Monoglyceride Blend Reduces Mortality, Improves Nutrient Digestibility, and Intestinal Health in Broilers Subjected to Clinical Necrotic Enteritis Challenge
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Feed Additives
2.3. Design and Animal Husbandry
2.4. Necrotic Enteritis Challenge
2.5. Performance Measurement
2.6. Sampling and Intestinal Lesion Scoring
2.7. Cecal Bacterial Quantification
2.8. RNA Extraction and cDNA Synthesis
2.9. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.10. Apparent Ileal Nutrient Digestibility
2.11. Data Analysis
3. Results
3.1. Bird Performance
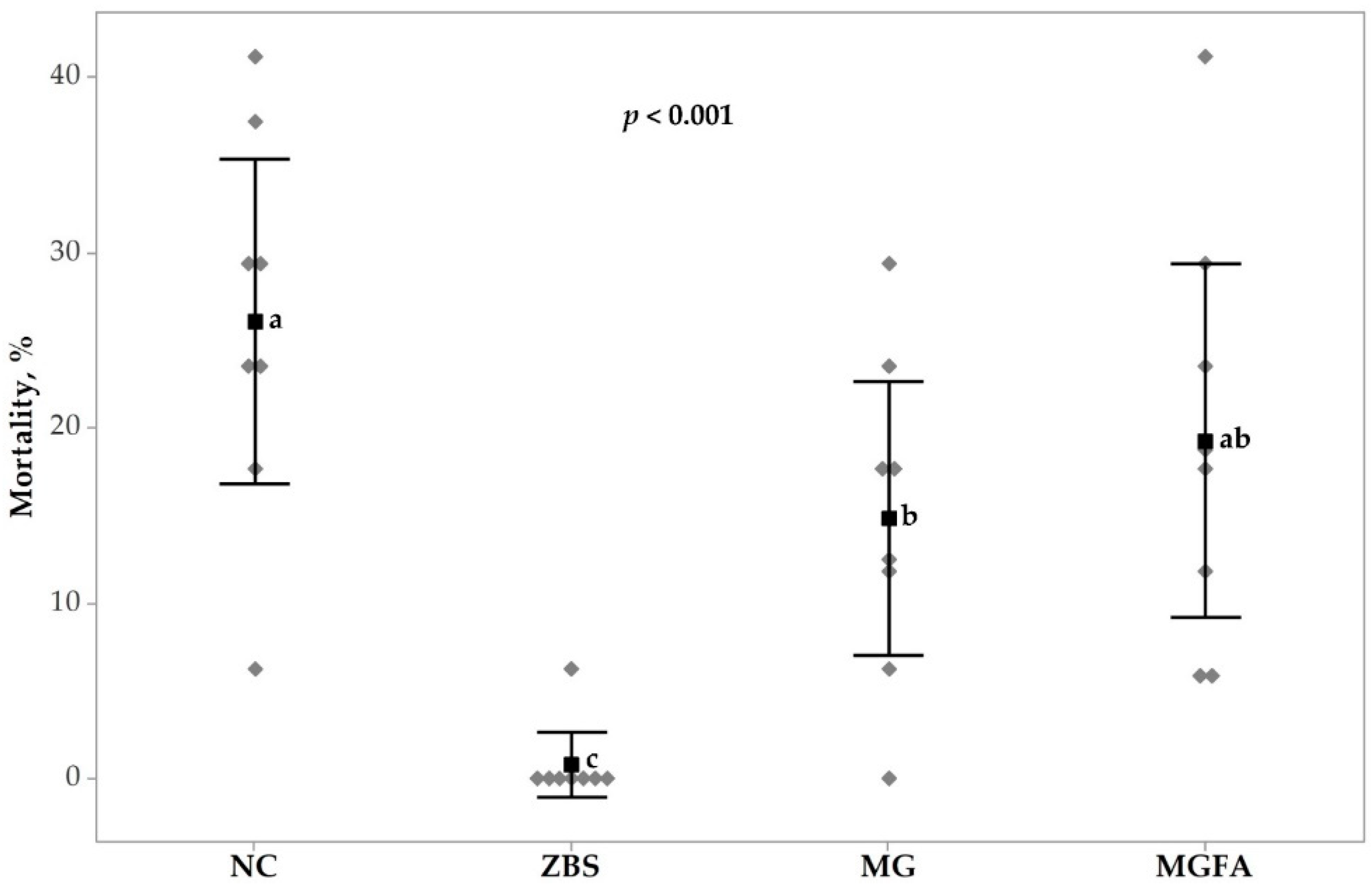
3.2. Necrotic Enteritis Caused Mortality and Lesion Scores
3.3. Cecal Bacterial Quantification
3.4. Expression of Jejunal Genes
3.5. Apparent Ileal GE and CP Digestibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, K.K.; Songer, J.G.; Uzal, F.A. Diagnosing Clostridial enteric disease in poultry. J. Vet. Diagn. Invest. 2013, 25, 314–327. [Google Scholar] [CrossRef]
- Wade, B.; Keyburn, A. The true cost of necrotic enteritis. Poult. World 2015, 31, 16–17. [Google Scholar]
- Immerseel, F.V.; Buck, J.D.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathol. 2004, 33, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Kaldhusdal, M.; Schneitz, C.; Hofshagen, M.; Skjerve, E. Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Dis. 2001, 149–156. [Google Scholar] [CrossRef]
- Hofacre, C.; Mathis, G.; Quiroz, M. Natural alternatives to prevent necrotic enteritis. Int. Poult. Prod. 2005, 13, 7–9. [Google Scholar]
- M’Sadeq, S.A.; Wu, S.; Swick, R.A.; Choct, M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim. Nutr. 2015, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef]
- Seal, B.S.; Lillehoj, H.S.; Donovan, D.M.; Gay, C.G. Alternatives to antibiotics: A symposium on the challenges and solutions for animal production. Anim. Health Res. Rev. 2013, 14, 78–87. [Google Scholar] [CrossRef]
- Adil, S.; Banday, T.; Bhat, G.A.; Mir, M.S.; Rehman, M. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet. Med. Int. 2010, 479485. [Google Scholar] [CrossRef]
- Polycarpo, G.V.; Andretta, I.; Kipper, M.; Cruz-Polycarpo, V.C.; Dadalt, J.C.; Rodrigues, P.H.M.; Albuquerque, R. Meta-analytic study of organic acids as an alternative performance-enhancing feed additive to antibiotics for broiler chickens. Poult. Sci. 2017, 96, 3645–3653. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef]
- Namkung, H.; Yu, H.; Gong, J.; Leeson, S. Antimicrobial activity of butyrate glycerides toward Salmonella Typhimurium and Clostridium perfringens. Poult. Sci. 2011, 90, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Patten, J.; Waldroup, P. Use of organic acids in broiler diets. Poult. Sci. 1988, 67, 1178–1182. [Google Scholar] [CrossRef]
