1. Introduction
Lake Sturgeon,
Acipenser fulvescens, are a species ancestral to the teleost fishes, in the division Osteichthyes (bony fishes), and part of the class Actinopterygii (ray-finned fishes). Lake Sturgeon have a rich history in America; until the 1870s, they were considered a “nuisance” with little to no value. Not much care was put into the conservation of Lake Sturgeon populations and when they were caught by fishermen, they were killed, thrown back, or left on the river/lake banks [
1]. It was not until they were used for caviar that Lake Sturgeon were considered of value, and this change contributed to additional population declines. Habitat disturbance and pollution in lakes further decreased the population levels [
2,
3]. Lake Sturgeon are one of the largest freshwater fish, and have one of the longest life spans. The oldest specimen recorded was caught in 1952 and was estimated to be 152 years old [
1,
4]. Female Lake Sturgeon reach sexual maturity in 14 to 24 years and males at approximately 14 to 16 years old [
5]. It was not until the mid-to-late 20th century that people started to realize that populations were declining at an alarming rate and research toward their conservation began in earnest. In 1986, Lake Sturgeon were listed as vulnerable on the International Union for Conservation of Nature (IUCN) red list due to populations declining. With the help of management and protection plans, in 2004 Lake Sturgeon were listed as a species of least concern by IUCN, and populations are increasing [
