Simple Summary
When working with laboratory animals, scientists need to ensure that animals are not suffering from natural infections. Therefore, hygienic standards have been developed over the last 100+ years. The key element of hygienic standardization is the monitoring of infectious agents that can compromise the animal’s health, are dangerous for the personnel or interfere with the research. However, scientists became aware that by eliminating such unwanted infectious agents the overall diversity of all microbes in research animals, the so-called microbiome, has also been reduced. Moreover, it became clear that the microbiome composition has an enormous impact on how research models react, e.g., to treatments. This might hinder the translation of findings in preclinical research to the clinical situation. Therefore, new concepts in hygienic standardization need to be developed that take animal welfare as well as scientific value into account. In this review, we will give an overview of how classical monitoring has been evolved and demonstrate concepts regarding how to handle the microbiome as a yet unknown variable in animal-based research in order to enhance the validity of research findings.
Abstract
The health monitoring of laboratory rodents is essential for ensuring animal health and standardization in biomedical research. Progress in housing, gnotobiotic derivation, and hygienic monitoring programs led to enormous improvement of the microbiological quality of laboratory animals. While traditional health monitoring and pathogen detection methods still serve as powerful tools for the diagnostics of common animal diseases, molecular methods develop rapidly and not only improve test sensitivities but also allow high throughput analyses of various sample types. Concurrently, to the progress in pathogen detection and elimination, the research community becomes increasingly aware of the striking influence of microbiome compositions in laboratory animals, affecting disease phenotypes and the scientific value of research data. As repeated re-derivation cycles and strict barrier husbandry of laboratory rodents resulted in a limited diversity of the animals’ gut microbiome, future monitoring approaches will have to reform—aiming at enhancing the validity of animal experiments. This review will recapitulate common health monitoring concepts and, moreover, outline strategies and measures on coping with microbiome variation in order to increase reproducibility, replicability and generalizability.
1. Introduction
The health monitoring (HM) of laboratory rodents builds the solid foundation for preserving animal health and ensuring the validity of biomedical research data. During the last century, enormous progress has been made regarding animal housing and gnotobiotic derivation processes, aiming at improving the microbiological quality of rodents bred in laboratory research facilities. Revisiting the last 140 years, the hygienic status of animals used for scientific purposes evolved from an early phase of domestication (~1880–1950), in which breeding stocks were established with animals naturally infected with diverse pathogens, over extensive gnotobiotic rederivation processes (~1960–1985), to a period where indigenous viruses and other specified pathogens were eliminated from laboratory mouse and rat colonies (~1980–1996). This roughly divided timeline, already chronicled by Steven H. Weisbroth and David G. Baker in the late 20th century [1,2], can be extended to the most recent and still ongoing situation, the phase of an isolated animal husbandry with limited microbial exposure. Though this development follows an intrinsic logical path, it turns out that the current situation also brings its own challenges.
Nowadays, widely used hygienic monitoring programs and sanitation procedures lead to enormous improvement of the microbiological quality of laboratory animals, producing breeding colonies, which are free of pathogens and even free of most opportunistic pathogens [3,4]. Together with strict barrier housing in commercial vendor and experimental user facilities, this allows for efficient control of infectious disease development. Undoubtedly, modern rodent research management has improved the overall health status of colonies, in line with the 3Rs [5], and enabled the breeding of even severely immunocompromised animal models without microbial-induced diseases. Importantly, as functions of genes and noncoding genome regions are still largely unknown, the vast and even rapidly growing production of genetically altered animals results in a yet unknown number of mutants with immune defects, making strict barrier housing inevitable.
However, this concept of increased isolation also shaped a limited diversity of the animals’ gut microbiome, which dramatically differs between distinct animal facilities [6,7,8]. Rapidly developing molecular methods, allowing for high throughput analyses of various sample types, have revealed a striking influence of microbiome compositions on animal models, e.g., influencing immune system maturation and disease phenotypes and therefore the validity of biomedical research [9,10,11,12]. Scientists have noted this phenomenon and developed ideas to overcome this situation, especially in fields where “too clean mice” do not produce scientifically valid results [13]. These approaches range from the use of pet-shop mice to intentionally rewilding research models (see below).