- Kabara, J.J. Antimicrobial agents derived from fatty acids. J. Am. Oil Chem. Soc. 1984, 61, 397–403. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, T.; Tsvetkova, V.; Najdenski, H.M. Antibacterial study of the medium-chain fatty acids and their 1-monoglycerides: Individual effects and synergistic relationships. Pol. J. Microbiol. 2009, 58, 43–47. [Google Scholar] [PubMed]
- Leeson, S.; Namkung, H.; Antongiovanni, M.; Lee, E. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult. Sci. 2005, 84, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rubio, C.; Ordonez, C.; Abad-González, J.; Garcia-Gallego, A.; Honrubia, M.P.; Mallo, J.J.; Balana-Fouce, R. Butyric acid-based feed additives help protect broiler chickens from Salmonella Enteritidis infection. Poult. Sci. 2009, 88, 943–948. [Google Scholar] [CrossRef]
- Gharib-Naseri, K.; Kheravii, S.K.; Wu, S.B. Buffered formic acid and a monoglyceride blend coordinately alleviate subclinical necrotic enteritis impact in broiler chickens. Poult. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Bedford, A.; Yu, H.; Hernandez, M.; Squires, E.; Leeson, S.; Gong, J. Effects of fatty acid glyceride product SILOhealth 104 on the growth performance and carcass composition of broiler chickens. Poult. Sci. 2018, 97, 1315–1323. [Google Scholar] [CrossRef]
- Bedford, A.; Yu, H.; Squires, E.; Leeson, S.; Gong, J. Effects of supplementation level and feeding schedule of butyrate glycerides on the growth performance and carcass composition of broiler chickens. Poult. Sci. 2017, 96, 3221–3228. [Google Scholar] [CrossRef]
- NHMRC. Australian Code for the Care and Use of Animals for Scientific Purposes, 8th ed.; National Health and Medical Research Council: Canberra, Australia, 2013. [Google Scholar]
- Aviagen. Ross Broiler Management Handbook; Aviagen Ltd.: Newbridge Midlothian, UK, 2014. [Google Scholar]
- Rodgers, N.J.; Swick, R.A.; Geier, M.S.; Moore, R.J.; Choct, M.; Wu, S.-B. A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2015, 59, 38–45. [Google Scholar] [CrossRef]
- Wu, S.-B.; Stanley, D.; Rodgers, N.; Swick, R.A.; Moore, R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014, 169, 188–197. [Google Scholar] [CrossRef]
- Keyburn, A.L.; Sheedy, S.A.; Ford, M.E.; Williamson, M.M.; Awad, M.M.; Rood, J.I.; Moore, R.J. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 2006, 74, 6496–6500. [Google Scholar] [CrossRef] [PubMed]
- Kheravii, S.K.; Swick, R.A.; Choct, M.; Wu, S.B. Coarse particle inclusion and lignocellulose-rich fiber addition in feed benefit performance and health of broiler chickens. Poult. Sci. 2017, 96, 3272–3281. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.G.; Siragusa, G.R. Quantitative analysis of the intestinal bacterial community in one- to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J. Appl. Microbiol. 2007, 102, 1138–1149. [Google Scholar] [CrossRef]
