Simple Summary
Necrotic enteritis (NE) is a common and devastating enteric bacterial disease prevalent in fast-growing broilers. It is a great concern to the global poultry industry as impaired performance and high flock mortality up to 50% in severe cases occur, leading to losses of over US$6 billion each year. Controlling NE in fast-growing broilers is crucial, particularly in the antibiotic-free era. Among many potential alternatives to in-feed antibiotics, fatty acid glycerides and formic acid supplementation in diets have shown promising effects in improving performance and intestinal health in broilers infected with subclinical NE. However, data are limited in clinical NE infected broilers. Thus, this study was conducted to evaluate the potential of monoglyceride blend (MG) and buffered formic acid (FA) as alternatives to antibiotics in the performance and intestinal health of broilers subjected to clinical NE challenge. The obtained results highlighted that the diet supplemented with MG has the potential to improve intestinal health and reduce the severity of clinical NE by reducing mortality. This study underpins the importance of additives in poultry production following the removal of antibiotics in poultry feed to alleviate the possible loss posed by enteric diseases such as NE.
Abstract
This study evaluated the potential of monoglyceride blend (MG) and buffered formic acid (FA) as alternatives to antibiotics in the performance and intestinal health of broilers under clinical necrotic enteritis (NE) challenge. A total of 544 as-hatched Ross 308 broiler chicks were randomly distributed to 32-floor pens housing 17 birds per pen. The four treatments were: NC—non-additive control; ZBS—antibiotic group supplemented with zinc bacitracin and salinomycin; MG—additive MG supplementation in the starter phase only; and MGFA—additive MG in starter phase and FA in grower and finisher phases. All birds were challenged with Eimeria spp. and Clostridium perfringens. Results showed that the NC group had lower BWG and higher FCR than the ZBS group in the grower and overall period (p < 0.05). The NC group had higher NE-caused mortality (days 14 to 17) than the ZBS group (p < 0.05). Birds fed MG had lower NE-caused mortality than the NC group (p < 0.05). Birds fed MG had upregulated jejunal tight junction protein1 (TJP1) and immunoglobulin (IgG) on day 16 and improved gross energy digestibility on day 24 than the NC group (p < 0.05). These findings suggest that supplementation of MG may improve intestinal health and protect birds from clinical NE occurrence.
1. Introduction
Necrotic enteritis (NE) is one of the world’s most economically important and severe enteric poultry diseases caused by NetB producing Clostridium perfringens [1]. The economic costs to the world poultry industry by NE have been estimated to be over US $6 billion per annum associated with disease control measures and production losses [2]. The NE is typified by reduced body weight gain (BWG), feed intake (FI) and digestibility, increased feed conversion ratio (FCR), intestinal lesions and increased incidence of wet litter, and diarrhea [3,4]. In addition, NE damages intestinal epithelial cells and the mucosa in general, impairing the function of tight junction genes, which leads to a disruption of microbial inhabitants. The acute clinical form of NE can cause sudden death and a high flock mortality rate of 2 to 10% and in severe cases up to 50% over several days, whereas the subclinical form of NE can significantly impair growth performance and reduce FCR [5]. The occurrence and the severity of NE are affected by the presence of predisposing factors (e.g., coccidiosis, fish meal, and poor management) [6,7].
The application of in-feed antibiotic growth promoters (AGP) has been banned in many countries (e.g., European Union or phasing-out worldwide in the poultry industry due to public concerns over bacterial antibiotic resistance). This, in turn, has contributed to a higher prevalence of economically important enteric diseases in livestock such as NE in poultry [8]. Consequently, the poultry industry faces challenges with the health and performance of the birds that have led to increased production costs for disease control and management purposes. Thus, the poultry and other livestock industries are in need of potential in-feed antibiotic alternatives to improve the performance and protect the intestinal health of the birds so as to minimize the production cost and profit losses in the post-antibiotic era.
To achieve improved production in animals, certain bioactive ingredients have been used to supplement diets as alternatives to in-feed AGP. Organic acids (OA) have been used in poultry feed as a preservative over several decades and increased interest in recent years as a possible alternative to in-feed AGP due to its promising effects on bird performance and intestinal health [9,10]. Among the OA, dietary addition of short-chain fatty acids (SCFA) and medium-chain fatty acids (MCFA) in different forms such as salts and glycerides alone and their blends have been used to protect gut barrier integrity and control the balance of microbiota by their bactericidal and bacteriostatic characteristics, resulting in better animal performance [10,11,12]. The efficacy of OA and their blends varies due to the chemical composition, pKa value, form, molecular weight, and experimental conditions [13]. A widely accepted form of OA is glycerides, an esterified product of fatty acids. Fatty acid glycerides are free from unpleasant odors and thus easier to handle. They can be released to the lower part of the digestive tract under their lipase actions. Glyceride products are known to have enhanced antibacterial activities due to the improved availability in the lower part of the intestine [14,15]. Previous studies have shown that the diet supplemented with butyric acid or its glyceride derivatives could replace antibiotics and maintain optimal bird performance [12,16] and reduce Salmonella enteritidis caused infection [17]. A recent study has shown that birds fed different dosages of butyric acid glycerides significantly improved overall FCR under subclinical NE challenge [18]. However, other studies have reported inconsistent growth performance results in birds supplemented with butyric acid glycerides alone or in combination with tributyrin, and a combination of mono- and di-glyceride products [19,20]. The types of butyric acid derivatives, forms of delivery, amount of active compounds, dosage, diet composition, management, bird health, and environmental conditions or disease may contribute to the different results observed in the literature.
Moreover, a recent study reported that the birds fed a monoglyceride blend (MG) at a high dose had improved FCR compared to the challenged control group in the grower phase, but the low dose did not [18]. Buffered formic acid (FA) at a high dose improved FCR in the finisher phase, but had no effect in the starter and grower phases. Birds fed MG at a low dose had improved FCR compared to the birds fed FA at a high dose in the starter phase. Therefore, this study was to investigate whether the supplementation of MG and FA at appropriate doses in different feeding phases may help to achieve optimal bird performance under diseased conditions [18]. It was hypothesized that (a) the supplementation of MG at a high dose in the starter phase improves performance and protects birds from NE in the later phases; and (b) FA supplementation in grower and finisher phases provides additional benefits to improve performance and protect the intestinal health of birds from the negative effects of NE.
The current study was designed to evaluate the effects of MG supplementation in the starter phase and the effects of FA supplementation in grower and finisher phases in the mitigation of NE.
2. Materials and Methods
2.1. Ethics Statement
The experimental procedures were approved by the Animal Ethics Committee of University of New England, Australia (Approval No.: AEC18-007) and conducted according to the guidelines for the care and use of laboratory and farm animals for scientific purposes accredited by the Australian Bureau of Animal Health [21].