At a first glance, there is an obvious dilemma of two divergent goals, which both are fundamental in animal-based research. On the one hand, there is the desired goal to maintain standardized research animals infectious-disease-free, following the basic principle of animal welfare with the 3Rs as its foundation. On the other hand, scientists aim to produce findings that can be reproduced, replicated and finally translated to the human situation, following the principle of ensuring scientific value. The latter has recently been laid down in the concept of the 6Rs, which takes both animal welfare and scientific value into consideration [14]. In this review, we will outline both sides of this dilemma and describe current concepts and ideas regarding how to deal with this situation. All in all, these factors need to be respected to avoid loss of animal welfare or loss of scientific validity, both being indispensable in biomedical research.
2. Scope of Health Monitoring Management
As the microbiological quality of laboratory animals did evolve over time, so did the scopes of HM concepts (Figure 1). In the early phase of rodent maintenance, naturally infected breeding stocks potentially harbored various pathogens, causing severe clinical diseases and sudden deaths of whole animal cohorts. During this time, the main task was simply to keep live animals and, later, to identify causative agents, giving rise to the question: how can one keep them alive? With the help of gnotobiotic rederivation procedures carried out by hysterectomy or embryo transfer of the offsprings from infected donor animals and subsequent rearing by uninfected foster dams, typical pathogens were removed from colonies, redirecting to the question: how can one keep them healthy? [1,2]. With the development of reliable and more sensitive diagnostic (screening) methods, the scientific community became more and more aware that even subclinical infections with certain pathogens have a pronounced impact on different animal models, shifting the focus of HM towards aspects of research quality (how can one ensure quality?). Concrete recommendations for systematic HM programs [15,16,17] aimed at a high level of hygienic standardization by defining specific pathogens, which should be included in the HM procedure. This “inclusion” of microbial agents in the procedure often shaped the view of a maximal “exclusion” from the colonies, and repeated re-derivation cycles led to a situation that some rodents harbor an artificially limited microbiome [8,13]. Further improvement of diagnostic methods and the rapid development of molecular techniques currently create increasing awareness of the striking influence of the microbiome composition on animal models [9,10]. Modern HM concepts have to take this impact into account to ensure the value of biomedical science (how can one ensure validity?). This requires, among others, the inclusion of metagenomics techniques as well as a differentiated view on hygienic management.
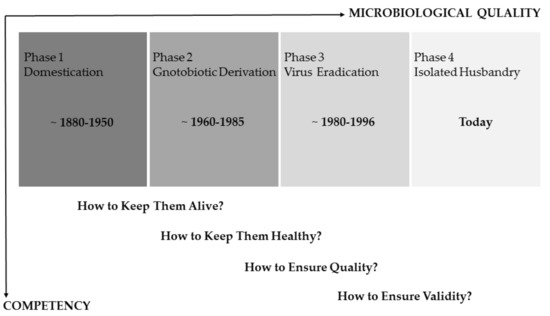
Figure 1.
Schematic timeline roughly dividing the last 140 years of experimental rodent work into four different phases. The main scopes of health monitoring concepts evolved analogous to the improvement of the microbiological quality of laboratory rodents. The underlying questions reflect the competency necessary to implement/realize these concepts (modified from [1,2]).
In principle, all these historical stages still need to be respected in modern health-monitoring programs, as the impacts of pathogens on the one hand and the microbiome on the other hand are equally important. Therefore, responsible personnel needs to be proficient in the underlying competencies and skills, ranging from knowledge of microbial agents critical for animal’s health or research, necropsy and diagnostic techniques, basic understanding in epidemiology, up to the impact of the microbiome on research models.
3. How to Keep Them Alive
Before rederivation techniques became available, laboratory rodents were naturally infected with various pathogens, affecting their overall health status. Deaths due to infectious diseases became rare with improvement in husbandry techniques and declining prevalence of agents. However, enzootic outbreaks still pose a risk to experimental and breeding colonies, as barrier leaks can never be completely excluded, live animals are transported internationally for exchange, and immune-compromised animals are highly susceptible even to prevalent opportunistic pathogens. Accidental pathogen introduction also occurs via biological material, which is injected or otherwise intentionally applied to animals during experiments.