- Requena, T.; Burton, J.; Matsuki, T.; Munro, K.; Simon, M.A.; Tanaka, R.; Watanabe, K.; Tannock, G.W. Identification, detection, and enumeration of human Bifidobacterium species by PCR targeting the transaldolase gene. Appl. Environ. Microbiol. 2002, 68, 2420–2427. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Yu, B.; He, J.; Yu, J.; Mao, X.; Wang, J.; Luo, J.; Huang, Z.; Cheng, G. Spray-dried chicken plasma improves intestinal digestive function and regulates intestinal selected microflora in weaning piglets. J. Anim. Sci. 2015, 93, 2967–2976. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Lee, D.-H.; Zo, Y.-G.; Kim, S.-J. Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism. Appl. Environ. Microbiol. 1996, 62, 3112–3120. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, 1–14. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Gharib-Naseri, K.; Kheravii, S.; Keerqin, C.; Swick, R.A.; Choct, M.; Wu, S.-B. Differential expression of intestinal genes in necrotic enteritis challenged broiler chickens with two different Clostridium perfringens strains. Poult. Sci. 2020. [Google Scholar] [CrossRef]
- Du, E.; Wang, W.; Gan, L.; Li, Z.; Guo, S.; Guo, Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-Q.; Zhang, Z.-W.; Yao, H.-D.; Wang, L.-L.; Liu, T.; Yu, X.-Y.; Li, S.; Xu, S.-W. Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res. Vet. Sci. 2013, 95, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, S.; Liu, G.; Zhao, J.; Jiao, H.; Wang, X.; Song, Z.; Lin, H. Vitamin A deficiency impairs mucin expression and suppresses the mucosal immune function of the respiratory tract in chicks. PLoS ONE 2015, 10, e0139131. [Google Scholar] [CrossRef]
- Yang, F.; Lei, X.; Rodriguez-Palacios, A.; Tang, C.; Yue, H. Selection of reference genes for quantitative real-time PCR analysis in chicken embryo fibroblasts infected with avian leukosis virus subgroup. J. BMC Res. Notes 2013, 6, 1–5. [Google Scholar] [CrossRef]
- Kuchipudi, S.V.; Tellabati, M.; Nelli, R.K.; White, G.A.; Perez, B.B.; Sebastian, S.; Slomka, M.J.; Brookes, S.M.; Brown, I.H.; Dunham, S.P. 18S rRNA is a reliable normalisation gene for real-time PCR based on influenza virus-infected cells. Virol. J. 2012, 9, 1–7. [Google Scholar] [CrossRef]
- Short, F.; Gorton, P.; Wiseman, J.; Boorman, K. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996, 59, 215–221. [Google Scholar] [CrossRef]
- Base SAS® 9.3 Procedures Guide: Statistical Procedures; SAS Institute. Inc.: Cary, NC, USA, 2010.