1]. The northernmost range of Lake Sturgeon is Hudson Bay in Canada, while the southernmost populations of Lake Sturgeon in North America are found in the Coosa River in Georgia and Alabama. Populations have been declining in the Southern United States region [
6,
7] and to re-establish the extirpated population, the Georgia Department of Natural Resources (GA-DNR) established a conservation stocking program for Lake Sturgeon into the Coosa River in 2002.
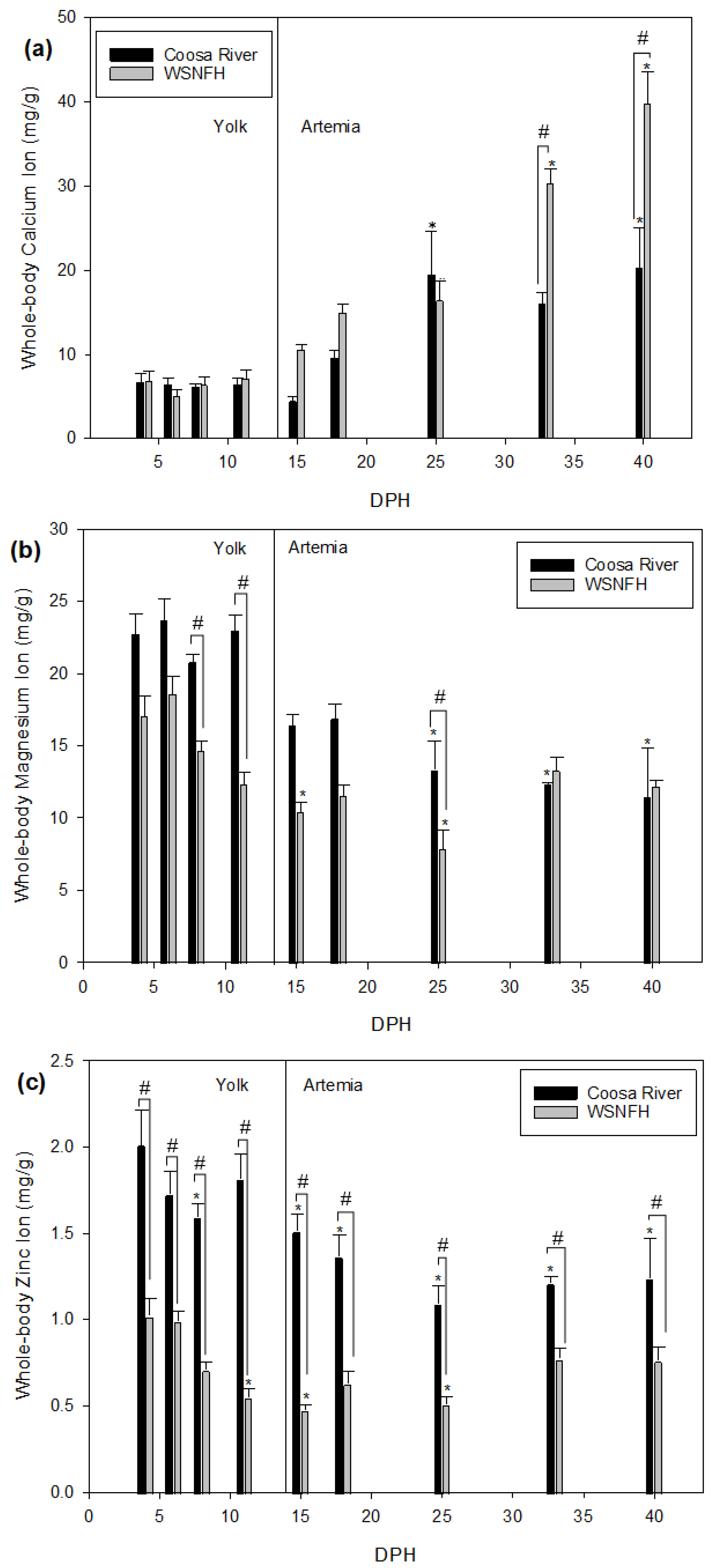
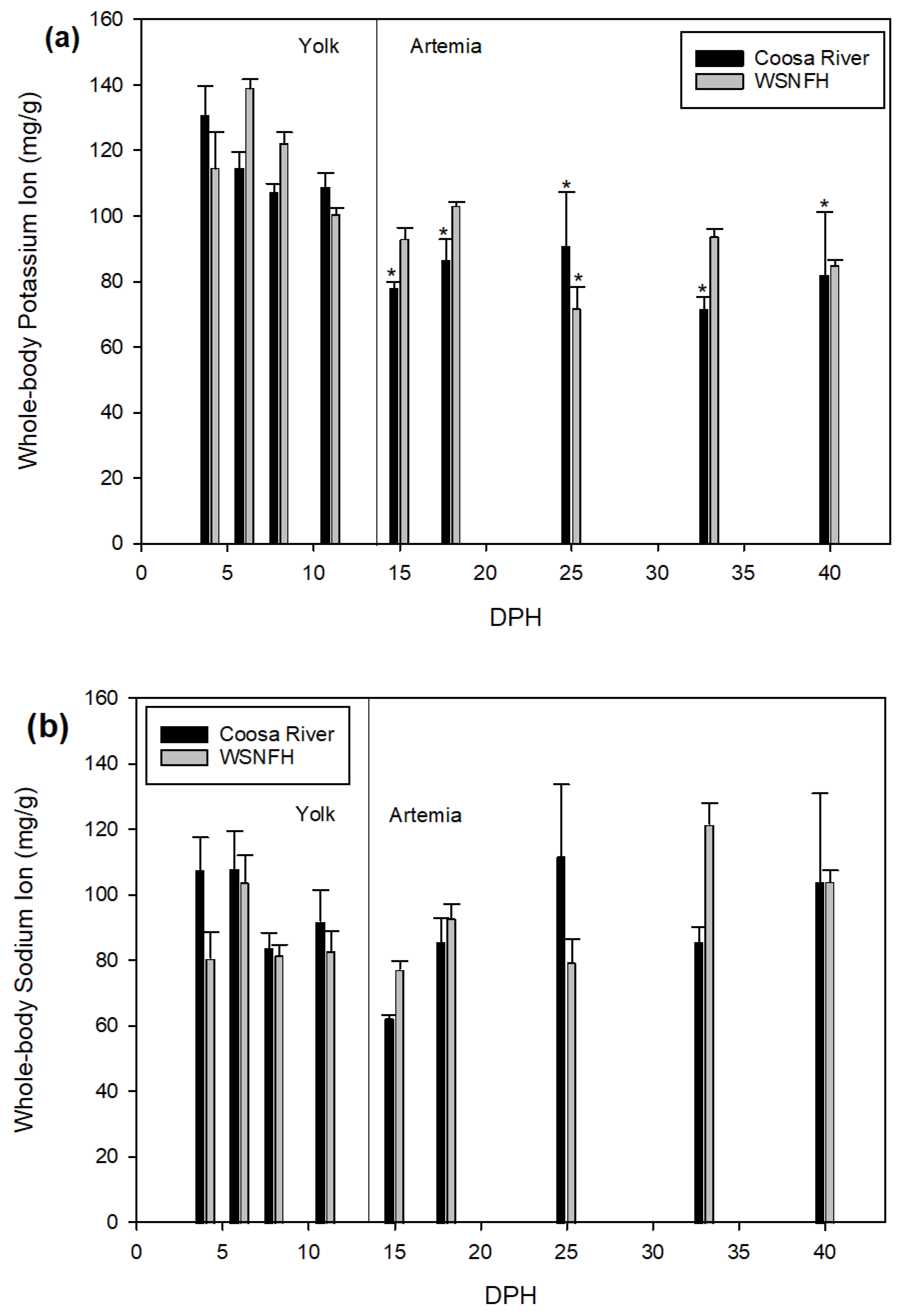
It is important to observe what differences in development may exist between the river where Lake Sturgeon naturally occur and the hatchery where Lake Sturgeon are reared for stocking. There are some important similarities and differences between the Warm Springs National Fish Hatchery (WSNFH) and Coosa River water. In the Coosa River water, magnesium, zinc, and sodium are significantly higher (
Table 1) [
8]. In WSNFH water, the levels of calcium are almost double the concentration found in Coosa River water. Potassium levels are almost the same between the two systems (Coosa River: 1.59 ± 0.06 vs. WSNFH: 1.62 ± 0.16 mg/L). This study used water collected from the Coosa River to see what effect it will have on Lake Sturgeon when they are developing in order to provide insight into how stocked juveniles may differ from naturally recruited young of the year. We also measured ions in the water from the Warm Springs National Fish Hatchery because young-of-year Lake Sturgeon are being kept and grown annually by that facility so we could compare the water at WSNFH to the Coosa River water. Knowing how Lake Sturgeon develop in the wild could help conservation projects in the future.