3.1. Microbial Agents Compromising the Animal´s Life
Classically, the sudden death of larger animal cohorts were to be expected in scenarios of an (acute) epizootic infection with viral agents, such as the Murine Respirovirus, also known as Sendai Virus (SeV), Lymphocytic Choriomeningitis Virus (LCMV), enterotropic variants of Mouse Hepatitis virus (MHV), or Ectromelia Virus. The last two viruses only infect mouse colonies. In contrast, SeV and LCMV have a broader host spectrum, causing disease in most rodent species and—in the case of LCMV—can also be transmitted to primates, including humans [1,4,18,19,20]. While most bacterial infections do not result in acute mortality, infection with Clostridium (Cl.) piliforme, the causative agent of Tyzzer’s disease, may lead to the sudden death of young animals, especially in the phase of weaning [1,4,21,22]. As an endo-spore-forming bacterium, Cl. piliforme is infective for long time periods in the environment, which—together with the ability to infect most mammalian species—caused high prevalences in laboratory rodent colonies [23,24,25]. Since Mongolian Gerbils are highly susceptible for Tyzzer’s disease, even infections of adult animals cause severe clinical symptoms in this species [18]. Fatal bacterial pneumoniae is one common cause of seasonal occurring acute death in laboratory guinea pigs. Bordetella bronchiseptica can frequently be isolated from infected lung or tracheal tissue often co-associated with other opportunistic pathogens such as Pasteurella multocida or Streptococcus pneumoniae [26]. Considering that mouse and rat colonies are very often subclinically infected with the aforementioned bacteria, different animal species should be housed separately in individual hygienic units within a facility.
3.2. Diagnostic Measures to Enable Disease Control
For effective disease control in animal facilities, clinical examination and necropsy of sick or deceased animals should be regularly performed by veterinarians, which are competent in basic diagnostic methods. Patho-histological evaluation of organ morphology serves as a powerful tool to identify common disease phenotypes and discriminate those likely caused by infectious etiology from typical genetically or environmentally induced disorders, such as hydronephrosis, ulcerative dermatitis or the lactation associated ileus [18,19]. Therefore, the training of staff in basic diagnostic skills and the possibility to perform on-site examinations is essential and should be the substantial part of every solid health monitoring program.
Particular attention needs to be given to the use of biological materials in in vivo research, as agents endangering animals’ lives and even zoonoses can be transferred into research vivaria. Collaborating researchers might share infected tissues and so involuntarily contribute to the spread of agents between facilities all over the world. Two different surveys systematically analyzed biological material (in this case: tumors) in the USA and Europe and consistently found that more than 50% of all analyzed mouse tumors harbored pathogens, mostly viruses, but also mycoplasmas and protozoa [27,28,29]. Actual data suggest that the prevalence is decreasing [30]; however, material might still remain in freezers and infections due to contaminated material still occur. Several outbreaks of Mousepox were reported to be caused by the introduction of the Ectromelia Virus to the Naval Medical Research Institute and Cornell University in the USA via contaminated, commercially purchased serum [31,32]. Unfortunately, the same scenario repeatedly happened a few years later, pointing out that the prospective screening of all biological material, which is to be introduced into an animal, is indispensable [33]. Besides the risk of causing severe disease in animals, there are also major human health concerns, as there are several reports of accidental infection of facility research employees with zoonotic agents such as Hantaviruses and LCMV due to the handling of subclinically infected research animals [34,35,36]. Traditionally, biological material can be screened by the use of so-called antibody production tests (mouse/MAP or rat/RAP tests) which are based on serological analysis of animals, several weeks after they were injected with the respective biological material [37,38,39]. As this in vivo testing strategy not only requires the use of animals but may also potentially fail since it relies on a complete immune response of injected individuals [33], it is nowadays nearly completely replaced by direct analysis of the biological material using Polymerase Chain Reaction (PCR) assays for defined pathogens [40,41,42].
4. How to Keep Them Healthy
Very few pathogens come along with high mortality rates in immunocompetent animals, but may cause clinical forms of disease, with symptoms exerting when individuals are susceptible to an infection with the respective pathogens. Nowadays, a bewildering number of mouse and rat models are available from a wide variety of commercial and non-commercial sources. Due to advances in transgenic techniques and especially the development of genome editing technologies [43,44], their number is constantly increasing. These models can substantially differ in their immunological response according to their genetic background and/or their genetic alterations and therefore may show different symptoms and vary in the severity of disease development after infection [45,46,47,48,49,50].