- Van Immerseel, F.; Russell, J.; Flythe, M.; Gantois, I.; Timbermont, L.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R. The use of organic acids to combat Salmonella in poultry: A mechanistic explanation of the efficacy. Avian Pathol. 2006, 35, 182–188. [Google Scholar] [CrossRef]
- Papatsiros, V.; Katsoulos, P.-D.; Koutoulis, K.; Karatzia, M.; Dedousi, A.; Christodoulopoulos, G. Alternatives to antibiotics for farm animals. CAB Rev. Ag. Vet. Sci. Nutr. Res. 2013, 8, 1–15. [Google Scholar] [CrossRef]
- Sampugna, J.; Quinn, J.; Pitas, R.; Carpenter, D.; Jensen, R. Digestion of butyrate glycerides by pancreatic lipase. Lipids 1967, 2, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Geier, M.; Mikkelsen, L.; Torok, V.; Allison, G.; Olnood, C.; Boulianne, M.; Hughes, R.; Choct, M. Comparison of alternatives to in-feed antimicrobials for the prevention of clinical necrotic enteritis. J. Appl. Microbiol. 2010, 109, 1329–1338. [Google Scholar] [CrossRef]
- Kumar, A.; Toghyani, M.; Kheravii, S.K.; Pineda, L.; Han, Y.; Swick, R.A.; Wu, S.-B. Potential of blended organic acids to improve performance and health of broilers infected with necrotic enteritis. Anim. Nutr. 2021. [Google Scholar] [CrossRef]
- Fascina, V.B.; Sartori, J.R.; Gonzales, E.; Carvalho, F.B.; Souza, I.M.G.P.; do Valle Polycarpo, G.; Stradiotti, A.C.; Pelícia, V.C. Phytogenic additives and organic acids in broiler chicken diets. Rev. Cent. Am. Odontol. 2012, 41, 2189–2197. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Y.; Chen, J.; Ni, A.; Jiang, Y.; Li, Y.; Huang, Z.; Shi, L.; Xu, H.; Chen, C. Effect of replacing in-feed antibiotics with synergistic organic acids on growth performance, health, carcass, and immune and oxidative status of broiler chickens under Clostridium perfringens type A challenge. Avian Dis. 2020. [Google Scholar] [CrossRef]
- Ritzi, M.M.; Abdelrahman, W.; Mohnl, M.; Dalloul, R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014, 93, 2772–2778. [Google Scholar] [CrossRef]
- De Medina, F.S.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. Ther. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Medina, R.; Rahner, C.; Mitic, L.; Anderson, J.; Van Itallie, C. Occludin localization at the tight junction requires the second extracellular loop. J. Memb. Biol. 2000, 178, 235–247. [Google Scholar] [CrossRef]
- Barekatain, R.; Nattrass, G.; Tilbrook, A.J.; Chousalkar, K.; Gilani, S. Reduced protein diet and amino acid concentration alter intestinal barrier function and performance of broiler chickens with or without synthetic glucocorticoid. Poult. Sci. 2019, 98, 3662–3675. [Google Scholar] [CrossRef]
- Vicuña, E.; Kuttappan, V.; Tellez, G.; Hernandez-Velasco, X.; Seeber-Galarza, R.; Latorre, J.; Faulkner, O.; Wolfenden, A.; Hargis, B.; Bielke, L. Dose titration of FITC-d for optimal measurement of enteric inflammation in broiler chicks. Poult. Sci. 2015, 94, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Kan, L.; Huang, J.; Geng, Y.; Zhen, W.; Guo, Y.; Abbas, W.; Wang, Z. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J. Anim. Sci. Biotechnol. 2020, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Johansen, F.E. Mucosal B cells: Phenotypic characteristics, transcriptional regulation, and homing properties. Immunol. Rev. 2005, 206, 32–63. [Google Scholar] [CrossRef] [PubMed]