Lake Sturgeon can absorb calcium, magnesium, zinc, potassium, and sodium in more ways than simply ingesting them through their food. They can also intake calcium at the gills by mitochondria-rich cells (MRC), also known as chloride cells or ionocytes [
9,
10,
11]. In teleost fishes, the gills are where most of the calcium is absorbed [
10]. In larval fish, ions can be also absorbed through the skin [
11,
12]. For all the ions (calcium, magnesium, zinc, potassium, and sodium), where they are absorbed depends on the amounts of ions in the water. If the water ion concentrations are low, they may absorb more from the food to make up for the low levels [
13]. It is estimated that about 30% of calcium is absorbed through the intestines in fishes such as
Gadus morhua [
9]. There is not a lot known regarding the natural diet of exogenously feeding larval Lake Sturgeon. It is believed that young sturgeon eat small invertebrates and are omnivorous [
6]. This study focused on the calcium uptake from the water and controlled dietary ion input in an attempt to isolate effects that reflect only branchial, rather than intestinal, absorption. We also focused on zinc because this heavy metal has been found at sub-lethal levels in WSNFH water and Coosa River water and accumulates in Lake Sturgeon tissues. A buildup of zinc in the body of a Lake Sturgeon that absorbs or consumes it may cause overall growth to slow over time, as is the case in other bony fishes [
14]. It has also been shown that a decrease in the calcium levels also decreases the zinc levels [
15].
Early in a Lake Sturgeons’ life, they have a high requirement for calcium to support their development [
16], but the freshwater lakes and rivers in which they are found typically do not have high levels of calcium [
13]. An important question is how do Lake Sturgeon in the Southeastern United States get enough calcium to grow and develop normally? Available information on calcium regulation in larval and juvenile Lake Sturgeon is very limited, and we still do not fully understand the mechanisms driving uptake of calcium in Lake Sturgeon [
17]. While in the pre-larval stage, Lake Sturgeon obtain the calcium they need from their yolk and subsequently rely on branchial and intestinal transport, similar to other fishes [
11,