In fact, most rodents experience subclinical forms of infection, meaning that they become a carrier of pathogens, without developing clinical symptoms. When subclinical infected animals remain undiagnosed, they may uncontrollably shed infectious agents in the environment and infect other individuals of the colony. The infection may then become enzootic, resulting in pathogen persistence. The introduction of new strains to the animal room will then lead to an epizootic infection of the naïve animals, causing more or less severe forms of diseases, depending on their susceptibility to the respective pathogen.
4.1. Agents Affecting the Animal’s Health Status
Most infectious agents are causing the most pronounced symptoms in very young animals, typically in the phase of weaning, when other stressors and the absence of maternal antibodies allow uncontrolled pathogen proliferation [1,4]. A classic example of age-dependent disease development is an infection with Rotaviruses, resulting in the epizootic diarrhea of infant mice (EDIM), which is caused by the Mouse Rotavirus Type A, or the infectious diarrhea of infant rats (IDIR), caused by the Rat Rotavirus Type B [51,52,53,54,55]. Although animals of all age groups are susceptible, clinical disease in the form of watery stool, lethargy and distended abdomens exclusively occurs in animals infected between birth and about two weeks of age, which differentiates EDIM/IDIM from other infections associated with diarrhea, such as Tyzzer’s Disease or enterotropic forms of MHV. Those substantial age-dependent differences regarding virus susceptibility are major advantages in terms of sanitation procedures, as virus eradication may be relatively simply achieved by a prolonged breeding cessation and strict hygienic measurements, as it was successfully conducted after an EDIM outbreak within an experimental barrier housing mice [56]. Due to sanitation procedures and strict hygienic measures, the actual prevalence of mouse and rat Rotaviruses is nowadays low; however, outbreaks occasionally occur, as was recently reported after exposure of exported mice to contaminated shipping boxes [57].
Generally, most rodents are relatively prone to develop chronic respiratory diseases. Rats are especially susceptible to infections with Filobacterium rodentium, formerly known as Cilia-associated respiratory (CAR) Bacillus or Mycoplasma pulmonis, which both can cause severe airway infection, particularly when animals are predisposed due to poor husbandry conditions or co-infections with other (opportunistic) pathogens [58,59,60,61,62,63,64]. Since viral pathogens were mostly eradicated from laboratory rodent colonies, opportunistic bacteria are probably the most common threat to the animal’s health status. In this context, members of the family Pasteurellaceae can be considered as classical pathobionts, since they are often part of the normal flora of mucosal sites, but may also induce inflammatory conditions such as conjunctivitis, dacryoadenitis or multiple abscess formations in susceptible mouse and rat strains [65,66,67,68,69,70]. Recently, rodent Pasteurellaceae were reclassified into the novel genus Rodentibacter, with Rodentibacter pneumotropicus and R. heylii (formerly known as [Pasteurella] pneumotropica) as the most prevalent species in laboratory mouse and rat colonies [71]. Staphylococcus aureus and Klebsiella sp. are also highly prevalent opportunists in laboratory rodents and as both are zoonotic agents, infection may be transmitted from animals to humans—and also the other way round. However, two comprehensive studies predominantly found host-adapted Staphylococcus aureus isolates in laboratory mouse and rat colonies, which were sensitive to Methicillin treatment [72,73]. Although both agents are not necessarily harmful to the animals, susceptible strains may develop clinical symptoms due to natural bacterial colonization. Development of otitis media caused by Klebsiella oxytoca was repeatedly described for C3H/HeJ mice, which express a dysfunctional Toll-like receptor 4 and therefore exhibit impaired innate immune mechanisms due to insufficient pathogen recognition [74,75]. Furthermore, Klebsiella induced urogenital infections and pneumonia in LEW.1AR1iddm rats, which are prone to develop type1 diabetes mellitus mediated by autoreactive T-cell populations [74]. While in these cases disease development occurred in somehow immune-modulated animals, two independent studies described the development of Staphylococcus aureus-induced Botryomycosis and facial abscesses in susceptible mouse outbred strains [76,77].