- Konashi, S.; Takahashi, K.; Akiba, Y. Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. Br. J. Nutr. 2000, 83, 449–456. [Google Scholar] [CrossRef]
- Wang, H.; Ni, X.; Qing, X.; Liu, L.; Lai, J.; Khalique, A.; Li, G.; Pan, K.; Jing, B.; Zeng, D. Probiotic enhanced intestinal immunity in broilers against subclinical necrotic enteritis. Front. Immunol. 2017, 8, 1592. [Google Scholar] [CrossRef]
- Stefanello, C.; Rosa, D.P.; Dalmoro, Y.K.; Segatto, A.L.; Vieira, M.S.; Moraes, M.L.; Santin, E. Protected Blend of Organic Acids and Essential Oils Improves Growth Performance, Nutrient Digestibility, and Intestinal Health of Broiler Chickens Undergoing an Intestinal Challenge. Front. Vet. Sci. 2020, 6, 491. [Google Scholar] [CrossRef] [PubMed]
- Golder, H.; Geier, M.; Forder, R.; Hynd, P.; Hughes, R. Effects of necrotic enteritis challenge on intestinal micro-architecture and mucin profile. Br. Poult. Sci. 2011, 52, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Applegate, T.J.; Lossie, A.C. Cloning, annotation and developmental expression of the chicken intestinal MUC2 gene. PLoS ONE 2013, 8, e53781. [Google Scholar] [CrossRef]
- Chen, J.; Tellez, G.; Richards, J.D.; Escobar, J. Identification of potential biomarkers for gut barrier failure in broiler chickens. Front. Vet. Sci. 2015, 2, 14. [Google Scholar] [CrossRef]
- Balda, M.S.; Matter, K. Tight junctions at a glance. J. Cell Sci. 2008, 121, 3677–3682. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Klasing, K. An immunologist’s perspective on nutrition, immunity, and infectious diseases: Introduction and overview. J. Appl. Poult. Res. 2009, 18, 103–110. [Google Scholar] [CrossRef]
- Palamidi, I.; Paraskeuas, V.; Theodorou, G.; Breitsma, R.; Schatzmayr, G.; Theodoropoulos, G.; Fegeros, K.; Mountzouris, K.C. Effects of dietary acidifier supplementation on broiler growth performance, digestive and immune function indices. Anim. Product. Sci. 2017, 57, 271–281. [Google Scholar] [CrossRef]

| Treatments 1 | Product Name | Inclusion Level; Starter Phase (days 0 to 10), Grower Phase (days 10 to 24) and Finisher Phase (days 24 to 35), % | Necrotic Enteritis Challenge 2 |
|---|---|---|---|
| NC | - | - | Challenged |
| ZBS | Zinc bacitracin and Salinomycin | 0.033 and 0.05, respectively; in all phases | Challenged |
| MG | BalanGutTM LS P | Starter: 0.5; Grower and Finisher: 0 | Challenged |
| MGFA | BalanGutTM LS P ** and Amasil® NA * | Starter: 0.5 (MG); Grower and Finisher: 0.3 (FA) | Challenged |
| Item | Starter (days 0 to 10) | Grower (days 10 to 24) | Finisher (days 24 to 35) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ingredients 2 | NC | ZBS | MG | MGFA | NC | ZBS | MG | MGFA | NC | ZBS | MG | MGFA |
| Wheat | 42.0 | 42.0 | 42.0 | 42.0 | 45.1 | 45.0 | 45.1 | 45.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Sorghum | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Soybean meal | 32.0 | 32.0 | 32.0 | 32.0 | 28.0 | 28.0 | 28.0 | 28.0 | 24.0 | 24.0 | 24.0 | 24.0 |
| Meat and bone meal | 2.43 | 2.43 | 2.43 | 2.43 | 1.610 | 1.608 | 1.610 | 1.610 | 0.862 | 0.862 | 0.862 | 0.862 |
| Cottonseed Oil | 0.561 | 0.550 | 0.100 | 0.100 | 1.900 | 1.902 | 1.900 | 1.901 | 2.57 | 2.57 | 2.57 | 2.53 |
| Limestone | 1.100 | 1.100 | 1.100 | 1.100 | 1.076 | 1.075 | 1.076 | 1.075 | 1.065 | 1.065 | 1.065 | 1.065 |
| Salt | 0.147 | 0.147 | 0.147 | 0.147 | 0.196 | 0.197 | 0.196 | 0.167 | 0.183 | 0.183 | 0.183 | 0.183 |
