romania, 23–25 October 2014; Filodiritto Editore: Iași, Romania, 2019; pp. 288–293. [Google Scholar]
- Marinescu, L.; Ficai, D.; Oprea, O.; Marin, A.; Ficai, A.; Andronescu, E.; Holban, A.-M. Optimized Synthesis Approaches of Metal Nanoparticles with Antimicrobial Applications. J. Nanomater. 2020, 2020, 6651207. [Google Scholar] [CrossRef]
- Devatha, C.P.; Thalla, A.K. Green Synthesis of Nanomaterials. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Noah, N. Green synthesis: Characterization and application of silver and gold nanoparticles. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Cele, T. Preparation of Nanoparticles. In Engineered Nanomaterials—Health and Safety; IntechOpen: London, UK, 2020. [Google Scholar]
- Raj, S.; Mali, S.C.; Trivedi, R. Green synthesis and characterization of silver nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem. Biophys. Res. Commun. 2018, 503, 2814–2819. [Google Scholar] [CrossRef] [PubMed]
- Das, P.E.; Abu-Yousef, I.A.; Majdalawieh, A.F.; Narasimhan, S.; Poltronieri, P. Green Synthesis of Encapsulated Copper Nanoparticles Using a Hydroalcoholic Extract of Moringa oleifera Leaves and Assessment of Their Antioxidant and Antimicrobial Activities. Molecules 2020, 25, 555. [Google Scholar] [CrossRef]
- Kamath, V.; Chandra, P.; Jeppu, G.P. Comparative study of using five different leaf extracts in the green synthesis of iron oxide nanoparticles for removal of arsenic from water. Int. J. Phytoremediat. 2020, 22, 1278–1294. [Google Scholar] [CrossRef] [PubMed]
- Muthu, K.; Priya, S. Green synthesis, characterization and catalytic activity of silver nanoparticles using Cassia auriculata flower extract separated fraction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Kuča, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials 2020, 10, 766. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Devanesan, S.; Alfuraydi, A.A.; Vishnubalaji, R.; Munusamy, M.A.; Murugan, K.; Nicoletti, M.; Benelli, G. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: Antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. Int. J. Nanomed. 2016, 11, 4439–4449. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Gupta, A.; Kim, B.S. Ultrasound Assisted Green Synthesis of Silver and Iron Oxide Nanoparticles Using Fenugreek Seed Extract and Their Enhanced Antibacterial and Antioxidant Activities. BioMed Res. Int. 2019, 2019, 1714358. [Google Scholar] [CrossRef]
- Yuan, C.; Huo, C.; Gui, B.; Cao, W. Green synthesis of gold nanoparticles using Citrus maxima peel extract and their catalytic/antibacterial activities. IET Nanobiotechnol. 2017, 11, 523–530. [Google Scholar] [CrossRef]
- Tan Sian Hui Abdullah, H.S.; Aqlili Riana Mohd Asseri, S.N.; Khursyiah Wan Mohamad, W.N.; Kan, S.-Y.; Azmi, A.A.; Yong Julius, F.S.; Chia, P.W. Green synthesis, characterization and applications of silver nanoparticle mediated by the aqueous extract of red onion peel. Environ. Pollut. 2021, 271, 116295. [Google Scholar] [CrossRef]
- Behravan, M.; Panahi, A.H.; NaghiZadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F.; Álvarez-Suarez, J.M. Green Synthesis of Silver Nanoparticles Using Astragalus tribuloides Delile. Root Extract: Characterization, Antioxidant, Antibacterial, and Anti-Inflammatory Activities. Nanomaterials 2020, 10, 2383. [Google Scholar] [CrossRef]