18]. Larval Lake Sturgeon are able to increase their calcium absorption rates if their environments have low levels to try and combat low levels of calcium, as is the case for most bony fishes [
11]. Since Lake Sturgeon are not typically found in water that is high in calcium, we are interested in how naturally recruited Lake Sturgeon larvae are surviving with these lower levels of environmental calcium. They might be utilizing these uptake mechanisms to obtain calcium from their environment and then storing the calcium in body tissues. Understanding the storage of calcium in developing Lake Sturgeon can help the population in the future, since calcium is important to their development and growth.
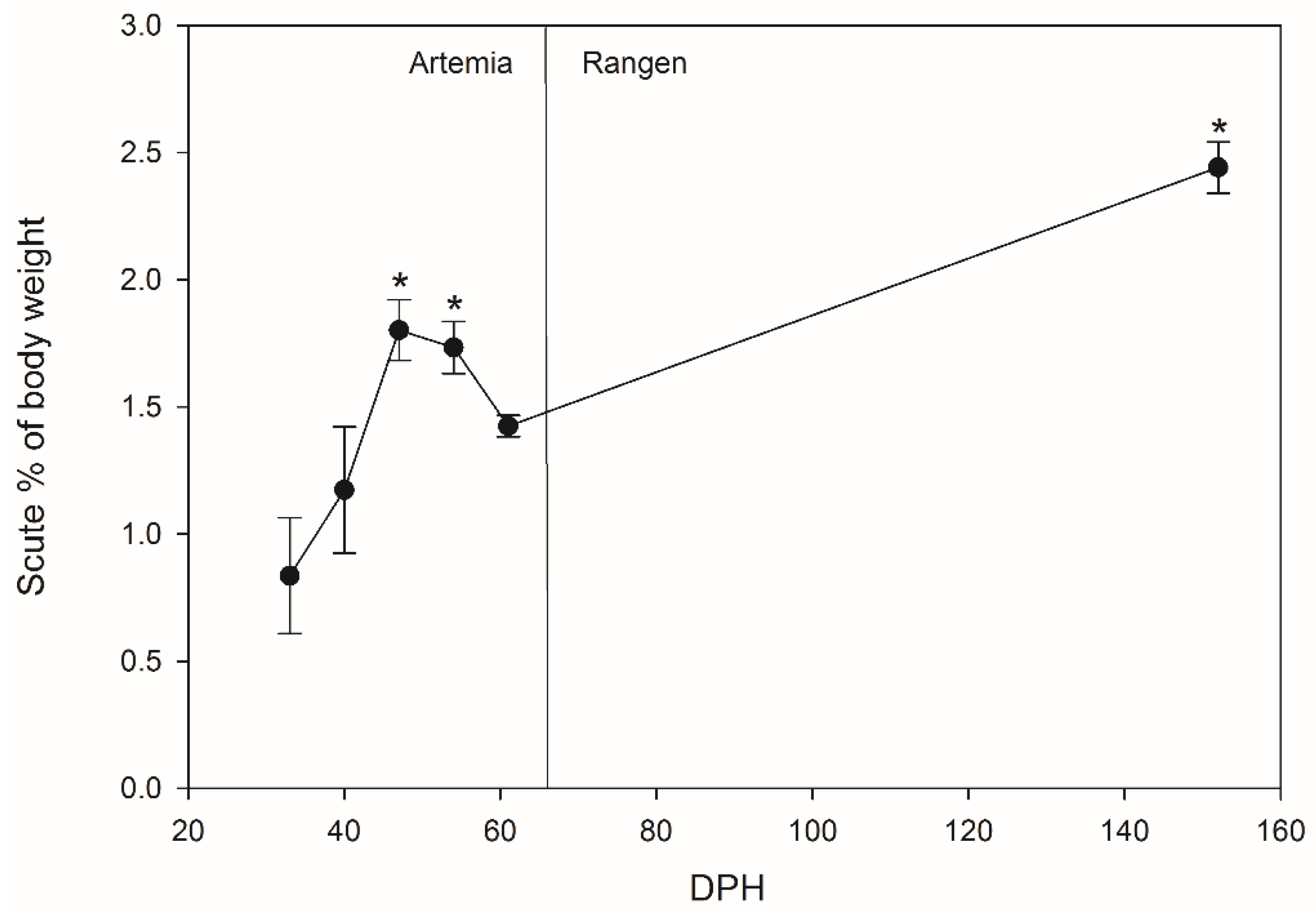
A possible storage reservoir for absorbed calcium may be the bony scutes developed in juvenile Lake Sturgeon. Sturgeon scutes are a form of ganoid scales, with five rows arranged longitudinally along the dorsal and lateral lines of Lake Sturgeon. In Lake Sturgeon, calcium is utilized to produce defensive dorsal scutes, which are mostly made up of calcium-based minerals, most likely hydroxyapatite, with substantial phosphate and carbonate content [
19]. When they are still developing, Lake Sturgeon rely on their scutes for protection [
20]. For hatchery-reared Lake Sturgeon, stocking survival is thought to be associated with fish size [
21]; fish are therefore reared to a relatively large size (>10 cm total length) in the hatchery prior to release. If Lake Sturgeon develop scutes faster and larger while young, this could help the stocked fingerlings to have better protection when they are released into the river system.
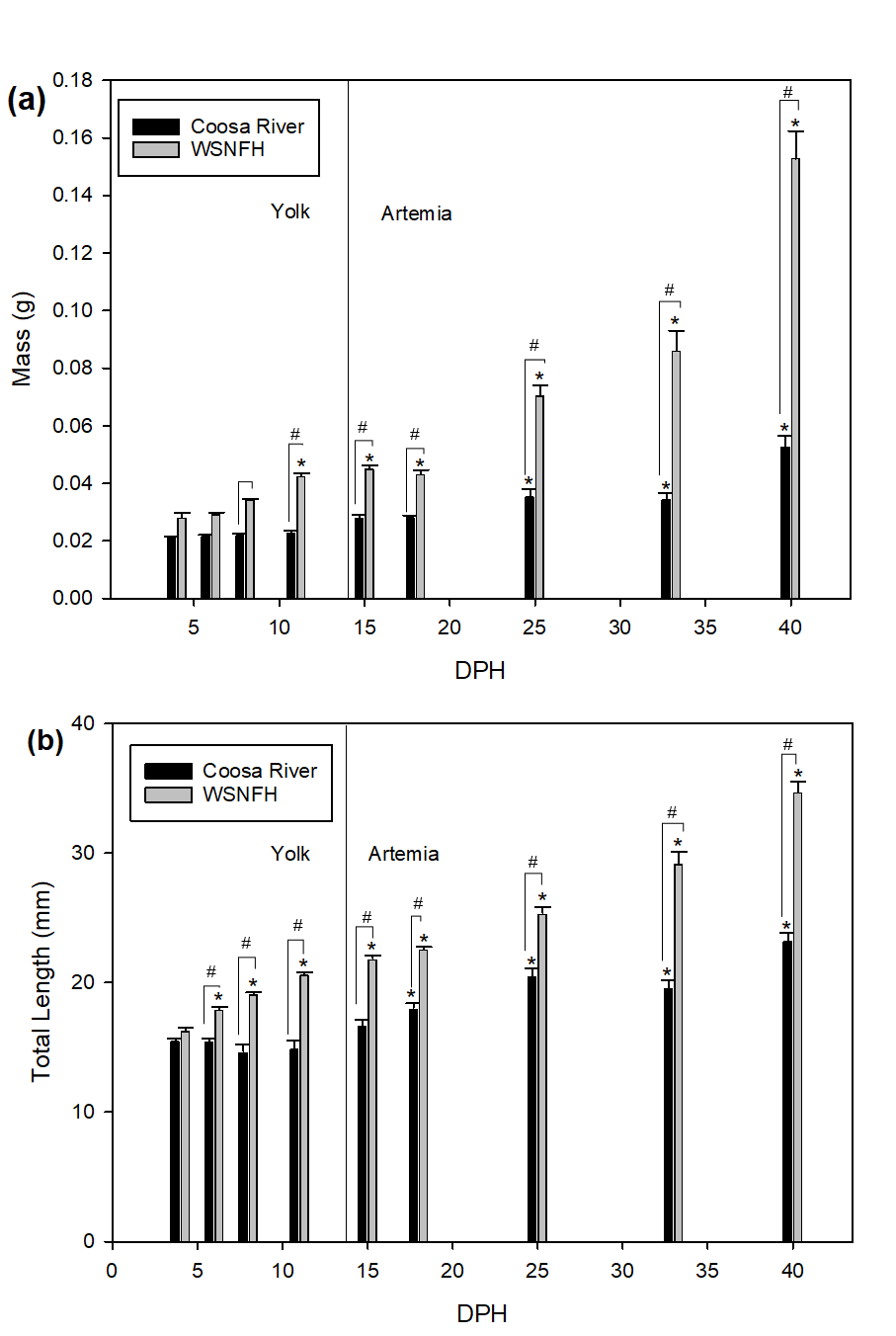
This study examined the growth, ion storage, and scute development of Lake Sturgeon, via three specific hypotheses: (1) ion accumulation in tissues of larvae will be directly correlated with environmental concentrations, (2) the period of active larval growth, measured as the rate of increase of body mass and total length, will stabilize earlier in water with higher calcium compared to lower calcium, and (3) Lake Sturgeon in water with higher calcium levels will develop scutes before Lake Sturgeon in water with lower levels of calcium.
2. Materials and Methods
2.1. Aquarium System
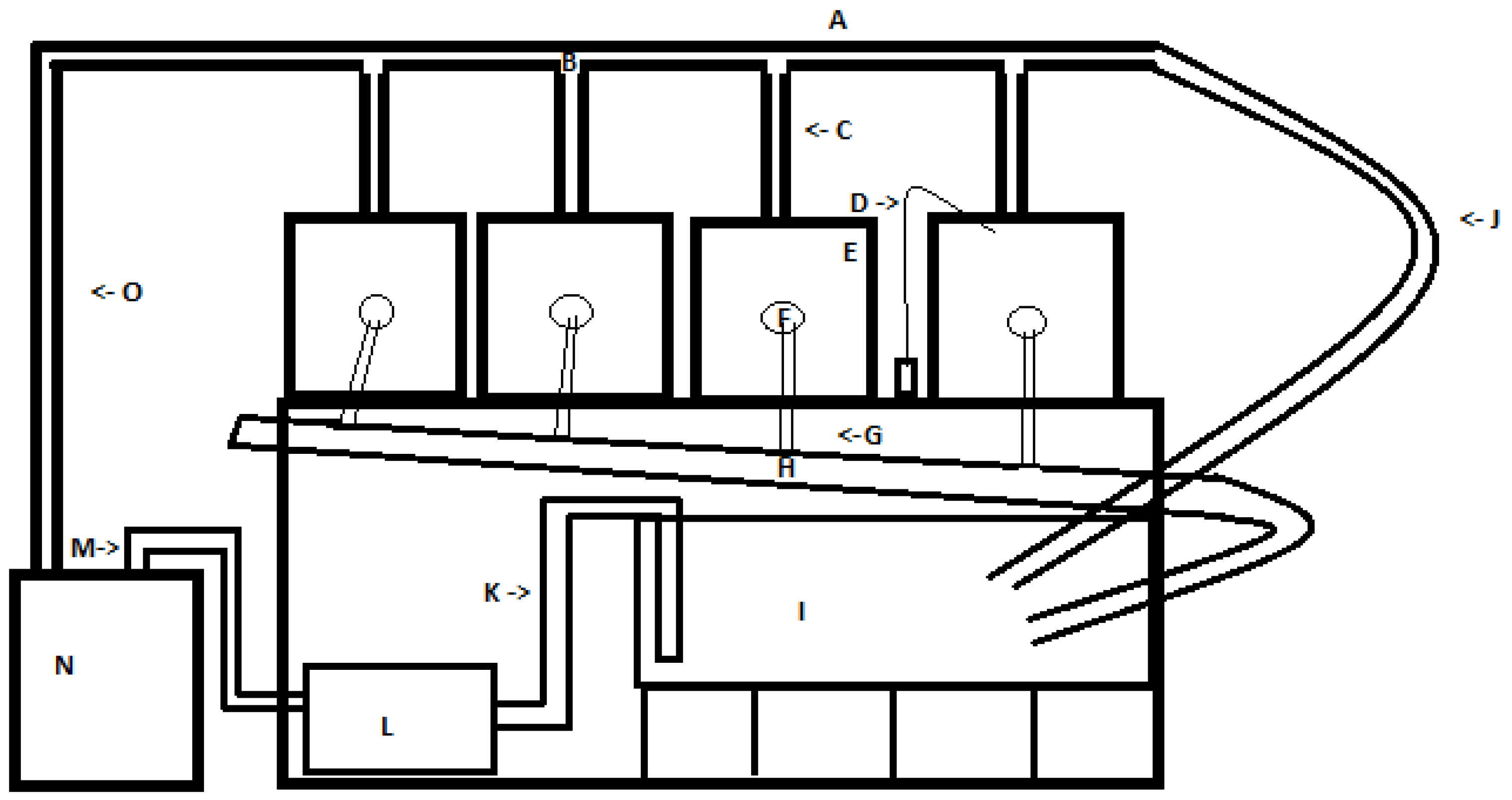
We set up a recirculating aquarium system (