| Sodium bicarbonate | 0.172 | 0.172 | 0.172 | 0.172 | 0.200 | 0.200 | 0.200 | 0.200 | 0.240 | 0.240 | 0.240 | 0.166 |
| Sand | 0.305 | 0.230 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.220 | 0.300 | 0.100 |
| Vitamins premix 3 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 |
| Minerals premix 4 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 |
| Choline chloride 60 | 0.043 | 0.042 | 0.040 | 0.040 | 0.038 | 0.039 | 0.038 | 0.039 | 0.033 | 0.033 | 0.033 | 0.033 |
| Kynofos 21P/16Ca | 0.100 | 0.100 | 0.100 | 0.100 | 0.080 | 0.080 | 0.080 | 0.080 | 0.060 | 0.060 | 0.060 | 0.060 |
| L-lysine HCl | 0.385 | 0.385 | 0.385 | 0.385 | 0.355 | 0.355 | 0.355 | 0.355 | 0.330 | 0.330 | 0.330 | 0.330 |
| DL-methionine | 0.331 | 0.331 | 0.331 | 0.331 | 0.296 | 0.296 | 0.296 | 0.296 | 0.262 | 0.262 | 0.262 | 0.262 |
| L-threonine | 0.230 | 0.230 | 0.230 | 0.230 | 0.197 | 0.197 | 0.197 | 0.197 | 0.171 | 0.171 | 0.171 | 0.171 |
| Phytase (Natuphos® E) | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.0200 | 0.020 | 0.020 |
| Monoglyceride blend (MG) | - | - | 0.500 | 0.500 | - | - | - | - | - | - | - | - |
| Buffered formic acid (FA) | - | - | - | - | - | - | - | 0.300 | - | - | - | 0.300 |
| Zinc bacitracin | - | 0.033 | - | - | - | 0.033 | - | - | - | 0.033 | - | - |
| Salinomycin | - | 0.050 | - | - | - | 0.050 | - | - | - | 0.050 | - | - |
| Titanium di-oxide | - | - | - | - | 0.500 | 0.500 | 0.500 | 0.500 | - | - | - | - |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Starter (days 0 to 10) | Grower (days 10 to 24) | Finisher (days 24 to 35) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrients | NC | ZBS | MG | MGFA | NC | ZBS | MG | MGFA | NC | ZBS | MG | MGFA |
| Calculated nutrients | ||||||||||||
| AME kcal/kg | 3000 | 3000 | 3000 | 3000 | 3100 | 3100 | 3100 | 3100 | 3200 | 3200 | 3200 | 3200 |
| Crude Protein | 24.0 | 24.0 | 24.0 | 24.0 | 23.0 | 23.0 | 23.0 | 23.0 | 21.0 | 21.0 | 21.0 | 21.0 |
| Crude fat | 2.74 | 2.73 | 2.28 | 2.28 | 3.96 | 4.01 | 3.96 | 4.00 | 4.65 | 4.65 | 4.65 | 4.61 |
| Crude fiber | 3.15 | 3.15 | 3.15 | 3.15 | 3.01 | 3.01 | 3.01 | 3.01 | 2.92 | 2.92 | 2.92 | 2.92 |
| Digestible Arginine | 1.370 | 1.370 | 1.370 | 1.370 | 1.230 | 1.230 | 1.230 | 1.230 | 1.100 | 1.100 | 1.100 | 1.100 |
| Digestible Lysine | 1.280 | 1.280 | 1.280 | 1.280 | 1.150 | 1.150 | 1.150 | 1.150 | 1.030 | 1.030 | 1.030 | 1.030 |
| Digestible Methionine | 0.604 | 0.603 | 0.604 | 0.604 | 0.546 | 0.546 | 0.546 | 0.546 | 0.490 | 0.490 | 0.490 | 0.490 |
| Digestible Methionine+Cystine | 0.950 | 0.950 | 0.950 | 0.950 | 0.870 | 0.870 | 0.870 | 0.870 | 0.800 | 0.800 | 0.800 | 0.800 |
| Digestible Tryptophan | 0.232 | 0.232 | 0.232 | 0.232 | 0.212 | 0.212 | 0.212 | 0.212 | 0.200 | 0.200 | 0.200 | 0.200 |
| Digestible Isoleucine | 0.912 | 0.912 | 0.912 | 0.912 | 0.832 | 0.831 | 0.832 | 0.831 | 0.760 | 0.760 | 0.760 | 0.760 |
| Digestible Threonine | 0.860 | 0.860 | 0.860 | 0.860 | 0.770 | 0.770 | 0.770 | 0.770 | 0.690 | 0.690 | 0.690 | 0.690 |
| Digestible Valine | 1.007 | 1.007 | 1.007 | 1.007 | 0.923 | 0.923 | 0.923 | 0.923 | 0.848 | 0.848 | 0.848 | 0.848 |
| Calcium | 0.960 | 0.960 | 0.960 | 0.960 | 0.870 | 0.870 | 0.870 | 0.870 | 0.790 | 0.790 | 0.790 | 0.790 |
| Phosphorus avail | 0.480 | 0.480 | 0.480 | 0.480 | 0.435 | 0.435 | 0.435 | 0.435 | 0.395 | 0.395 | 0.395 | 0.395 |