- Sinsinwar, S.; Sarkar, M.K.; Suriya, K.R.; Nithyanand, P.; Vadivel, V. Use of agricultural waste (coconut shell) for the synthesis of silver nanoparticles and evaluation of their antibacterial activity against selected human pathogens. Microb. Pathog. 2018, 124, 30–37. [Google Scholar] [CrossRef]
- Neculai-Valeanu, A.S.; Ariton, A.M.; Madescu, B.; Ghe, V.; Creanga, S. Harnessing Agri-Waste for Green Synthesis of Silver Nanoparticles. In Proceedings of the ISB-INMA TEH’ 2020 Proceedings, Bucharest, Romania, 30 October 2020; pp. 172–175. [Google Scholar]
- Vasyliev, G.; Vorobyova, V.; Skiba, M.; Khrokalo, L. Green Synthesis of Silver Nanoparticles Using Waste Products (Apricot and Black Currant Pomace) Aqueous Extracts and Their Characterization. Adv. Mater. Sci. Eng. 2020, 2020, 4505787. [Google Scholar] [CrossRef]
- Okpara, E.C.; Fayemi, O.E.; Sherif, E.-S.M.; Junaedi, H.; Ebenso, E.E. Green Wastes Mediated Zinc Oxide Nanoparticles: Synthesis, Characterization and Electrochemical Studies. Materials 2020, 13, 4241. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Salayová, A. Green-Synthesized Silver Nanoparticles and Their Potential for Antibacterial Applications. In Bacterial Pathogenesis and Antibacterial Control; InTech: London, UK, 2018. [Google Scholar]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Atapour, A.; Amani, A.M.; Savar Dashtaki, A.; Babapoor, A.; Arjmand, O. Green synthesis of silver nanoparticles toward bio and medical applications: Review study. Artif. Cells Nanomed. Biotechnol. 2018, 46, S855–S872. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Sedki, M. Green Synthesis of Chitosan-Silver/Gold Hybrid Nanoparticles for Biomedical Applications. Breast Cancer 2019, 2000, 79–84. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, Z.; Manzoor, M.Z.; Mujahid, M.; Faheem, Z.; Adnan, A. Optimization for biogenic microbial synthesis of silver nanoparticles through response surface methodology, characterization, their antimicrobial, antioxidant, and catalytic potential. Sci. Rep. 2021, 11, 770. [Google Scholar] [CrossRef]
- Ekaji, F.A.; Akujobi, C.O.; Umeh, S.I. Optimization of Selected Process Parameters Affecting Yield of Green Synthesized Silver Nanoparticles and Their Antibacterial Activity. Biotechnol. J. Int. 2021, 25, 25–36. [Google Scholar] [CrossRef]
- Wady, A.F.; Machado, A.L.; Foggi, C.C.; Zamperini, C.A.; Zucolotto, V.; Moffa, E.B.; Vergani, C.E. Effect of a Silver Nanoparticles Solution onStaphylococcus aureusandCandidaspp. J. Nanomater. 2014, 2014, 545279. [Google Scholar] [CrossRef]
- Mocan, L.; Matea, C.; Tabaran, F.A.; Mosteanu, O.; Pop, T.; Puia, C.; Agoston-Coldea, L.; Gonciar, D.; Kalman, E.; Zaharie, G.; et al. Selective in vitro photothermal nano-therapy of MRSA infections mediated by IgG conjugated gold nanoparticles. Sci. Rep. 2016, 6, 39466. [Google Scholar] [CrossRef]
- Hibbitts, A.; O’Leary, C. Emerging Nanomedicine Therapies to Counter the Rise of Methicillin-Resistant Staphylococcus aureus. Materials 2018, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, N.K.; Ammar, A.M.; El-Naenaeey, E.-S.Y.M.; El Damaty, H.M.; Elazazy, A.A.; Hefny, A.A.; Shaker, A.; Eldesoukey, I.E. Antimicrobial and antibiofilm potentials of cinnamon oil and silver nanoparticles against Streptococcus agalactiae isolated from bovine mastitis: New avenues for countering resistance. BMC Vet. Res. 2021, 17, 136. [Google Scholar] [CrossRef]
- Orellano, M.S.S.; Bohl, L.P.; Breser, M.L.; Isaac, P.; Falcone, R.D.; Porporatto, C. A comparative study of antimicrobial activity of differently-synthesized chitosan nanoparticles against bovine mastitis pathogens. Soft Matter 2021, 17, 694–703. [Google Scholar] [CrossRef]