2.2. Feed Additives
The current study evaluated the potential of two different types of feed additives supplied by BASF, Germany to improve performance and intestinal health in broilers as antibiotic alternatives using the clinical NE challenge model. The feed additives were: (A) monoglyceride blend (MG), a blend of mono-, di- and tri-glycerides with the main component being 1-monoglycerides (BalanGutTM LS P), primarily composed of approximately 45% mono-, di- and tri-glycerides of butyric, caprylic, and capric acids; and (B) buffered formic acid (FA), primarily composed of approximately 61% formic acid and 20.5% sodium formate (Amasil® NA).
2.3. Design and Animal Husbandry
A total of as-hatched 544 mixed-sex Ross 308 broiler chicks were obtained on the day of hatching from Baiada Hatchery in Tamworth, NSW, Australia. Birds were vaccinated against Marek’s disease and infectious bronchitis disease at the hatchery. Upon arrival, the gender of birds was determined by feather sexing and allocated to four treatments in 32-floor pens measuring 75 × 120 cm, based on a completely randomized design (CRD). Each of the four treatment groups had eight replicate pens with 17 birds per pen (eight males and nine females). Birds were raised in an environmentally controlled facility with softwood shavings as litter material. Clean water and feed were provided ad libitum with the temperature, relative humidity, and lighting following Ross 308 guidelines [22].
Birds in all treatment groups were challenged with Eimeria spp. and C. perfringens as shown in Table 1.

Table 1.
Treatment groups with additives applied in this study.
The treatments were: T1—non-additive control, without additives or in-feed antibiotics (NC); T2—control diet supplemented with in-feed zinc bacitracin (0.033%) and salinomycin (0.050%) in starter, grower and finisher phases (ZBS); T3—feed additive group supplemented with additive MG at concentrations of 0.5% in starter only (MG); and T4—MG and FA supplementation treatment (MGFA) with additive MG at a concentration of 0.5% in starter and additive FA at a concentration of 0.3% in grower and finisher phases (MGFA). All diets were formulated based on wheat, soybean meal, sorghum, and meat and bone meal where the feed additives and phytase were formulated with nutrient and the matrix values, respectively (Table 2 and Table 3). Titanium dioxide was added as an indigestible marker at 0.5% in the grower diets. The diets were made individually based on the formulation of each diet. The nutrient contents of feed ingredients were measured using near-infrared spectroscopy (NIRS, Evonik AminoProx, Germany) before feed formulation. Cold pelleted diets were fed in the starter phase (days 0 to 10; crumbled), grower phase (days 10 to 24) and finisher phase (days 24 to 35) followed by Ross 308 feeding standards for broilers.

Table 2.
Diet composition used in this study (percentage unless mentioned) 1

Table 3.
Nutrient contents of thee diets (as-fed basis, percentage unless mentioned) 1.
2.4. Necrotic Enteritis Challenge
The NE challenge model was executed in this study following previously described challenge protocols [23,24] with modification. In brief, on day 9, all birds were orally gavaged with field strains of Eimeria spp. oocysts in 1 mL dose consisting of E. acervulina (5000), E. maxima (5000), and E. brunetti (2500) (Eimeria Pty Ltd., Werribee, VIC, Australia). On day 14, all birds were orally gavaged with approximately 108 CFU/mL of C. perfringens EHE-NE18 strain in 1 mL dose (CSIRO Livestock, Geelong, VIC, Australia).
2.5. Performance Measurement
Pen weight and feed intake were recorded on days 0, 10, 24, and 35. Body weights of dead birds were recorded daily and FCR was corrected for the mortalities. Necropsies were carried out to determine the reason for deaths. Dead birds, sampled birds, and birds left at the end of the study (day 35) were opened to further confirm their sex by visual inspection of genital organs.
2.6. Sampling and Intestinal Lesion Scoring
On days 16 and 24, two randomly chosen birds (one male and one female) from each pen were weighed, electrically stunned (JF poultry equipment, Weltevreden Park, South Africa), and euthanized by cervical dislocation to collect intestinal samples and perform post mortem analysis. On day 16, cecal contents from two sampled birds per pen were collected in 2 mL Eppendorf tubes and stored at −20 ℃ for microbiota analysis. On day 16, approximately 2 cm of the proximal jejunal tissue from one male bird per pen was excised, flushed with chilled phosphate-buffered saline (PBS), and collected in 2 mL Eppendorf tubes containing RNA later (Invitrogen, Thermo Fisher Scientific, California, USA) and kept at 4 ℃ for 4 h before stored in −20 ℃ for further analysis. On day 24, ileal content from one male bird per pen was collected in 2 mL Eppendorf tube and stored at −20 ℃ for CP and GE digestibility measurements.
On days 16 and 24, all intestinal sections of sampled birds were excised for NE lesions. Intestinal lesions of the duodenum, jejunum, and ileum were scored by visual examination using a scale ranging from 0 to 6 following a previously described lesion scoring system [25].
2.7. Cecal Bacterial Quantification
The cecal bacterial DNA extraction method described by Kheravii et al. [26] was used for this study with minor modifications. The DNA of frozen cecal samples collected on d 16 was extracted using QIAxtractor DNA reagents and QIAxtractor DNA plasticware kits (Qiagen, Inc., Doncaster, VIC, Australia). Approximately 130 mg of defrosted cecal samples and 300 mg of glass beads (0.1 mm) were placed in a 2 mL Eppendorf tube. Then, 300 μL Qiagen Lysis Buffer (270 µL DXL and 30 µL digestive enzyme) was added to Eppendorf tubes containing samples and placed into a bead beater mill (Retsch GmbH and Co., Haan, Germany) at a frequency of 30/S for 5 min. The samples were placed in a heating block and incubated at 55 ℃ for 2 h and followed by centrifugation at 20,000× g for 5 min. An aliquot of 200 μL supernatant was placed into the loading block and extraction was carried out using the CAS-1820 Xtractor Gene (Corbett, Sydney, Australia) following the manufacturer’s instruction. In brief, the reactions (DXB, DXW, DXF, or DXE) were placed into assigned locations inside the robotics machine. An aliquot of 400 μL of binding buffer (DXB) was added in the loading block containing 200 μL of supernatant and mixed appropriately, and incubated for 6 min. A volume of 500 μL lysed samples were transferred into the capture columns and vacuumed for 3 min at 30 kPa. An additional 200 μL of DXB was loaded to the capture columns and vacuumed at 35 kPa. Next, an aliquot of 600 μL DXW was added into the capture columns and vacuumed for 2 min at 30 kPa, after that, 600 μL DXF washing buffer was loaded to capture columns and vacuumed for 1 min at 35 kPa and the extracted DNA was dried by vacuuming for 5 min at 25 kPa. At the end of the extraction process, an elution block was applied to elute the cecal DNA by adding 60 μL DXE and elution blocks containing samples were vacuumed for 2 min at 30 kPa. The purity and quantity of the resulting DNA samples were measured with a Nanodrop 8000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). The DNA with ratios of A260/A280 being greater than 1.8 was considered as of high quality and stored at −20 ℃ for further analysis.