Figure 1) to mimic the system in use at the Warm Springs National Fish Hatchery (WSNFH), consisting of four, 38 L tanks (
Figure 1E) which drained to a 208 L sump tank (
Figure 1I) which was elevated off the ground to allow proper flow to the filter and help with temperature control. Tank water was maintained at 15 ± 1 °C at both the University of West Georgia aquatics research lab (UWG) and WSNFH using a chiller (AquaEuroUSA Max-Chill, Aquacave Inc., Lake Forest, IL, USA) (
Figure 1N), and recirculated with an external water pump (Little Giant Pump, Franklin Electric Co., Fort Wayne, IN, USA) (
Figure 1L). Two air pumps (Penn-Plax Silent-air XS, Hauppauge, NY, USA) (
Figure 1D), and one canister filter (Penn-Plax Cascade 700, Hauppauge, NY, USA) were used to maintain consistently high oxygen levels and low nitrogen levels in the system.
In each 38 L tank, we had an outflow drain from the top of the tank (
Figure 1F). The outflow grill was covered with a piece of fine mesh fabric to make sure only water would go down the drain. Water was returned to the upper tanks from the sump using an external pump, connected to the chiller for temperature control. The pipes above the tanks we called the upper outflow, which included not only the water input for the upper tanks, but also a water line for surplus water to bypass the upper tanks and drain into the sump to control pressure and water flow coming out of the upper outflow (
Figure 1J). For the lower outflow of water from the four tanks to the sump, half-inch pipes connected each tank to a collection pipe (
Figure 1H), which was slanted at an angle to keep water flowing down towards the sump. To connect all the PVC pipes, we used water-safe PVC cement (Oatey rain-R-shine medium blue). Door screening fabric was used to build tank dividers to keep larvae from getting near the overflow drain. We slid the fabric into plastic clips and used aquarium-safe sealant (Marineland Aquarium) to attach the holders into the tank. Before we received Lake Sturgeon embryos, we filled all the tanks with water collected from the Coosa River and ran the recirculating system for 2 weeks to make sure it was working properly and establish the biological filters prior to the introduction of the Lake Sturgeon. Water was tested for appropriate water quality parameters before putting larvae in the system.