- Aguayo, P.R.; Larenas, T.B.; Godoy, C.A.; Rivas, B.C.; González-Casanova, J.; Rojas-Gómez, D.; Fuentes, N.C. Antimicrobial and Antibiofilm Capacity of Chitosan Nanoparticles against Wild Type Strain of Pseudomonas sp. Isolated from Milk of Cows Diagnosed with Bovine Mastitis. Antibiotics 2020, 9, 551. [Google Scholar] [CrossRef]
- Orellano, M.S.; Isaac, P.; Breser, M.L.; Bohl, L.P.; Conesa, A.; Falcone, R.D.; Porporatto, C. Chitosan nanoparticles enhance the antibacterial activity of the native polymer against bovine mastitis pathogens. Carbohydr. Polym. 2019, 213, 1–9. [Google Scholar] [CrossRef]
- Hozyen, H.F.; Ibrahim, E.S.; Khairy, E.A.; El-Dek, S.I. Enhanced antibacterial activity of capped zinc oxide nanoparticles: A step towards the control of clinical bovine mastitis. Vet. World 2019, 12, 1225–1232. [Google Scholar] [CrossRef]
- Yu, L.; Shang, F.; Chen, X.; Ni, J.; Yu, L.; Zhang, M.; Sun, D.; Xue, T. The anti-biofilm effect of silver-nanoparticle-decorated quercetin nanoparticles on a multi-drug resistant Escherichia coli strain isolated from a dairy cow with mastitis. PeerJ 2018, 6, e5711. [Google Scholar] [CrossRef]
- Omara, S.T. MIC and MBC of Honey and Gold Nanoparticles against methicillin-resistant (MRSA) and vancomycin-resistant (VRSA) coagulase-positive S. aureus isolated from contagious bovine clinical mastitis. J. Genet. Eng. Biotechnol. 2017, 15, 219–230. [Google Scholar] [CrossRef]
- Sankar, P. New Therapeutic Strategies to Control and Treatment of Bovine Mastitis. Vet. Med. Open J. 2016, 1, e7–e8. [Google Scholar] [CrossRef]
- Machado, G.T.P.; Veleirinho, M.B.; Mazzarino, L.; Filho, L.C.P.M.; Maraschin, M.; Cerri, R.L.A.; Kuhnen, S. Development of propolis nanoparticles for the treatment of bovine mastitis: In vitro studies on antimicrobial and cytotoxic activities. Can. J. Anim. Sci. 2019, 99, 713–723. [Google Scholar] [CrossRef]
- Seven, P.T.; Seven, I.; Baykalir, B.G.; Mutlu, S.I.; Salem, A.Z.M. Nanotechnology and nano-propolis in animal production and health: An overview. Ital. J. Anim. Sci. 2018, 17, 921–930. [Google Scholar] [CrossRef]
- Kazemi, F.; Divsalar, A.; Saboury, A.A.; Seyedarabi, A. Propolis nanoparticles prevent structural changes in human hemoglobin during glycation and fructation. Colloids Surf. B Biointerfaces 2019, 177, 188–195. [Google Scholar] [CrossRef] [PubMed]
- El Hamid, H.M.A.; Abdel-Aziz, M.S.; Abu Naeem, F.M. Antimicrobial Efficacy of Nanopropolis Coated Vs Silver-Curcumin Nanoparticles Coated Gutta-Percha Points on Various Microbial Species. A Comparative In Vitro Study. Egypt. Dent. J. 2020, 66, 1893–1902. [Google Scholar] [CrossRef]
- Seven, P.T.; Seven, I.; Karakus, S.; Mutlu, S.I.; Arkali, G.; Sahin, Y.M.; Kilislioglu, A. Turkish Propolis and Its Nano Form Can Ameliorate the Side Effects of Cisplatin, Which Is a Widely Used Drug in the Treatment of Cancer. Plants 2020, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Soni, G.; Yadav, K.S. Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi Pharm. J. 2016, 24, 133–139. [Google Scholar] [CrossRef]
- Krishna, A.N.; Reddy, M.V.Y.; Reddy, M.C.B.; Padmini, I. Formulation, Evaluation of Nano Copper Gel for Treatment of Clinical Mastitis. J. Pharmacol. Res. 2017, 11, 547–554. [Google Scholar]