| Sodium | 0.160 | 0.160 | 0.160 | 0.160 | 0.180 | 0.180 | 0.180 | 0.190 | 0.180 | 0.180 | 0.180 | 0.180 |
| Potassium | 0.994 | 0.994 | 0.994 | 0.994 | 0.913 | 0.912 | 0.913 | 0.912 | 0.838 | 0.838 | 0.838 | 0.838 |
| Chloride | 0.230 | 0.230 | 0.230 | 0.230 | 0.248 | 0.24 | 0.248 | 0.230 | 0.230 | 0.230 | 0.230 | 0.230 |
| Choline mg/kg | 1700 | 1700 | 1700 | 1700 | 1600 | 1600 | 1600 | 1600 | 1500 | 1500 | 1500 | 1500 |
| Linoleic acid | 0.888 | 0.883 | 0.658 | 0.658 | 1.530 | 1.550 | 1.530 | 1.550 | 1.900 | 1.900 | 1.900 | 1.880 |
| Analyzed nutrients | ||||||||||||
| Gross Energy, kcal/kg | 3847 | 3874 | 3848 | 3862 | 3933 | 3928 | 3940 | 3950 | 3998 | 3994 | 3992 | 4004 |
| Crude protein | 24.7 | 24.7 | 24.7 | 24.6 | 23.5 | 23.2 | 23.3 | 23.4 | 21.4 | 21.2 | 21.2 | 21.4 |
| Calcium | 0.973 | 0.962 | 0.963 | 0.968 | 0.879 | 0.870 | 0.881 | 0.881 | 0.761 | 0.763 | 0.769 | 0.763 |
| Phosphorus | 0.687 | 0.686 | 0.686 | 0.698 | 0.612 | 0.603 | 0.610 | 0.613 | 0.559 | 0.542 | 0.549 | 0.542 |
| Target Group of Bacteria | Primer Sequence (5′–3′) | Annealing Temperature (℃) | Reference |
|---|---|---|---|
| Lactobacillus spp. | F-CAC CGC TAC ACA TGG AG R-AGC AGT AGG GAA TCT TCC A | 63 | Wise and Siragusa [27] |
| Bifidobacterium spp. | F-GCG TCC GCT GTG GGC R-CTT CTC CGG CAT GGT GTT G | 63 | Requena et al. [28] |
| Bacillus spp. | F-GCA ACG AGC GCA ACC CTT GA R-TCA TCC CCA CCT TCC TCC GGT | 63 | Zhang et al. [29] |
| Ruminococcus spp. | F-GGC GGC YTR CTG GGC TTT R-CCA GGT GGA TWA CTT ATT GTG TTA A | 63 | Ramirez-Farias et al. [30] |
| Clostridium perfringens | F-ATG CAA GTC GAG CGA KG R-TAT GCG GTA TTA ATC TYC CTT T TaqMan Probe-5′-FAM-TCA TCA TTC AAC CAA AGG AGC AAT CC-TAMRA-3′ | 60 | Rinttilä et al. [31] |
| Total bacteria | F-CGG YCC AGA CTC CTA CGG G R-TTA CCG CGG CTG CTG GCA C | 63 | Lee et al. [32] |
| Item | Sequence | Size (pb) | Annealing T° | Reference |
|---|---|---|---|---|
| TJP1 | F-GGATGTTTATTTGGGCGGC R-GTCACCGTGTGTTGTTCCCAT | 187 | 60 | Gharib-Naseri et al. [35] |
| OCLN | F-ACGGCAGCACCTACCTCAA R-GGGCGAAGAAGCAGATGAG | 123 | 60 | Du et al. [36] |
| CLDN1 | F-CTTCATCATTGCAGGTCTGTCAG R-AAATCTGGTGTTAACGGGTGTG | 103 | 60 | Gharib-Naseri et al. [35] |
| CLDN5 | F-GCAGGTCGCCAGAGATACAG R-CCACGAAGCCTCTCATAGCC | 162 | 61 | This study |
| JAM2 | F-AGACAGGAACAGGCAGTGCTAG R-ATCCAATCCCATTTGAGGCTAC | 135 | 60 | This study |
| CASP3 | F-TGGTGGAGGTGGAGGAGC R-GTTTCTCTGTATCTTGAAGCACCA | 110 | 62 | Gharib-Naseri et al. [35] |
| CASP8 | F-GGAGCTGCTATCGGATCAAT R-GGAGCTGCTCTATCGGATCAAT | 126 | 60 | Gharib-Naseri et al. [35] |
| IgG | F-ATCACGTCAAGGGATGCCCG R-ACCAGGCACCTCAGTTTGG | 118 | 60 | Zhao et al. [37] |
| IgM | F-GCATCAGCGTCACCGAAAGC R-TCCGCACTCCATCCTCTTGC | 98 | 60 | Zhao et al. [37] |
| MUC2 | F-CCCTGGAAGTAGAGGTGACTG R-TGACAAGCCATTGAAGGACA | 143 | 60 | Fan et al. [38] |
| MUC5AC | F-AAGACGGCATTTATTTCTCCAC R-TCATTACCAACAAGCCAGTGA | 244 | 60 | Fan et al. [38] |
| HPRT1 | F-ACTGGCTGCTTCTTGTG R-GGTTGGGTTGTGCTGTT | 245 | 62 | Yang et al. [39] |
| GAPDH | F-GAAGCTTACTGGAATGGCTTTCC R-CGGCAGGTCAGGTCAACAA | 66 | 61 | Kuchipudi et al. [40] |
| Treatment 2 | NC | ZBS | MG | MGFA | SEM | p-Value |
|---|---|---|---|---|---|---|
| Starter phase (d 0 to 10) | ||||||
| BWG, g | 270 | 273 | 267 | 266 | 4 | 0.423 |
| FI, g | 301 | 298 | 301 | 301 | 4 | 0.883 |