The cecal bacterial DNA quantification methods were applied following previously described procedures [27]. The cecal DNA was diluted 20 times (1:20 dilution) with nuclease-free water and the quantitative real-time polymerase chain reaction (PCR) of six bacterial groups was executed to quantify with a real-time PCR system, Rotorgene 6000 (Corbett, Sydney, Australia). The SYBR-Green containing mix (SensiMix SYBR No-Rox, Bioline, Sydney, Australia) was applied for quantitative polymerase chain reaction (qPCR) and the qPCR was performed in duplicate for each sample. The reaction in an amount of 10 μL contained 2 μL of diluted cecal DNA, 300 mmol/L of forward and reverse primers, and 5 μL of 2 × SensiMix™ SYBR® No-ROX. The genomic DNA copies of Lactobacillus spp., Bifidobacterium spp., Bacillus spp., Ruminococcus spp., total anerobic bacteria, and SensiFAST Probe SYBR No-ROX (Bioline, Sydney, Australia) was used for C. perfringens for the Taqman-based assay. The specific primers used for quantifying these six bacterial groups are presented in Table 4. The target DNA copies were calculated and the quantified bacterial amount was expressed as log10 (genomic DNA copy number)/g digesta.

Table 4.
The specific primers applied for quantifying bacteria in cecal contents.
2.8. RNA Extraction and cDNA Synthesis
Total RNA from each jejunal tissue sample collected on day 16 was extracted after homogenization in TRIsureTM (Bioline, Sydney, Australia) according to the manufacturer’s instructions. The extracted RNA samples were purified using the Rneasy Mini Kit, (Qiagen, Hilden, Germany) based on the manufacturer’s instructions. The quantity and purity of total RNA samples were measured using a NanoDrop ND-8000 spectrophotometer (Thermo Fisher Scientific, Waltham, USA). An RNA 6000 Nano Kit was applied to determine the RNA integrity number (RIN) using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Waldbronn, Germany). The purified RNA samples were considered as high-quality if the value of 260/230 was higher than 1.8, 260/280 value between 2.0 to 2.2, and the RIN number was greater than 7.0. The isolated RNA of the tissue sample was reverse-transcribed using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. In brief, one µg of each total RNA sample was incubated at 42 ℃ for 2 min in 2 µL of 7 × genomic DNA (gDNA) Wipeout Buffer to avoid gDNA contamination. After that, the gDNA elimination reaction was added to reverse-transcription reaction components containing one µL of Quantiscript Reverse Transcriptase, 4 µL of 7 × Quantiscript RT Buffer, and one µL of RT Primer Mix and mixed appropriately. The Rotorgene 6000 real-time PCR machine (Corbett, Sydney, Australia) was applied to incubate the mixture at 42 ℃ for 15 min followed by 95 ℃ for 3 min to convert the RNA into cDNA. The cDNA samples were then diluted 10 times with Nuclease-free water and kept at −20 ℃ for further analysis.
2.9. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Amplification and detection were performed in duplicates using an SYBR Green Kit SensiFAST™ SYBR® No-ROX (Bioline, Sydney, Australia) with a Rotorgene 6000 real-time PCR machine (Corbett Research, Sydney, Australia). The PCR reaction was carried out in a volume of 10 µL containing 2 µL of 10 × diluted cDNA template, 400 mM of each primer, and 5 µL of 2 × SensiFAST™ SYBR® No-ROX. A total of eight house-keeping genes, namely, 18S, ACTB, GAPDH, YWHAZ, HMBS, SDHA, HPRT1, and TBP, were used for the optimization of reference genes using the gene expression stability measure (geNorm M) module in qbase+ software version 3.0 (Biogazelle, Zwijnbeke, Belgium). The two most stable house-keeping genes with the lowest M- value (<0.5), GAPDH and HPRT1, were chosen as optimized reference genes to normalize the expression of the target genes. The amplification cycle (Cq) values for candidate target genes were collected and imported into qBase+ version 3.0 software (Biogazelle, Zwijnbeke, Belgium) and analyzed against the reference genes GAPDH and HPRT1. The qbase + employed the arithmetic mean method to transform logarithmic Cq values to linear relative quantity, applying the exponential function for relative quantification of genes [33,34] and the output data were exported for the statistical analysis. The normalized relative quantities (NRQ) values were calculated and analyzed across all samples for each target gene. The primers employed in this study were either sourced from previously published studies in chickens or designed using the NCBI Primer-BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/ accessed on 6 April 2018) as presented in Table 5. An Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Waldron, Germany) was used to determine the specificity of each primer pair prior to qPCR analysis using an Agilent DNA 1000 Kit (Agilent Technologies, Inc., Waldron, Germany), and only specific primers amplifying target fragments were used in the qPCR assay.

Table 5.
Sequences of primers used for quantitative real-time PCR.
2.10. Apparent Ileal Nutrient Digestibility
Previously stored ileal digesta samples were freeze-dried. The diet and digesta samples were then ground to pass through a 0.5 mm sieve and analyzed for nitrogen (N) content using a combustion analyzer (LECO Corp., St. Joseph, MI). An adiabatic bomb calorimeter (IKA, Werke C7000, GMBH, and Co., Staufen, Germany) with benzoic acid as a calibration standard was applied to determine gross energy (GE) contents of diets and ileal digesta samples in duplicates. A previously described procedure [41] was used to determine titanium dioxide (TiO2), an indigestible marker in diets, and digesta samples in duplicates by the colorimetric method.
Apparent ileal digestibility (AID) of GE and CP (N × 6.25) was determined using the following equation:
2.11. Data Analysis
The normally distributed data were subjected to one-way ANOVA analysis using the general linear model procedure of SAS 9.3 package [42] in a completely randomized design. The pen was considered as an experimental unit (n = 32) for the performance data analysis and the values presented in the tables are means with a pooled standard error of the mean (SEM). Performance data were analyzed for the treatment effect with male percentage (corrected to dead birds) set as a covariate. When a treatment effect was detected, the significant differences between means were separated by the Tukey HSD test at the level of p < 0.05. Intestinal lesion scores and NE-caused mortality data were analyzed by the non-parametric Kruskal–Wallis test as the data were not normally distributed.