Water was collected from the Coosa River (location: 34.380306, −85.123845) by GA-DNR personnel and delivered to UWG using a fish-stocking truck. The water was transferred by pump to four large (>550 L) holding tanks for storage. We then placed a water filter and airline (Penn-Plax Silent-air X5, Hauppauge, NY, USA) in each tank. The water stayed in these holding tanks until it was needed for water changes and to top off the water in the tanks after feedings to keep a consistent total water volume and maintain good water quality.
The room lights were kept off in the lab during the entire study duration. Lab personnel used headlamps on the low setting during feeding or other work near the tanks. Nearby overhead lights were set to a light timer to turn on at 7:30 am every morning and to go off at 9 pm every night. This matched up with the natural sunrise and sunset times for the region in May/June. To mimic their natural environment of light only coming from above the larvae, and to minimize stress, we blacked out the sides of the tanks with black contact paper to keep any light from coming in on the sides of the tanks.
2.2. Larval Rearing
Procedures involving live animals used in this study were approved by the Institutional Animal Care and Use Committee of the University of West Georgia (protocol #1201). Lake Sturgeon eggs were obtained from the Wolf River, WI by USFWS personnel. Immediately after transport to WSNFH (Warm Springs, GA, USA), a subset of 700 eggs was transported in coolers to the aquatics lab at UWG and placed in the prepared tanks filled with water collected from the Coosa River. In this way, two subsets of a single cohort were reared from the egg stage in either Coosa River water (at UWG) or under standard hatchery conditions (at WSNFH).
We placed Lake Sturgeon eggs into two egg jars made with two 2 L bottles, 1″ PVC tubing, and aquarium-safe sealant (Marineland Aquarium), following a previously described procedure [
22]. We placed half of the eggs in each egg jar. Each egg jar had a water pipe that went down into it, generating a small flow to gently circulate the eggs within the bottom third of the jar. The egg jars were checked multiple times a day to make sure no fungi or dead eggs had accrued. Eggs that were not viable were removed to keep any fungi from developing.