Amongst that, some animals are highly susceptible to infections with Helicobacter sp. [78,79,80,81], e.g., immunodeficient mouse and rat strains suffer from severe intestinal inflammation. Here, especially Helicobacter hepaticus may also induce further pathologies such as hepatitis and inflammation-induced colon carcinogenesis, as Mangerich et al. described it after infection of Rag2-deficient mice [82]. Until now, at least nine distinct Helicobacter species (namely, H. hepaticus, H. typhlonius, H. bilis, H. rodentium, H. ganmani, H. muridarum, H. mastomyrinus, H. rappini, H. trogontium) were isolated from the gastrointestinal tract of laboratory rodents, which is why co-infections are not uncommon and may worsen disease development [83,84,85]. Since most genetically modified immunodeficient mouse and rat strains lack sufficient pathogen defense mechanisms, those animals are generally prone to develop infectious diseases and, as a result, unspecific intestinal inflammation may spontaneously occur after exposure to mixed opportunistic agents [86]. For the same reasons, immunodeficient rodents may develop other pathologies after infection with microorganisms, which are generally not harmful to immunocompetent animals. In this context, it was recently described that NSG and NRG mice developed ascending pyelonephritis after surgery, which could be followed up to an infection with Candida albicans, picked up after i.v. injection within a restrainment device [87]. Other reports are numerous and involve the development of hyperkeratotic dermatitis induced by Corynebacterium bovis [88,89], otitis media caused by Ralstonia picketii [90] or even the development of severe septicemia after bacterial translocation of commensal bacteria from the normal intestinal flora [91]. One of the greatest health threats of immunodeficient animals, when housed together with immunocompetent strains, is certainly respiratory disease and chronic wasting due to infection with Pneumocystis sp., a fungus causing severe interstitial pneumonia [92,93]. Although lung inflammation may occasionally also occur in immunocompetent animals [94], an intact adaptive immune response will normally prevent disease development. However, subclinically colonized animals may transmit infectious agents to susceptible hosts, leading to severe clinical symptoms in immunodeficient members of the colony [95], while co-infections with other opportunistic agents may worsen overall pathology [96,97].
4.2. Hygienic Measures to Maintain the Health Status of Laboratory Rodents
Today it is standard that laboratory rodents and especially all animals with impaired immune functions are housed within strict barrier systems, preserving a protective biocontainment, which allows for breeding healthy animals. It is of major importance to establish routine screening programs, aiming at pathogen detection at an early time point of infection. Here, the use of traditional bacteriological, parasitological and virological diagnostics methods in combination with both prospective and indicative sampling procedures allows for routine pathogen screening as well as clearing up observed abnormalities within a colony and detection of causal infectious agents. In this context, regularly performed necropsy of sick or deceased animals, together with classic patho-histological analyses, is of major importance, as it is the fundament for the discovery of novel pathogens, especially viral agents, which otherwise will remain unidentified. Based on this, the Murine Norovirus (MNV) as well as the Mouse Kidney Parvovirus (MKPV), which was discovered recently as the causative agents for inclusion body nephropathy in immunodeficient mouse strains, could be identified as novel murine viruses [98,99]. Since it has been unknown and therefore not included in routine diagnostics for a long time, prevalence of MNV is typically high in the facilities [100,101,102,103,104,105], and colonies may yet have to be re-derived by hygienic sanitation procedures to establish a virus-free status.
5. How to Ensure Quality
Beside the sheer intention to improve the overall health condition of laboratory animals, it is also of major importance to standardize the hygienic conditions within animal experiments to ensure the quality of biomedical research data. The number of publications reporting the influence of infectious agents on experimental results is countless, which gets even more problematic in subclinical infected animals when the infection remains undiagnosed. Therefore, standardized and up-to-date health monitoring strategies need to be in place at each animal facility to ensure all of the following: life, health and quality.
5.1. Microbial Agents Impacting “Quality”
Infections with mouse (Mouse Parvovirus (MPV1–5), Minute Virus of Mice (MMV)) and rat (Kilham Rat Virus (KRV), Rat Minute Virus (RMV), Rat Parvovirus (RPV), Toolan’s H–1 Virus) parvoviruses may be one of the most prevalent but also frequently underestimated natural viral contaminations of laboratory mice and rat colonies. As most Parvoviridae, rodent parvoviruses require actively dividing or differentiating cells for survival and therefore predominantly replicate in endothelial cells, lymphocytes and hematopoietic precursors [1,49,106,107]. Although natural infections usually remain asymptomatic, there are several reports of reproductive abnormalities, when infection occurred during fetal development [108]. Furthermore, experimental infections with MVM led to myelosuppressive and lysis of T–Lymphocytes in neonatal and immunodeficient animals, which even became lethal dependent on the administered virus dose [109,110,111]. Likewise, Toolan’s H–1 Virus was found to experimentally induce CNS malformations and deformations of the skeletal system as well as increased hepatocellular necrosis after provoked liver injury [1].