3. Results
3.1. Bird Performance
The impacts of NE challenge and feed additives on growth performance in broilers are shown in Table 6. One-way ANOVA analysis demonstrated that FCR on days 0 to 10 (p < 0.001), 10 to 24 (p < 0.001), 24 to 35 (p = 0.043), and 0 to 35 (p = 0.003), BWG on days 10 to 24 and 0 to 35 (p < 0.001 and 0.001, respectively) and FI on days 10 to 24 and 0 to 35 (p = 0.011 and 0.036, respectively) showed significant differences.

Table 6.
Performance of necrotic enteritis challenged broilers in response to additive treatments in different phases 1.
In the starter phase (d 0 to 10), birds in the ZBS group had significantly lower FCR compared to all the treatment groups. Body weight gain and FI were not different among the treatment groups.
In the grower phase (days 10 to 24), birds treated with ZBS had a significantly lower FCR and higher BWG compared to all other treatment groups. Birds fed additives MG and MGFA had a significantly lower FI compared to the ZBS group, but not from the NC group. During the onset of NE (d 10 to 24), BWG and FCR were not significantly different between birds supplemented with feed additives and NC groups.
In the finisher phase (days 24 to 35), the negative effect of the NE challenge disappeared on BWG and FI from the feed additive and NC groups compared to the ZBS treated group. Birds fed additives (MG and MGFA) had a similar FCR compared to the birds in the NC and ZBS groups, and the NC group had a lower FCR compared to the ZBS group, indicating fast recovery of the surviving birds in the NC and additive groups.
Considering the overall study period (d 0 to 35), birds fed ZBS had a higher BWG and lower FCR compared to all other treatment groups. Birds fed MG had a lower FI compared to the ZBS group, but not different from the NC and MGFA groups. Moreover, the overall bird performance showed that BWG, FI, and FCR were not affected by the diets supplemented with additives MG and MGFA compared to the diet without additive supplementation (NC group).
3.2. Necrotic Enteritis Caused Mortality and Lesion Scores
The impacts of NE challenge and feed additives on mortality (days 14 to 17) and lesion scores (day 16) in broilers are shown in Figure 1 and Figure 2. Non- parametric Kruskal–Wallis test showed that mortality due to NE was significantly different (p < 0.001). Birds treated with ZBS protected birds from NE caused mortality and had the lowest mortality compared to all other treatment groups, whereas the highest mortality was observed in the NC group. Birds fed additive MG reduced the occurrence of mortality (−11.3%) due to NE compared to the NC group (14.8% vs. 26.1%), but not different from birds in the additive MGFA group. Additionally, birds treated with MGFA showed a numeric reduction of mortality and reduced mortality by 6.8% compared to the NC group (19.3% vs. 26.1%).
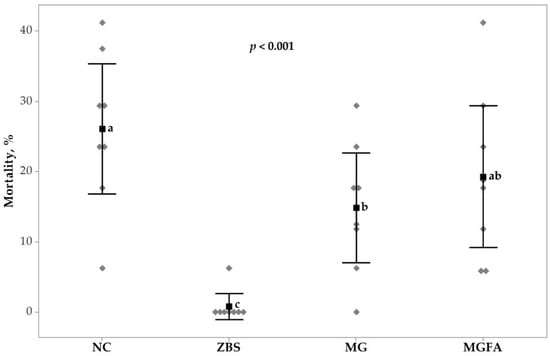
Figure 1.
Necrotic enteritis (NE) caused mortality of broilers in response to additive treatments from d 14 to 17. All birds were gavaged with Eimeria spp. on day 9 and C. perfringens on day 14. NC, non-additive control; ZBS, zinc bacitracin and salinomycin; MG, monoglyceride blend; MGFA, MG in starter phase and buffered formic acid (FA) in grower and finisher phases. a–c values in a row with no common superscripts differ significantly (p < 0.05).
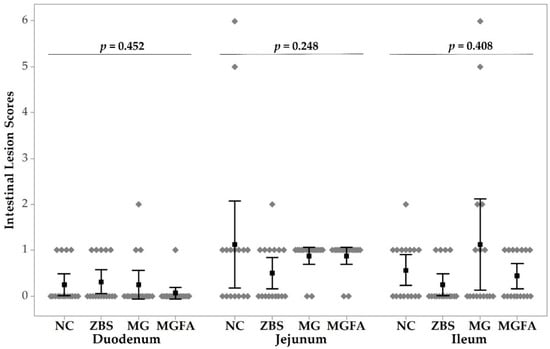
Figure 2.
Intestinal lesions of necrotic enteritis (NE) challenged broilers in response to additive treatments on day 16. All birds were gavaged with Eimeria spp. on day 9 and C. perfringens on day 14. NC, non-additive control; ZBS, zinc bacitracin and salinomycin; MG, monoglyceride blend; MGFA, MG in starter phase and buffered formic acid (FA) in grower and finisher phases.
Non- parametric Kruskal–Wallis test indicated no significant differences of duodenal, jejunal, and ileal lesions in different treatment groups (p = 0.452, 0.248, and 0.408, respectively). Neither birds treated with ZBS nor feed additives (MG and MG+FA) used had any significant effects on intestinal lesions compared to the NC group. Moreover, there were no NE-caused intestinal lesions observed in any of the treatment groups on day 24.
3.3. Cecal Bacterial Quantification
The impacts of NE challenge and feed additives on cecal microbiota on day 16 in broilers are presented in Table 7. One-way ANOVA analysis showed that the quantification of Lactobacillus spp. and C. perfringens in cecal content indicated significant differences (p = 0.010 and 0.022, respectively). Birds treated with ZBS had lower Lactobacillus spp. and not significantly but numerically lower C. perfringens in the ceca compared to the NC group. Birds fed additive MGFA had a higher amount of Lactobacillus spp. compared to the birds in the ZBS group, but not different from NC and MG groups. Birds treated with MG and MGFA had significantly higher C. perfringens compared to the birds fed ZBS and numerically higher from the NC group. Bifidobacteria spp., Bacillus spp., Ruminococcus spp., and total bacteria were not different between treatment groups.

Table 7.
Cecal bacterial loads (log10 genomic DNA copies/g digesta) in response to additive treatments in NE challenged broilers on day 16 1.