After the Lake Sturgeon hatched out, they exhibited swim-up behavior, and we placed them into submersible breeder boxes with mesh around the inside to keep them from escaping through the drain holes on the sides. Once the Lake Sturgeon began to consume live feed (
Artemia spp.), they were placed in two larval baskets secured to the inside of the tank above the water line of the main tank (
Figure 1E) to ensure water could flow in from the top of the larval holder and then drain to the sump for mechanical and biological filtering. These baskets had six, 0.5’’ holes down the side with mesh covering each hole so water and excess food could flush out but not the larvae, while maintaining a water depth of approximately 4 inches. We would turn the water off when we were feeding the Lake Sturgeon. Out of the four tanks, only two were used to hold larvae at any given time.
We performed a weekly water change of 38 L. We pulled the water from the sump or drained one of the smaller tanks to do a tank cleaning and water change at the same time. We did not clean tanks that had a sturgeon holder in them to make certain we did not disturb the larvae. We then obtained water from the holding tanks to replace the water in the tank system. Commercial testing kits (API) were used regularly to check for nitrogenous compounds such as nitrate, nitrite, or ammonia in the water sample; any evidence of nitrogenous waste buildup would be followed by a water change to keep levels as near to zero as possible.
At 12 days post-hatch (dph), the larvae were offered Artemia as the initial feed (Argent Aquaculture Argentamin Gold Grade I Brine shrimp eggs 90% hatch rate). We made a solution of salt-water to hatch the Artemia cysts according to the provided recommendations by dissolving 25 g of non-iodized salt and 2 g of sodium bicarbonate into 950 mL of deionized water. The salinity was confirmed as 28–30 ppt using a portable refractometer. Large batches of the saltwater were made every three days. We used a water bottle hatching system to incubate the Artemia to feed the Lake Sturgeon. To do this, we placed six water bottles (500 mL each) upside down in a metal holder with the bottoms cut out of the bottles. Each water bottle was filled with 500 mL saltwater and 1.2 g of Artemia cysts per jar, so that one hatched jar was sufficient for each larval feeding. Once filled, the bottoms of the bottles that were cut off were placed back on the bottle as a “lid” to reduce evaporation, with small holes in them so they would not build up pressure and pop off. We replaced the bottle caps with a cap that had two out ports: one for air, which also provided circulation of the cysts, and the other to retrieve the hatched Artemia. The temperature of the jars was maintained at 28 °C by using two 100 watt bulbs in terrarium reflecting lamps at a set distance from the bottles. A temperature gauge in the bottle was used to monitor the temperature. Hatch out of Artemia occurred within 24 h, at which point they were retrieved by turning off the airlines and moving the light towards the cap (bottom) of the bottles. After 20 min or when most of the Artemia had moved towards the cap, we collected them from the bottles using the siphon line with a spigot at the end. The Artemia were emptied out over a plastic beaker and strained into a fine-mesh net. We cleaned off the saltwater by dipping the net into another plastic beaker filled with water from the tank system. At each feeding time, we evenly distributed the Artemia from one entire jar to the larval holders using plastic transfer pipettes. Frozen cubes of baby brine shrimp (Hikari Bio-Pure) were used for supplementary feeding as needed.
We fed the Lake Sturgeon larvae four times a day at 2 am, 8 am, 2 pm, and 9 pm. At each feeding, the Lake Sturgeon were given an entire jar of hatched
Artemia ranging in volume from 6 mL to 12 mL and allowed to feed to satiation for 1–2 h. In general, the nutritional value of
Artemia with regard to the ions of interest in this study has been reported as: sodium (2.1–51.1 mg·g
−1), potassium (0.73–12.7 mg·g
−1), magnesium (1.05–6.8 mg·g
−1), calcium (0.2–4.8 mg·g
−1), and zinc (75–241 µg·g
−1) [
23]. After each feeding event, any leftover food was gently siphoned out of the larval holder using a modified tank gravel cleaner. We measured how much water was removed from the recirculating water system and replaced the same volume of water from the reservoir tanks into the system at the sump.
2.3. Water Sampling
We collected 40 mL aliquots of water from the sump, water held in the river water reservoir tanks, and from WSNFH each time Lake Sturgeon samples were taken (
Appendix A (
Table A1)). We used water testing strips (Tetra Eazy Strips 6-in-1) to do quick testing of the water quality. We also took samples of the water and tested it for ammonia (NH
3/NH
4+), nitrite (NO
2−), nitrate (NO
3−), and general and carbonate hardness (GH and KH) using commercial testing kits (API). We used a portable pH meter (Cole Parmer 423) accurate to ±0.01 units to test water pH. Following these initial water quality checks, the water samples were stored in a refrigerator at 4 °C for later analysis of ion content (Na
+, Ca
2+, Mg
2+, K
+, and Zn
2+) via Inductively Coupled Plasma Optical Emission Spectrometry (ICP:OES).