- Mohsenabadi, N.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A. Physical and Antimicrobial Properties of Starch-Carboxy Methyl Cellulose Film Containing Rosemary Essential Oils Encapsulated in Chitosan Nanogel. Int. J. Biol. Macromol. 2018, 112, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H. Recent Advances in Experimental Basic Research on Graphene and Graphene-Based Nanostructures. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 023001. [Google Scholar] [CrossRef]
- Xia, M.-Y.; Xie, Y.; Yu, C.-H.; Chen, G.-Y.; Li, Y.-H.; Zhang, T.; Peng, Q. Graphene-based nanomaterials: The promising active agents for antibiotics-independent antibacterial applications. J. Control. Release 2019, 307, 16–31. [Google Scholar] [CrossRef]
- Teodorescu, F.; Quéniat, G.; Foulon, C.; Lecoeur, M.; Barras, A.; Boulahneche, S.; Medjram, M.S.; Hubert, T.; Abderrahmani, A.; Boukherroub, R.; et al. Transdermal skin patch based on reduced graphene oxide: A new approach for photothermal triggered permeation of ondansetron across porcine skin. J. Control. Release 2017, 245, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 8. [Google Scholar] [CrossRef]
- Prasad, K.; Lekshmi, G.S.; Ostrikov, K.; Lussini, V.; Blinco, J.; Mohandas, M.; Vasilev, K.; Bottle, S.; Bazaka, K.; Ostrikov, K. Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci. Rep. 2017, 7, 1591. [Google Scholar] [CrossRef] [PubMed]
- El-Shafai, N.; El-Khouly, M.E.; El-Kemary, M.; Ramadan, M.; Eldesoukey, I.; Masoud, M. Graphene oxide decorated with zinc oxide nanoflower, silver and titanium dioxide nanoparticles: Fabrication, characterization, DNA interaction, and antibacterial activity. RSC Adv. 2019, 9, 3704–3714. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Ejaz, M.; Mullah, M. Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. Int. J. Biol. Macromol. 2018, 107, 194–203. [Google Scholar] [CrossRef]
- De Moraes, A.C.M.; Lima, B.A.; de Faria, A.F.; Brocchi, M.; Alves, O.L. Graphene oxide-silver nanocomposite as a promising biocidal agent against methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2015, 10, 6847–6861. [Google Scholar] [CrossRef]
- Yousefi, M.; Dadashpour, M.; Hejazi, M.; Hasanzadeh, M.; Behnam, B.; de la Guardia, M.; Shadjou, N.; Mokhtarzadeh, A. Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater. Sci. Eng. C 2017, 74, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene Oxide-Based Nanocomposites Decorated with Silver Nanoparticles as an Antibacterial Agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Bugli, F.; Cacaci, M.; Palmieri, V.; Di Santo, R.; Torelli, R.; Ciasca, G.; Di Vito, M.; Vitali, A.; Conti, C.; Sanguinetti, M.; et al. Curcumin-loaded graphene oxide flakes as an effective antibacterial system against methicillin-resistant Staphylococcus aureus. Interface Focus 2018, 8, 20170059. [Google Scholar] [CrossRef]
- Shin, Y.C.; Lee, J.H.; Jin, L.; Kim, M.J.; Kim, Y.-J.; Hyun, J.K.; Jung, T.-G.; Hong, S.W.; Han, D.-W. Stimulated myoblast differentiation on graphene oxide-impregnated PLGA-collagen hybrid fibre matrices. J. Nanobiotechnol. 2015, 13, 21. [Google Scholar] [CrossRef]
- Vijaya Sekhar, K.; Debroy, S.; Miriyala, V.P.K.; Acharyya, S.G.; Acharyya, A. Self-Healing Phenomena of Graphene: Potential and Applications. Open Phys. 2016, 14, 364–370. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Lasocka, I.; Jastrzębska, E.; Szulc-Dąbrowska, L.; Skibniewski, M.; Pasternak, I.; Hubalek Kalbacova, M.; Skibniewska, E.M. The effects of graphene and mesenchymal stem cells in cutaneous wound healing and their putative action mechanism. Int. J. Nanomed. 2019, 14, 2281–2299. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Fusco, L.; León, V.; Martín, C.; Criado, A.; Sosa, S.; Vázquez, E.; Tubaro, A.; Prato, M. Differential cytotoxic effects of graphene and graphene oxide on skin keratinocytes. Sci. Rep. 2017, 7, 40572. [Google Scholar] [CrossRef]