3.4. Expression of Jejunal Genes
The impacts of NE challenge and feed additives on the expression of jejunal genes on day 16 in broilers are presented in Table 8. One-way ANOVA analysis showed the significant differences of TJP1 (p = 0.028), CLDN5 (p < 0.001), IgG (p < 0.001), and IgM (p = 0.001) genes in the jejunum, whereas no differences were observed in other genes examined, namely, OCLDN, CLDN1, CASP3, CASP8, E-CADH, MUC2, MUC5AC, and JAM2 (p > 0.05). The expression of jejunal TJP1 was upregulated in the birds fed MG compared to the NC group, but not different from the birds fed ZBS and MGFA. The expression of jejunal CLDN5 gene was upregulated in the NC and MG groups compared to the ZBS group, but not different from the MGFA group. Birds fed additives MG and MGFA had upregulated IgG gene compared to the NC and ZBS groups. The expression of the IgM gene was upregulated in the MG and MGFA groups compared to the ZBS group, but not different from the NC group.

Table 8.
The mRNA expression of jejunal genes in response to additive treatments in NE challenged broilers on day 16 1.
3.5. Apparent Ileal GE and CP Digestibility
The impacts of NE challenge and feed additives on apparent ileal GE and CP digestibility in broilers on day 24 are shown in Table 9. One-way ANOVA analysis showed that the apparent ileal GE digestibility exhibited significant differences (p = 0.013), but no differences of apparent ileal CP digestibility were present among the treatment groups. Birds fed additive MG had a higher GE digestibility compared to the NC group, but not different from the ZBS and MGFA groups.

Table 9.
Apparent ileal nutrient digestibility of NE challenged broilers in response to additive treatments on day 24 1.
4. Discussion
The clinical form of NE is disastrous to the broiler industry as occurred with impaired performance and high mortality [5,7]. Traditionally, antibiotics have been used to control NE. However, with the ban or phasing out of in-feed AGP from the poultry feed industry, worldwide, there have been concerted efforts to find a comparable alternative to AGP to ameliorate the adverse impacts of NE. Organic acids to some extent have shown to be effective against NE in subclinical form. To be a possible replacement of the in-feed AGP in the commercial broiler industry, it is essential to evaluate the feed additives under more severe diseased conditions. The current study examined the potentials of MG and FA to ameliorate the detrimental impacts of clinical NE on performance, mortality, and intestinal health in broilers. The successful introduction of clinical NE challenge was illustrated by the typical signs of NE observed in birds without supplementation (e.g., presence of intestinal lesions, reduced FI and BWG, increased FCR, and high mortality). Although the challenge was severe in this study, antibiotics were able to protect birds against clinical NE as indicated by very low mortality and improved performance. Results showed that diet supplemented with MG reduced mortality and upregulated TJP1 and IgG genes in the jejunum and improved apparent ileal GE digestibility whereas MGFA fed birds increased the expression of jejunal IgG gene compared to the NC group. Altogether, these findings support our hypothesis that birds supplemented with the MG product under investigation improve intestinal health, so provide better protection of the birds from clinical NE indicated by reduced mortality. However, in contrast to our hypothesis, dietary supplementation of FA in grower and finisher phases did not add benefits compared to the MG fed birds alone in the present conditions. Therefore, these results reject our hypothesis that FA supplementation in the grower and finisher phases may add beneficial effects in controlling birds from clinical NE.
The dietary addition of individual OA and their blends in different forms (e.g., calcium, sodium, and potassium salts with or without esterification) have the potential to improve FCR and weight gain, and enhance protection against enteric diseases. In general, the mode of action of OA is believed to be associated with their pH decreasing abilities and antibacterial activities [43]. The OA supplemented in diets mitigate the deleterious effect of enteric diseases on intestinal health via a pH reducing mechanism that decreases pathogenic bacterial load by bactericidal and bacteriostatic activities, resulting in improved microbial inhabitants in the OA supplemented birds compared to the birds without OA supplementation. Supplementation of OA in diets improves intestinal integrity by protecting the disruption of intestinal epithelial cells. Dietary inclusion of OA also improves the digestibility of nutrients by increasing pancreatic enzyme activities [9,44]. Therefore, OA supplemented to diets can have a positive impact on bird performance. On the other hand, monoglycerides are made from the esterification of fatty acids with a glycerol molecule and are harmless without stringent smells by nature. They can be released to the lower part of the intestine via the action of lipase and act against Gram-negative and Gram-positive bacteria [12,45]. Studies have shown that the esterification of fatty acids with glycerol molecules can increase the antibacterial activities, resulting in better microbial inhabitants [14,15]. As a result, monoglycerides supplemented in diets can positively affect bird performance. The current study showed that diet supplemented with MG in the starter phase reduced mortality and increased the expression of TJP1 and IgG genes in jejunum on day 16 and apparent ileal GE digestibility on day 24 compared to the NC group. Birds fed MG in the starter phase and FA in the grower and finisher phases had numerically reduced mortality, significantly increased the expression of jejunal IgG gene on day 16 compared to the NC group. These effects indicated the reduced NE severity and improved intestinal health status of birds under clinical NE. However, BWG or FCR were not improved during the entire period of study (days 0 to 35). On the other hand, although FCR was not statistically different between NC and feed additive groups, MG and MGFA treatment groups had lower FCR by 6.0 and 6.8 points compared to the NC group during the onset of NE (days 10 to 24), indicating the positive impact of additive supplementation. Due to the nature of clinical NE present in the current study, the NC group had much higher mortality and thus the entire experimental period showed mostly the performance of survived birds, whose performance was quickly recovered, thus less performance effects of OA were detectable following the recovery. Similar to our results, birds fed blended OA had no effects on BWG but improved FCR under a clinical NE challenge [46] and subclinical NE challenge condition [18]. Moreover, Geier et al. [46] also reported the high mortality in OA-fed birds and not inconsistent from the control group, which is inconsistent with our findings. Reduced mortality in birds fed MG compared to the NC group was possibly due to the higher immune responses and improved gut integrity observed in this study. These improvements of intestinal health in birds fed additives may have a positive impact on nutrient digestibility, indicated by increased apparent ileal GE digestibility. Previous studies have shown that birds supplemented with OA in diets improved apparent ileal GE digestibility [47,48], which further supports our findings. Moreover, additive supplemented birds had similar intestinal lesions compared to birds fed antibiotics, confirming the protective effects of additives as also shown before [47,49]. However, the results observed in this study also revealed that the dietary addition of FA in grower and finisher phases did not have positive impacts on performance and intestinal health over the diet supplemented with MG only in the starter phase as indicated by similar BWG, FCR, intestinal lesions, apparent ileal digestibility, bacterial quantification, and gene expression results in birds fed both additive supplemented diets under the present conditions. Similar to our results, a recent study reported that birds supplemented with FA (high dose) had no effects on performance and mortality in birds under sub-clinical NE challenge [18]. Cumulatively, the findings of this study indicated the beneficial effects of additive MG in reducing the severity of clinical NE on intestinal health, evidenced by improved immunity, gut integrity, digestibility, and reduced mortality.