2.4. Larval Sampling
We made a pH-buffered solution of tricanine methanesulfonate (MS-222) in 150 mL water by adding 0.33 g of MS-222 and 0.105 g of sodium bicarbonate. Larvae identified for sampling were quickly transferred using a wide-mouth pipette to 40 mL of water and then 10 mL of the MS-222 stock solution was added to dilute to the final concentration (440 mg/L). All sampled fish were euthanized simultaneously for each sampling point. Prior to taking physical measurements, mortality was confirmed by watching for any movement within a 10 min period after flipping the bottle over. Our first three samples were taken in the first week, after which samples were taken two times a week for two weeks (
n = 10–12 per sample). After this, samples were taken once a week. We sampled four weeks longer at WSNFH than we did at the UWG lab (
Table A1), due to higher mortality at UWG. All Lake Sturgeon had fasted for 12 h before sampling to make sure subsequent analysis represented the tissue of the fish only and did not include any gut content.
Sampling protocols until 11 dph were identical at WSNFH and UWG. Fish were euthanized, measured for total length with an electronic digital caliper to the nearest 0.01 mm, and placed in individual pre-weighed microcentrifuge tubes, and then reweighed to determine the mass of each sampled fish. After the Lake Sturgeon began feeding on
Artemia (15 dph,
Table A1), we began to bring them back to the UWG lab alive to check for any scute development instead of taking measurements at WSNFH. We brought the Lake Sturgeon back in a plastic bag placed in water within a cooler, and continuously aerated with a portable air pump. An ice pack was placed in the bottom of the cooler to keep the temperature below 19 °C.
After doing the initial anatomical measurements, we placed the Lake Sturgeon on a Petri dish and placed the dish on ice to prevent tissue degradation during dissection. While on the ice, the samples were placed under a dissecting stereoscope (Practum sartorius) and the scutes were cut off using a dissecting blade. The scutes were placed in their own pre-weighed tubes and weighed. The fish was also reweighed to confirm no weight was lost during the dissection apart from the removed scutes.
2.5. Sample Ion Analysis Using Inductively Coupled Plasma Optical Emission Spectrometry (ICP:OES)
All Lake Sturgeon samples were placed in the freezer at −20 °C until they were processed for ion composition analysis. Samples were pulled out of the freezer at random to prevent sample bias within any given processing batch, and each box with samples was out of the freezer for less than 2 min while all samples that were needed for an individual round of analysis were pulled out to prevent freeze–thaw cycles.
The total mass of each fish was used to calculate how much nitric acid (70% HNO3) was needed. For a mass of 0.05 g or under we added 110 µL of nitric acid and scaled the volume by 100 µL increments per additional 0.05 g. After nitric acid was added, the test tubes were vortexed to mix the acid and sample together. The samples were left at room temperature for 24 h to ensure complete digestion of the tissues. During the 24 h, the samples were vortexed twice to aid acid digestion. After the samples were completely dissolved, they were centrifuged at 12,000× g for 2 min to pellet any non-soluble components to the bottom of the tube. We removed 100 µL of the sample from the test tube and placed it in a 15 mL test tube with 100 µL of DI water and then added 100 µL of 30% H2O2 to keep the proteins from precipitating out of the solution. The last step was to add 9.7 mL of 4% nitric acid to the test tube to have a total volume of 10 mL with a final acid concentration of 4.5% by volume.
Natural water samples were prepared for ICP-OES using a simplified procedure similar to the fish sample preparation. First, the sample tube was centrifuged to remove particulates. Then, the sample was stabilized at pH < 2 by combining the sample with HNO3 to a final concentration of 5% acid by volume (1 mL 70% HNO3 added to 13 mL sample).
We followed EPA method 200.7 for sample preparation and analytical protocols [
24]. The ICP:OES instrument used five different concentrations to set a standard curve for each ion of interest ranging from 0.001 to 10.0 mg/L. If necessary, samples were diluted to be within this range then back-calculated to know the final ion concentration by using the dilution factor. Samples were pumped using an autosampler from the 15 mL test tubes into the ICP:OES at approximately 1 mL per minute. We analyzed emission spectra for calcium at two wavelengths (393.366 nm; 317.933 radial and axial), along with magnesium (270.553), potassium (766.490), sodium (589.592), and zinc (213.857). The ICP:OES provided the ion concentration of the sample in mg/L using the calibration standard curve, and these values were then standardized to the total mass of each individual fish (mg/g).
2.6. Statistical Analysis
All data are reported as the mean ± SEM (standard error of the mean). The statistical software Sigmaplot was used for all data analysis. The water quality and ionic composition of the two rearing treatments were compared using an unpaired t-test. A two-way ANOVA was used to compare between treatment groups for each parameter measured for larval samples (mass, ion concentration) and time as the second factor, as well as any interaction effects. For our post hoc test, we used a Holm–Sidak test. Values below p < 0.05 were considered to be significant.