| FCR | 1.117 a | 1.090 b | 1.128 a | 1.134 a | 0.006 | <0.001 |
| Grower phase (d 10 to 24) | ||||||
| BWG, g | 776 b | 938 a | 781 b | 789 b | 15 | <0.001 |
| FI, g | 1240 ab | 1274 a | 1192 b | 1200 b | 18 | 0.011 |
| FCR | 1.590 a | 1.362 b | 1.530 a | 1.522 a | 0.024 | <0.001 |
| Finisher phase (d 24 to 35) | ||||||
| BWG, g | 1187 | 1155 | 1144 | 1169 | 20 | 0.450 |
| FI, g | 1805 | 1824 | 1756 | 1787 | 25 | 0.272 |
| FCR | 1.521 b | 1.579 a | 1.534 ab | 1.531 ab | 0.014 | 0.043 |
| Overall period (d 0 to 35) | ||||||
| BWG, g | 2239 b | 2380 a | 2202 b | 2229 b | 28 | <0.001 |
| FI, g | 3283 ab | 3369 a | 3220 b | 3235 ab | 37 | 0.036 |
| FCR | 1.467 a | 1.416 b | 1.463 a | 1.453 a | 0.009 | 0.003 |
| Treatment 2 | NC | ZBS | MG | MGFA | SEM | p-Value |
|---|---|---|---|---|---|---|
| Lactobacillus spp. | 8.48 a | 7.81 b | 8.09 ab | 8.61 a | 0.17 | 0.010 |
| Ruminococcus spp. | 8.79 | 9.00 | 8.83 | 9.03 | 0.27 | 0.887 |
| Bacillus spp. | 6.82 | 6.77 | 6.51 | 7.20 | 0.28 | 0.379 |
| Bifidobacteria spp. | 7.51 | 7.50 | 7.68 | 7.58 | 0.18 | 0.893 |
| Clostridium pefringens | 8.46 ab | 7.56 b | 8.81 a | 8.87 a | 0.31 | 0.022 |
| Total bacteria | 10.12 | 10.09 | 10.10 | 10.41 | 0.15 | 0.394 |
| Treatment 2 | NC | ZBS | MG | MGFA | SEM | p-Value |
|---|---|---|---|---|---|---|
| TJP1 | 0.902 b | 1.018 ab | 1.306 a | 1.032 ab | 0.092 | 0.028 |
| OCLN | 1.300 | 0.895 | 1.057 | 1.284 | 0.209 | 0.469 |
| CLDN1 | 1.149 | 0.836 | 1.301 | 0.945 | 0.168 | 0.230 |
| CLDN5 | 1.059 a | 0.632 b | 1.097 a | 0.840 ab | 0.077 | <0.001 |
| JAM2 | 1.022 | 0.789 | 1.064 | 0.953 | 0.093 | 0.195 |
| CASP3 | 0.923 | 1.236 | 1.123 | 0.780 | 0.142 | 0.129 |
| CASP8 | 1.001 | 1.040 | 1.180 | 0.944 | 0.121 | 0.569 |
| E-CADH | 1.132 | 1.077 | 1.090 | 1.014 | 0.102 | 0.876 |
| IgG | 0.937 b | 0.997 b | 1.496 a | 1.456 a | 0.106 | <0.001 |
| IgM | 1.150 ab | 0.811 b | 1.600 a | 1.374 a | 0.126 | <0.001 |
| MUC2 | 1.093 | 1.163 | 1.348 | 1.117 | 0.168 | 0.730 |
| MUC5AC | 1.221 | 1.122 | 1.244 | 1.140 | 0.127 | 0.879 |
| Item | NC 2 | ZBS | MG | MGFA | SEM | p-Value |
|---|---|---|---|---|---|---|
| Gross Energy, % | 71.0 b | 72.4 ab | 75.0 a | 73.5 ab | 0.008 | 0.013 |
| Protein, % | 81.2 | 82.3 | 82.0 | 80.6 | 0.010 | 0.637 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Kheravii, S.K.; Li, L.; Wu, S.-B. Monoglyceride Blend Reduces Mortality, Improves Nutrient Digestibility, and Intestinal Health in Broilers Subjected to Clinical Necrotic Enteritis Challenge. Animals 2021, 11, 1432. https://doi.org/10.3390/ani11051432
Kumar A, Kheravii SK, Li L, Wu S-B. Monoglyceride Blend Reduces Mortality, Improves Nutrient Digestibility, and Intestinal Health in Broilers Subjected to Clinical Necrotic Enteritis Challenge. Animals. 2021; 11(5):1432. https://doi.org/10.3390/ani11051432
Chicago/Turabian StyleKumar, Alip, Sarbast K. Kheravii, Lily Li, and Shu-Biao Wu. 2021. "Monoglyceride Blend Reduces Mortality, Improves Nutrient Digestibility, and Intestinal Health in Broilers Subjected to Clinical Necrotic Enteritis Challenge" Animals 11, no. 5: 1432. https://doi.org/10.3390/ani11051432
APA StyleKumar, A., Kheravii, S. K., Li, L., & Wu, S.-B. (2021). Monoglyceride Blend Reduces Mortality, Improves Nutrient Digestibility, and Intestinal Health in Broilers Subjected to Clinical Necrotic Enteritis Challenge. Animals, 11(5), 1432. https://doi.org/10.3390/ani11051432