An intact intestinal epithelium provides a major defense against the entry of pathogens and maintains homeostasis resulted in proper nutrient digestion, absorption, and utilization, leading to optimal intestinal health and growth performance [50,51]. Tight junction genes such as CLDN1, OCLN, and TJP1 are strongly connected with intestinal epithelial cells, and upregulation of these tight junction genes are associated with improved gut barrier integrity and permeability. The expression of TJP1 gene is correlated with other tight junction genes in the epithelium [52]. Enteric diseases such as NE damage intestinal integrity and downregulates tight junction gene expression (CLDN, OCLN, and TJP1), resulting in increased intestinal permeability [35,53,54]. However, it should be noted that damage in the intestinal epithelium and disturbances in the function of genes regulating tight junctions and immunity can be due to the Eimeria-caused infections prior to C. perfringens challenge of the birds. The application of Eimeria spp. prior to the C. perfringens challenge in the NE challenge model was to predispose birds for the successful induction of NE. Therefore, it is anticipated that the Eimeria caused infections would affect the intestinal health of the birds negatively. The results observed in the current study showed that the mRNA expression of tight junction gene TJP1 was upregulated in birds fed MG compared to the NC group, but not different from ZBS and MGFA fed birds, indicating the potentiality of MG to improve intestinal barrier function, as also shown before in butyrate supplemented birds [55]. Similar to our results, the upregulation of TJP1 in broilers supplemented with essential oil and OA containing butyric acid was previously reported by Pham et al. [56].
Tight junction genes, immunoglobulin genes (e.g., IgA, IgG, and IgM) produced by mucosal plasma cells in the lamina propria are acting as the first line of defense to protect the luminal surfaces and small intestine against diseases [57]. It has been indicated that NE damages intestinal epithelium and lamina propria, resulting in reduced nutrient uptake that in turn impaired immune responses [58]. Wang et al. [59] confirmed the significant effect of NE on immunoglobulin genes evidenced by the reduced expression of IgA+ B cells in birds infected with NE. Similarly, Gharib-Naseri et al. [35] reported a reduced expression of IgG and IgM genes in NE challenged birds. In the current study, upregulation of IgG gene in birds fed MG and MGFA compared to the NC group indicates that the birds fed additives had a greater immune response. Furthermore, birds fed MG had upregulated MUC2 gene (1.306) compared to the NC group (1.093), which is consistent with the reports of Stefanello et al. [60]. Mucin-2 is the primary mucin produced by goblet cells and is considered a biomarker of intestinal health [61] and higher expression of MUC2 is known to have improved intestinal health as it protects pathogenic bacterial adhesion to the mucosa [62,63]. Therefore, increased expression of immunoglobulin genes and MUC2 (despite numerically) in the current study suggests that the additives are able to modulate intestinal health and immune protection under the NE challenged condition.
Intestinal health plays a key role in nutrient digestion and absorption. The status of the intestinal mucosa is a good indicator of intestinal health. Mucosa plays an important role in the protection of birds against pathogenic bacterial adhesion, resulting in improved barrier function. Previous studies have shown that birds infected with enteric diseases are deemed to have disrupted functions of mucosa and tight junction. Their proper functions are essential for the optimum digestion and absorption of nutrients, thus such disruption results in reduced feed intake and increased energy demands [52,64,65]. Therefore, enhanced tight junction genes and immune responses observed in this study may underlie the improved apparent ileal GE digestibility, as has been shown previously in broilers [48,49]. Moreover, improved apparent GE digestibility in MG fed birds compared to the birds without supplementation could further indicate the ameliorating effects of additive MG against enteric inflammation [66].
5. Conclusions
The current study demonstrated that a diet supplemented with monoglyceride blend (MG) has the potential to improve intestinal health and reduce the severity of clinical NE, as indicated by upregulated tight junction and immune genes, improved energy digestibility, and reduced mortality. However, supplementation of FA in grower and finisher phases did not have beneficial effects on the performance and intestinal health over the diet supplemented with MG alone. As expected, the antibiotic was more effective to control the disease outbreak, and thus the development of additives may need to consider a possible combination of different additives with appropriate dosages to improve their usefulness in practice, and recommendations for the poultry industry as alternatives to in-feed AGP. Further research for a better understanding of their mode of action in improved intestinal health and consequent performance, and disease amelioration would provide more information for the poultry industry to combat the challenge posed by antibiotic-free production.
Author Contributions
A.K.: Animal trial, feed formulation, laboratory analysis, statistical analysis, manuscript writing; S.K.K.: Study design, feed formulation, data evaluation, manuscript review; L.L.: Study design, Data evaluation, manuscript review; S.-B.W.: Coordinator, study design, data validation, critical manuscript review. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by BASF, Germany.
Institutional Review Board Statement
The experimental procedures were approved by the Animal Ethics Committee of University of New England, Australia (Approval No.: AEC18-007).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to acknowledge Shuyu Song, Leanne Lisle, Elizabeth Marshall, and CART team, University of New England for their technical assistance. The authors thank Petrina Young for providing Eimeria spp. oocysts and Robert Moore for providing Clostridium perfringens EHE-18.
Conflicts of Interest
L.L. is employed by BASF, which provided the feed additives used in the current study, namely, BalanGutTM LS P, and Amasil® NA. The authors declare there are no relevant financial or non-financial competing interests to report.