- Goyal, A.K.; Singh, R.; Chauhan, G.; Rath, G. Non-invasive systemic drug delivery through mucosal routes. Artif. Cells Nanomed. Biotechnol. 2018, 46, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Ng, K.W.; Lau, W.M.; Khutoryanskiy, V.V. Silica Nanoparticles in Transmucosal Drug Delivery. Pharmaceutics 2020, 12, 751. [Google Scholar] [CrossRef]
- Vllasaliu, D. Non-Invasive Drug Delivery Systems. Pharmaceutics 2021, 13, 611. [Google Scholar] [CrossRef]
- Singhal, M.; Lapteva, M.; Kalia, Y.N. Formulation challenges for 21st century topical and transdermal delivery systems. Expert Opin. Drug Deliv. 2017, 14, 705–708. [Google Scholar] [CrossRef]
- Boldeanu, L.; Boldeanu, M.V.; Bogdan, M.; Meca, A.D.; Coman, C.G.; Buca, B.R.; Tartau, C.G.; Tartau, L.M. Immunological approaches and therapy in burns (Review). Exp. Ther. Med. 2020, 20, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Iftime, M.-M.; Tartau, L.M.; Marin, L. New formulations based on salicyl-imine-chitosan hydrogels for prolonged drug release. Int. J. Biol. Macromol. 2020, 160, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Mititelu, L.T.; Marin, L. Drug delivery systems based on biocompatible imino-chitosan hydrogels for local anticancer therapy. Drug Deliv. 2018, 25, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Porzio, W.; Marin, L. Hydrogels Based on Imino-Chitosan Amphiphiles as a Matrix for Drug Delivery Systems. Polymers 2020, 12, 2687. [Google Scholar] [CrossRef]
- Mao, K.-L.; Fan, Z.-L.; Yuan, J.-D.; Chen, P.-P.; Yang, J.-J.; Xu, J.; ZhuGe, D.-L.; Jin, B.-H.; Zhu, Q.-Y.; Shen, B.-X.; et al. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf. B Biointerfaces 2017, 160, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Ozkahraman, B.; Emeriewen, K.; Saleh, G.M.; Thanh, N.T.K. Engineering hydrogel nanoparticles to enhance transdermal local anaesthetic delivery in human eyelid skin. RSC Adv. 2020, 10, 3926–3930. [Google Scholar] [CrossRef]
- Bashyal, S.; Shin, C.Y.; Hyun, S.M.; Jang, S.W.; Lee, S. Preparation, Characterization, and In Vivo Pharmacokinetic Evaluation of Polyvinyl Alcohol and Polyvinyl Pyrrolidone Blended Hydrogels for Transdermal Delivery of Donepezil HCl. Pharmaceutics 2020, 12, 270. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Stan, D.; Tanase, C.; Avram, M.; Apetrei, R.; Mincu, N.; Mateescu, A.L. Wound healing applications of creams and “smart” hydrogels. Exp. Dermatol. 2021. [Google Scholar] [CrossRef]
- Biondi, M.; Borzacchiello, A.; Mayol, L.; Ambrosio, L. Nanoparticle-Integrated Hydrogels as Multifunctional Composite Materials for Biomedical Applications. Gels 2015, 1, 162–178. [Google Scholar] [CrossRef]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Arno, M.C.; Inam, M.; Weems, A.C.; Li, Z.; Binch, A.L.A.; Platt, C.I.; Richardson, S.M.; Hoyland, J.A.; Dove, A.P.; O’Reilly, R.K. Exploiting the role of nanoparticle shape in enhancing hydrogel adhesive and mechanical properties. Nat. Commun. 2020, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Wang, C.; Wang, Y.; Xu, W.; Hu, J.; Cheng, Y. A Nanocomposite Hydrogel with Potent and Broad-Spectrum Antibacterial Activity. ACS Appl. Mater. Interfaces 2018, 10, 15163–15173. [Google Scholar] [CrossRef]