Despite those clinical manifestations, the impact of parvovirus infections on research results is huge, as it will massively influence processes linked to cell proliferation. While some authors observed immunomodulatory and myelosuppressive properties [112,113,114], mouse and rat parvoviruses also reveal a strong oncosupressive potential, leading to decreased growth rate and rejection of tumor cells [107,115,116]). Since parvoviruses may cause persistent infections of a colony, researchers should be aware of the parvovirus status of their animals and the striking influence that may have on their results, especially in the field of immunology and oncology. However, elimination of these agents from an infected colony may be challenging, due to its high environmental tenacity, residual risk of infection after hygienic re-derivation procedures and difficulties in diagnostics [107,117,118]. Mouse-and-rat-strain-dependent seroconversion and intermittency of viral shedding result in seemingly low prevalences within a given colony, which requires testing of quite large animal numbers to successfully reveal ongoing infections [107,117,119,120,121]. However, health surveys from all around the globe showed that seroprevalence of parvoviruses in laboratory rodents as well as in pet-shop animals is generally high, indicating that infections remain endemically persistent in most facilities [100,101,102,103,104,105]. The most prevalent virus within mouse colonies is the Murine Norovirus (MNV) [100,101,102,103,104,105], probably due to its relatively recent discovery in 2003 [98]. Until then, MNV infections remained undiagnosed and therefore mostly unaffected by rederivation processes. Generally, MNV infections are asymptomatic in immunocompetent mouse strains and clinical forms of disease are very rare, even in immunodeficient animals [122]. However, MNV infection can lead to gastrointestinal pathology in susceptible mouse models [123,124], e.g., MNV causes intestinal epithelial barrier disruption in Interleukin-10-deficient mouse strains, which spontaneously develop chronic typhlocolitis depending on microbiological stimuli and are commonly used as a murine model for inflammatory bowel disease. In a comprehensive study using a gnotobiotic mouse model, Basic et al. demonstrated that MNV exacerbated the inflammatory phenotype, serving as a potent colitogenic stimulus, pointing out its possible impact on gastrointestinal research data [124,125]. Therefore, it is inevitable that scientists are aware of the potential impact of certain agents on their specific field and consider them in the hygienic status of their colony.
Within the group of bacteria, Helicobacter sp., Staphylococcus aureus and Rodentibacter spp. are the most prevalent agents in laboratory rodent colonies, which not only affect animal health by causing opportunistic infections in susceptible strains, but may also interfere with research results in the case of subclinical forms of disease [126,127,128]. Parasitic infections are also common in laboratory rodents, mostly including endoparasites such as pinworms or protozoa [101,102,105]. Other agents such as Filobacterium rodentium and Mycoplasma pulmonis are less frequently found but may strongly interfere with immune responses, if present in the colony [129,130,131,132]. Concerns should generally come up regarding zoonotic agents, which are nowadays rarely, but still occasionally, detected in laboratory and also pet-shop rodents [133,134,135]. Actual infections of humans regularly occur, especially in people involved in the care and husbandry of subclinically infected animals [135,136,137] or after injury with contaminated material [138]. In this context, Hantaviruses may be most concerning, as acute infections are a serious threat for human health. A systematic literature survey, based on the serology of staff, determined that 180 persons suffered from clinical disease manifestation and 85 persons were subclinically infected and unknowingly acquired Hantavirus infections from working with rat colonies [137]. The same study investigated the prevalence of infections with LCMV and concluded that thousands of employees and pet-shop customers were probably unaware of their potentially exposure to infected animals due to a major LCMV outbreak in 2012 in the United States of America. Special attention should be paid to the fact that infection with both agents may be airborne, which makes protective measures indispensable, especially when persons are exposed to excretions of rodents. Infections with Streptobacillus moniliformis, the causative agent of the so-called “rat bite fever” are also relevant but can be diagnosed more easily, as most infections involve positive history of a rat bite [139,140].