References
- Cooper, K.K.; Songer, J.G.; Uzal, F.A. Diagnosing Clostridial enteric disease in poultry. J. Vet. Diagn. Invest. 2013, 25, 314–327. [Google Scholar] [CrossRef]
- Wade, B.; Keyburn, A. The true cost of necrotic enteritis. Poult. World 2015, 31, 16–17. [Google Scholar]
- Immerseel, F.V.; Buck, J.D.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathol. 2004, 33, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Kaldhusdal, M.; Schneitz, C.; Hofshagen, M.; Skjerve, E. Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Dis. 2001, 149–156. [Google Scholar] [CrossRef]
- Hofacre, C.; Mathis, G.; Quiroz, M. Natural alternatives to prevent necrotic enteritis. Int. Poult. Prod. 2005, 13, 7–9. [Google Scholar]
- M’Sadeq, S.A.; Wu, S.; Swick, R.A.; Choct, M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim. Nutr. 2015, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef]
- Seal, B.S.; Lillehoj, H.S.; Donovan, D.M.; Gay, C.G. Alternatives to antibiotics: A symposium on the challenges and solutions for animal production. Anim. Health Res. Rev. 2013, 14, 78–87. [Google Scholar] [CrossRef]
- Adil, S.; Banday, T.; Bhat, G.A.; Mir, M.S.; Rehman, M. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet. Med. Int. 2010, 479485. [Google Scholar] [CrossRef]
- Polycarpo, G.V.; Andretta, I.; Kipper, M.; Cruz-Polycarpo, V.C.; Dadalt, J.C.; Rodrigues, P.H.M.; Albuquerque, R. Meta-analytic study of organic acids as an alternative performance-enhancing feed additive to antibiotics for broiler chickens. Poult. Sci. 2017, 96, 3645–3653. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef]
- Namkung, H.; Yu, H.; Gong, J.; Leeson, S. Antimicrobial activity of butyrate glycerides toward Salmonella Typhimurium and Clostridium perfringens. Poult. Sci. 2011, 90, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Patten, J.; Waldroup, P. Use of organic acids in broiler diets. Poult. Sci. 1988, 67, 1178–1182. [Google Scholar] [CrossRef]
- Kabara, J.J. Antimicrobial agents derived from fatty acids. J. Am. Oil Chem. Soc. 1984, 61, 397–403. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, T.; Tsvetkova, V.; Najdenski, H.M. Antibacterial study of the medium-chain fatty acids and their 1-monoglycerides: Individual effects and synergistic relationships. Pol. J. Microbiol. 2009, 58, 43–47. [Google Scholar] [PubMed]
- Leeson, S.; Namkung, H.; Antongiovanni, M.; Lee, E. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult. Sci. 2005, 84, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rubio, C.; Ordonez, C.; Abad-González, J.; Garcia-Gallego, A.; Honrubia, M.P.; Mallo, J.J.; Balana-Fouce, R. Butyric acid-based feed additives help protect broiler chickens from Salmonella Enteritidis infection. Poult. Sci. 2009, 88, 943–948. [Google Scholar] [CrossRef]
- Gharib-Naseri, K.; Kheravii, S.K.; Wu, S.B. Buffered formic acid and a monoglyceride blend coordinately alleviate subclinical necrotic enteritis impact in broiler chickens. Poult. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Bedford, A.; Yu, H.; Hernandez, M.; Squires, E.; Leeson, S.; Gong, J. Effects of fatty acid glyceride product SILOhealth 104 on the growth performance and carcass composition of broiler chickens. Poult. Sci. 2018, 97, 1315–1323. [Google Scholar] [CrossRef]
- Bedford, A.; Yu, H.; Squires, E.; Leeson, S.; Gong, J. Effects of supplementation level and feeding schedule of butyrate glycerides on the growth performance and carcass composition of broiler chickens. Poult. Sci. 2017, 96, 3221–3228. [Google Scholar] [CrossRef]
- NHMRC. Australian Code for the Care and Use of Animals for Scientific Purposes, 8th ed.; National Health and Medical Research Council: Canberra, Australia, 2013. [Google Scholar]
- Aviagen. Ross Broiler Management Handbook; Aviagen Ltd.: Newbridge Midlothian, UK, 2014. [Google Scholar]
- Rodgers, N.J.; Swick, R.A.; Geier, M.S.; Moore, R.J.; Choct, M.; Wu, S.-B. A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2015, 59, 38–45. [Google Scholar] [CrossRef]
- Wu, S.-B.; Stanley, D.; Rodgers, N.; Swick, R.A.; Moore, R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014, 169, 188–197. [Google Scholar] [CrossRef]
- Keyburn, A.L.; Sheedy, S.A.; Ford, M.E.; Williamson, M.M.; Awad, M.M.; Rood, J.I.; Moore, R.J. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 2006, 74, 6496–6500. [Google Scholar] [CrossRef] [PubMed]
- Kheravii, S.K.; Swick, R.A.; Choct, M.; Wu, S.B. Coarse particle inclusion and lignocellulose-rich fiber addition in feed benefit performance and health of broiler chickens. Poult. Sci. 2017, 96, 3272–3281. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.G.; Siragusa, G.R. Quantitative analysis of the intestinal bacterial community in one- to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J. Appl. Microbiol. 2007, 102, 1138–1149. [Google Scholar] [CrossRef]
- Requena, T.; Burton, J.; Matsuki, T.; Munro, K.; Simon, M.A.; Tanaka, R.; Watanabe, K.; Tannock, G.W. Identification, detection, and enumeration of human Bifidobacterium species by PCR targeting the transaldolase gene. Appl. Environ. Microbiol. 2002, 68, 2420–2427. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Yu, B.; He, J.; Yu, J.; Mao, X.; Wang, J.; Luo, J.; Huang, Z.; Cheng, G. Spray-dried chicken plasma improves intestinal digestive function and regulates intestinal selected microflora in weaning piglets. J. Anim. Sci. 2015, 93, 2967–2976. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Lee, D.-H.; Zo, Y.-G.; Kim, S.-J. Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism. Appl. Environ. Microbiol. 1996, 62, 3112–3120. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, 1–14. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Gharib-Naseri, K.; Kheravii, S.; Keerqin, C.; Swick, R.A.; Choct, M.; Wu, S.-B. Differential expression of intestinal genes in necrotic enteritis challenged broiler chickens with two different Clostridium perfringens strains. Poult. Sci. 2020. [Google Scholar] [CrossRef]
- Du, E.; Wang, W.; Gan, L.; Li, Z.; Guo, S.; Guo, Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-Q.; Zhang, Z.-W.; Yao, H.-D.; Wang, L.-L.; Liu, T.; Yu, X.-Y.; Li, S.; Xu, S.-W. Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res. Vet. Sci. 2013, 95, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, S.; Liu, G.; Zhao, J.; Jiao, H.; Wang, X.; Song, Z.; Lin, H. Vitamin A deficiency impairs mucin expression and suppresses the mucosal immune function of the respiratory tract in chicks. PLoS ONE 2015, 10, e0139131. [Google Scholar] [CrossRef]
- Yang, F.; Lei, X.; Rodriguez-Palacios, A.; Tang, C.; Yue, H. Selection of reference genes for quantitative real-time PCR analysis in chicken embryo fibroblasts infected with avian leukosis virus subgroup. J. BMC Res. Notes 2013, 6, 1–5. [Google Scholar] [CrossRef]
- Kuchipudi, S.V.; Tellabati, M.; Nelli, R.K.; White, G.A.; Perez, B.B.; Sebastian, S.; Slomka, M.J.; Brookes, S.M.; Brown, I.H.; Dunham, S.P. 18S rRNA is a reliable normalisation gene for real-time PCR based on influenza virus-infected cells. Virol. J. 2012, 9, 1–7. [Google Scholar] [CrossRef]
- Short, F.; Gorton, P.; Wiseman, J.; Boorman, K. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996, 59, 215–221. [Google Scholar] [CrossRef]
- Base SAS® 9.3 Procedures Guide: Statistical Procedures; SAS Institute. Inc.: Cary, NC, USA, 2010.