- Monerris, M.; Broglia, M.F.; Yslas, E.I.; Barbero, C.A.; Rivarola, C.R. Highly effective antimicrobial nanocomposites based on hydrogel matrix and silver nanoparticles: Long-lasting bactericidal and bacteriostatic effects. Soft Matter 2019, 15, 8059–8066. [Google Scholar] [CrossRef] [PubMed]
- Helmiyati, N.G.; Abbas, G.H.; Budianto, E. Nanocomposite hydrogel-based biopolymer modified with silver nanoparticles as an antibacterial material for wound treatment. J. Appl. Pharm. Sci. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- El-Hady, M.M.A.; Saeed, S.E.-S. Antibacterial Properties and pH Sensitive Swelling of Insitu Formed Silver-Curcumin Nanocomposite Based Chitosan Hydrogel. Polymers 2020, 12, 2451. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Aunkor, T.H.; Raihan, T.; Prodhan, S.H.; Metselaar, H.S.C.; Malik, S.U.F.; Azad, A.K. Antibacterial activity of graphene oxide nanosheet against multidrug resistant superbugs isolated from infected patients. R. Soc. Open Sci. 2020, 7, 200640. [Google Scholar] [CrossRef]
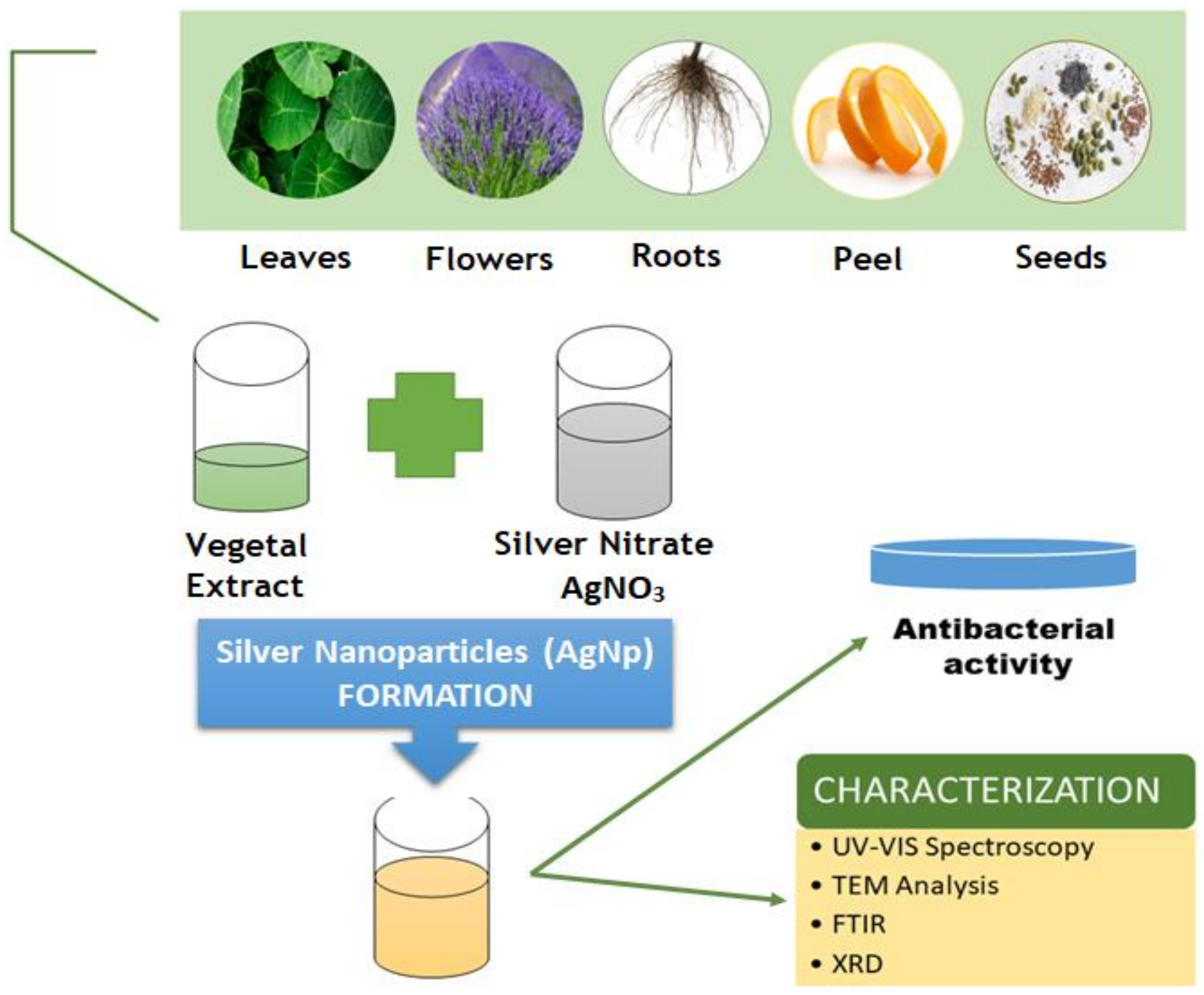
| Type of Oil/Plant Extract | Type of Study | Tested Pathogens | Effect | References |
|---|---|---|---|---|
| Siam weed EO (Chromolaena squalida) Guabiroba Verde EO (Campomanesia sessiliflora) Rapanea punctata EO (Myrsine guianensis) (Matayba guianensis) EO Negramina EO (Siparuna guianensis) Canelinha EO (Ocotea minarum) Endlicheria EO (Endlicheria paniculata) | In vitro | Staphylococcus aureusEscherichia coli Escherichia coli (β−lactamase producer) Pseudomonas aeruginosa | All of the tested oils demonstrated moderate to excellent activity against four bacterial species, including Salmonella Typhi and oxacillin-resistant Staphylococcus aureus. | de Jesus et al. 2020 [92] |
| FMexican Avocado Seed (Persea americana var. drymifolia) | In vitro | Staphylococcus aureus | Lipid extract from avocado seed inhibits the Staphylococcus aureus internalization into bovine mammary epithelial cells (bMECs) and modulates the innate immune response (IIR) | Báez-Magaña et al. 2019 [93] |
| Pink Powderpuff (Calliandra surinamensis) | In vitro | Staphylococcus isolates from either bovine (Ssp6PD and Sa) or caprine (Ssp5D and Ssp01) mastitic milk samples | Calliandra surinamensis leaf pinnulae lectin displayed a bacteriostatic and antibiofilm agent against certain bovine and caprine mastitis isolates. When used in conjunction with either ampicillin (against one isolate) or tetracycline (against two isolates), it showed synergistic effect. | Procópio et al. 2019 [94] |
| Southern blue gum (Eucalyptus globulus) Walnut (Juglans regia) | In vitro | Staphylococcus aureus | Eucalyptus globulus extract alone appeared to have a bacteriostatic effect against Staphylococcus aureus, up to 8 hours of incubation. When opposed to the positive control, Eucalyptus globulus and Juglans regia extracts alone had a minor inhibitory effect over time. | Gomes et al. 2019 [95] |
| Black Myrobalan (Terminalia chebula) extract | In Vitro | Staphylococcus aureus | The 500 µg/mL concentration of Terminalia chebula ethyl acetate extract was as effective as standard amoxicillin | Kher et al. 2019 [96] |
| Rose Myrtle Rhodomyrtus tomentosa (Rose myrtle) leaves | In vitro In vivo | Staphylococcus aureus | The ethanolic extract showed good antibacterial activity in vitro, a reduction of activity being observed in vivo. | Mordmuang et al. 2019 [97] |