- Van Immerseel, F.; Russell, J.; Flythe, M.; Gantois, I.; Timbermont, L.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R. The use of organic acids to combat Salmonella in poultry: A mechanistic explanation of the efficacy. Avian Pathol. 2006, 35, 182–188. [Google Scholar] [CrossRef]
- Papatsiros, V.; Katsoulos, P.-D.; Koutoulis, K.; Karatzia, M.; Dedousi, A.; Christodoulopoulos, G. Alternatives to antibiotics for farm animals. CAB Rev. Ag. Vet. Sci. Nutr. Res. 2013, 8, 1–15. [Google Scholar] [CrossRef]
- Sampugna, J.; Quinn, J.; Pitas, R.; Carpenter, D.; Jensen, R. Digestion of butyrate glycerides by pancreatic lipase. Lipids 1967, 2, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Geier, M.; Mikkelsen, L.; Torok, V.; Allison, G.; Olnood, C.; Boulianne, M.; Hughes, R.; Choct, M. Comparison of alternatives to in-feed antimicrobials for the prevention of clinical necrotic enteritis. J. Appl. Microbiol. 2010, 109, 1329–1338. [Google Scholar] [CrossRef]
- Kumar, A.; Toghyani, M.; Kheravii, S.K.; Pineda, L.; Han, Y.; Swick, R.A.; Wu, S.-B. Potential of blended organic acids to improve performance and health of broilers infected with necrotic enteritis. Anim. Nutr. 2021. [Google Scholar] [CrossRef]
- Fascina, V.B.; Sartori, J.R.; Gonzales, E.; Carvalho, F.B.; Souza, I.M.G.P.; do Valle Polycarpo, G.; Stradiotti, A.C.; Pelícia, V.C. Phytogenic additives and organic acids in broiler chicken diets. Rev. Cent. Am. Odontol. 2012, 41, 2189–2197. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Y.; Chen, J.; Ni, A.; Jiang, Y.; Li, Y.; Huang, Z.; Shi, L.; Xu, H.; Chen, C. Effect of replacing in-feed antibiotics with synergistic organic acids on growth performance, health, carcass, and immune and oxidative status of broiler chickens under Clostridium perfringens type A challenge. Avian Dis. 2020. [Google Scholar] [CrossRef]
- Ritzi, M.M.; Abdelrahman, W.; Mohnl, M.; Dalloul, R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014, 93, 2772–2778. [Google Scholar] [CrossRef]
- De Medina, F.S.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. Ther. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Medina, R.; Rahner, C.; Mitic, L.; Anderson, J.; Van Itallie, C. Occludin localization at the tight junction requires the second extracellular loop. J. Memb. Biol. 2000, 178, 235–247. [Google Scholar] [CrossRef]
- Barekatain, R.; Nattrass, G.; Tilbrook, A.J.; Chousalkar, K.; Gilani, S. Reduced protein diet and amino acid concentration alter intestinal barrier function and performance of broiler chickens with or without synthetic glucocorticoid. Poult. Sci. 2019, 98, 3662–3675. [Google Scholar] [CrossRef]
- Vicuña, E.; Kuttappan, V.; Tellez, G.; Hernandez-Velasco, X.; Seeber-Galarza, R.; Latorre, J.; Faulkner, O.; Wolfenden, A.; Hargis, B.; Bielke, L. Dose titration of FITC-d for optimal measurement of enteric inflammation in broiler chicks. Poult. Sci. 2015, 94, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Kan, L.; Huang, J.; Geng, Y.; Zhen, W.; Guo, Y.; Abbas, W.; Wang, Z. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J. Anim. Sci. Biotechnol. 2020, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Johansen, F.E. Mucosal B cells: Phenotypic characteristics, transcriptional regulation, and homing properties. Immunol. Rev. 2005, 206, 32–63. [Google Scholar] [CrossRef] [PubMed]
- Konashi, S.; Takahashi, K.; Akiba, Y. Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. Br. J. Nutr. 2000, 83, 449–456. [Google Scholar] [CrossRef]
- Wang, H.; Ni, X.; Qing, X.; Liu, L.; Lai, J.; Khalique, A.; Li, G.; Pan, K.; Jing, B.; Zeng, D. Probiotic enhanced intestinal immunity in broilers against subclinical necrotic enteritis. Front. Immunol. 2017, 8, 1592. [Google Scholar] [CrossRef]
- Stefanello, C.; Rosa, D.P.; Dalmoro, Y.K.; Segatto, A.L.; Vieira, M.S.; Moraes, M.L.; Santin, E. Protected Blend of Organic Acids and Essential Oils Improves Growth Performance, Nutrient Digestibility, and Intestinal Health of Broiler Chickens Undergoing an Intestinal Challenge. Front. Vet. Sci. 2020, 6, 491. [Google Scholar] [CrossRef] [PubMed]
- Golder, H.; Geier, M.; Forder, R.; Hynd, P.; Hughes, R. Effects of necrotic enteritis challenge on intestinal micro-architecture and mucin profile. Br. Poult. Sci. 2011, 52, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Applegate, T.J.; Lossie, A.C. Cloning, annotation and developmental expression of the chicken intestinal MUC2 gene. PLoS ONE 2013, 8, e53781. [Google Scholar] [CrossRef]
- Chen, J.; Tellez, G.; Richards, J.D.; Escobar, J. Identification of potential biomarkers for gut barrier failure in broiler chickens. Front. Vet. Sci. 2015, 2, 14. [Google Scholar] [CrossRef]
- Balda, M.S.; Matter, K. Tight junctions at a glance. J. Cell Sci. 2008, 121, 3677–3682. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Klasing, K. An immunologist’s perspective on nutrition, immunity, and infectious diseases: Introduction and overview. J. Appl. Poult. Res. 2009, 18, 103–110. [Google Scholar] [CrossRef]
- Palamidi, I.; Paraskeuas, V.; Theodorou, G.; Breitsma, R.; Schatzmayr, G.; Theodoropoulos, G.; Fegeros, K.; Mountzouris, K.C. Effects of dietary acidifier supplementation on broiler growth performance, digestive and immune function indices. Anim. Product. Sci. 2017, 57, 271–281. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).