| Wild cabbage (Brassica oleracea) | In vitro | Staphylococcus aureus Escherichia coli Klebsiella pneumoniae | Interferes in the mechanisms of action of genes such as MTOR and TP53, thus may be a possible alternative for developing herbal formulations for bovine mastitis. | Sobrinho Santos et al. 2019 [98] |
| Piperina EO (Minthostachys verticillata) | In vitro | Escherichia coli Bacillus pumilus Enterococcus faecium | EO affected the formation of biofilm and revealed the antibacterial capacity of EO and limonene. | Cerioli et al. 2018 [90] |
| Oregano EO (Origanum floribundu) Morrocan Thyme EO (Thymus ciliatus) Rosemary EO (Rosmarinus officinalis) | In vitro | Candida albicans | The three essential oils showed highly anticandidal activity, with values ranging from 15.02 to 31.08 g/mL. | Ksouri et al. 2017 [99] |
| Cinnamon EO (Cinnamomum zeylandicum) Geranium EO (Pelargonium graveolens) Clove EO (Syzygium aromaticum) Thyme EO (Thymus vulgaris) Lavender EO (Lavandula angustifolia) Basil EO (Ocimum basilicum) Rosemary EO (Rosmarinus officinalis) Clary sage EO (Salvia sclarea) | In vitro | Eight strains of Prototheca zopfii isolated from mastitic milk | Many of the oils tested were effective against algal strains, but cinnamon, clove, and thyme were the most effective. | Grzesiak et al. 2016 [91] |
| Oregano EO (Origanum vulgare) | In vivo | Staphylococcus aureus and Escherichia coli | In the group of cows treated intramammary with oregano essential oil (OEO), the number of somatic cells (SCCs) and number of white blood cells (WBC) were significantly decreased and Staphylococcus aureus and Escherichia coli were not present in milk samples. | Cho et al. 2015 [100] |
| Thyme EO (Thymus vulgaris); Lavender EO (Lavandula angustifolia) | In vitro In vivo Intramammary and External applications (oils mixed in vaseline) | Staphylococcus sp. And Streptococcus sp. | External use of these oils in vaseline resulted in a greater antibacterial action, for a 100% recovery rate with thymus essential oils. | Abboud et al. 2015 [51] |
| Cinnamon EO (Cinnamomum zeylanicum) Bergamot EO (Citrus bergamia Risso) Tasmanian blue gum EO (Eucalyptus globulus) Fennel EO (Foeniculum vulgare) Marjoram EO (Origanum majorana) Oregano EO (Origanum vulgare) Rosemary EO (Rosmarinus officinalis) Winter savory EO (Satureja montana) Thyme EO (Thymus vulgaris) | In vitro | Staphylococcus aureus Staphylococcus chromogenes Staphylococcus sciuri Staphylococcus warneri Staphylococcus xylosus Escherichia coli | The mixture containing Thymus vulgaris and Winter savory essential oils exhibited the best inhibitory activity against all the tested bacterial strains. The artificial mixtures composed of carvacrol/thymol, respectively carvacrol/thymol/p-cymene presented strong inhibition against Staphylococcus aureus and Staphylococcus sciuri | Fratini et al. 2014 [101] |
| Summer savory (Satureja hortensis) Silver fir (Abies alba) | In vitro | Prototheca zopfii isolates (from mastitic milk and bovine feces) Prototheca wickerhami | Fir oil is presented lower anti-algae activity as compared to summer savory | Bouari et al. 2011 [102] |
| Type of Nanoparticles | Type of Study | Tested Pathogens | Effect | References |
|---|---|---|---|---|
| Silver nanoparticles (AgNPs) | in vitro | Streptococcus agalactiae | AgNPs showed reasonable antimicrobial and relatively low antibiofilm activities, while cinnamon oil showed high antimicrobial and antibiofilm against biofilms of Streptococcus agalactiae isolates. | Abd El-Aziz et al. 2021 [160] |
| Chitosan nanoparticles (Ch-NPs) | in vitro | Staphylococcus aureus | The smaller Ch-NPs were active in preventing Staphylococcus aureus from entering the cells, but they did not stimulate the formation of pro-inflammatory cytokines. The results support the assertion that Ch-NPs are an excellent bacteriostatic agent, capable of preventing the replication of bovine mastitis pathogens in the udder. | Orellano et al. 2021 [161] |
| Chitosan nanoparticles (Ch-NPs) | in vitro | Pseudomonas sp. strain isolated from bovine milk samples | The nanoparticles inhibited biofilm formation and could eliminate pre-existing mature biofilms. | Rivera Aguayo et al. 2020 [162] |
| Chitosan nanoparticles (Ch-NPs) | in vitro | Staphylococcus aureus | The antimicrobial activity of Ch-NP was higher than that of the native polymer used in the nanocomposites’ preparation. Ch-NPs impaired bacterial cell membranes and prevented the development of bacterial biofilms without impacting the viability of bovine cells. | Orellano et al, 2019 [163] |
| inc oxide nanoparticles (ZnO-NPs) | in vitro | Staphylococcus aureus Escherichia coli Klebsiella pneumoniae isolated from milk of affected cows. | At the same concentrations, capped dispersed ZnO-NPs demonstrated greater antibacterial activity against Staphylococcus aureus, Escherichia coli Klebsiella pneumoniae than non-capped nanoparticles. Gram-positive Staphylococcus aureus showed higher resistance to ZnO-NPs synthesized as compared to Gram-negative Escherichia coli Klebsiella pneumoniae. | Hozyen et al. 2019 [164] |
| Silver-nanoparticle-decorated quercetin nanoparticles (QA NPs) | in vitro | Escherichia coli multi-drug resistant strain isolated from a dairy cow with mastitis | QA NPs showed higher antibacterial and anti-biofilm properties in a multi-drug resistant Escherichia coli strain isolated from a dairy cow with mastitis, as compared to Ag NPs and quercetin alone. | Yu et al. 2018 [165] |
| Honey and Gold Nanoparticles | in vitro | Methicillin-resistant (MRSA) and vancomycin-resistant (VRSA) coagulase-positive Staphylococcus aureus isolated from contagious bovine clinical mastitis | AuNPs, 30 nm in size, presented visible anti- Methicillin-resistant (MRSA) and anti-vancomycin-resistant (VRSA) activities in vitro | Omara et al. 2017 [166] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neculai-Valeanu, A.S.; Ariton, A.M.; Mădescu, B.M.; Rîmbu, C.M.; Creangă, Ş. Nanomaterials and Essential Oils as Candidates for Developing Novel Treatment Options for Bovine Mastitis. Animals 2021, 11, 1625. https://doi.org/10.3390/ani11061625
Neculai-Valeanu AS, Ariton AM, Mădescu BM, Rîmbu CM, Creangă Ş. Nanomaterials and Essential Oils as Candidates for Developing Novel Treatment Options for Bovine Mastitis. Animals. 2021; 11(6):1625. https://doi.org/10.3390/ani11061625
Chicago/Turabian StyleNeculai-Valeanu, Andra Sabina, Adina Mirela Ariton, Bianca Maria Mădescu, Cristina Mihaela Rîmbu, and Şteofil Creangă. 2021. "Nanomaterials and Essential Oils as Candidates for Developing Novel Treatment Options for Bovine Mastitis" Animals 11, no. 6: 1625. https://doi.org/10.3390/ani11061625
APA StyleNeculai-Valeanu, A. S., Ariton, A. M., Mădescu, B. M., Rîmbu, C. M., & Creangă, Ş. (2021). Nanomaterials and Essential Oils as Candidates for Developing Novel Treatment Options for Bovine Mastitis. Animals, 11(6), 1625. https://doi.org/10.3390/ani11061625