5.2. Measures to Ensure Quality—Standardized Health Monitoring
The Federation of European Laboratory Animal Science Associations (FELASA) has published a number of recommendations for the health monitoring of animals used for scientific purpose. Those recommendations are regularly updated and available for different species, including rodents, guinea pigs and rabbits [17], ruminants and pigs [141] and non-human primates [142]. The first publication for rodents was released in 1994 [15] with the primary intention of harmonizing the health monitoring procedures between animal facilities, especially to facilitate the exchange of animals between cooperating researchers. As knowledge about infectious agents increases over time, those recommendations were yet revised twice [16], with the last version published in 2014 by Mähler et al. [17]. Here, quality criteria are defined, which serve as a valuable orientation for systematic HM programs in facilities assigned with the breeding or experimental use of laboratory animals, facilitating processes harmonization and reporting of the hygienic status. According to those recommendations, the main goals of an HM program should include overall health improvement (exclude clinical infections), enhancement of general biosafety (exclude zoonotic infections) as well as prevention of interference of the hygienic status with research results (exclude subclinical infections). Therefore, this publication provides lists of relevant viruses, bacteria, fungi and parasites (choice of agents) including concrete recommendations for their test frequencies, depending on the respective risk of infecting the colony. Furthermore, commonly used test methods, selection of animals/sentinels and results interpretation are critically discussed, aiming to improve the reporting of the hygienic status and the writing of appropriate health certificates. A colony monitored according to these recommendations can be referred to as “specified pathogen free” (SPF). Unfortunately, it became more and more common to misinterpret the intention of this publication and to use it as a simple exclusion list. Contrarily, the authors intended to contribute compact information of current knowledge in laboratory animal medicine and emphasize that HM programs should be complexly designed and supervised by a competent person, educated in the field of infectious biology in veterinary medicine.
The most critical part of a systematic HM approach is probably the choice of agents, which should be primarily defined by their zoonotic potential, pathogenicity (obligate pathogens, opportunists, and pathobionts) and possible impact on results. The abovementioned recommendations serve here as a valuable tool, since they consider relevant agents on their individual risk. Hence, frequently found pathogens such as parvoviruses or MHV should be monitored at least quarterly, whereas an annual monitoring frequency should be sufficient for less prevalent agents, such as LCMV or Ectromelia. In addition to those obligatory agents, Mähler et al. also defined some additional agents, which do not necessarily have to be included in an HM program, depending on their individual relevance to the facility. Most importantly, the authors also pointed out that the panel of monitored agents has to be critically adapted to the current situation and that more agents should be included, if considered relevant. Therefore, the provided list should not be uncritically interpreted as carved in stone, but rather used as a solid foundation to work on.
Another essential a priori consideration is the appropriate size of a colony, for which the HM results have to be summarized. Therefore, hygienic units should be defined, which are characterized as those parts of a facility in which animals, personnel and material are transferred freely—without hygienic measures—and which can consequently be assumed to harbor the same germ spectrum. The size of such a hygienic unit can vary immensely, dependent on the applied hygienic measures. It can simply be a single animal room if animals are housed in open cage systems and every room has its individual equipment. However, it can also be a whole floor, consisting of several animal rooms, if personnel is allowed to switch between rooms without changing the protective clothing. The minimum size may be even a single cage, if animals are kept within micro-isolation conditions, as they exist in IVC housing systems (appropriate handling using a laminar flow safety cabinet and disinfection procedures implied). IVC cage systems can effectively prevent transmission of infections within a colony, but also kind of complicate routine HM procedures as monitoring obviously cannot be conducted on cage-containment level. To solve this problem, traditional health monitoring concepts often make use of sentinel animals (see below). As this approach has its limitations and is not suitable for all agents, it is essential to include direct testing of colony animals, especially when they show clinical signs of disease, to enhance the diagnostic success for infectious agents.
5.3. Key Figures of Standard Health Monitoring
In general, two factors affect the overall diagnostic success (suitability of the chosen method implied): test accuracy and sample size. The accuracy of a diagnostic test can be described by its sensitivity and specificity (Figure 2), with the first one certainly more important for the probability of simply finding a certain infectious agent and the latter one defining the diagnostic relevance of the results. The higher the diagnostic sensitivity, the lower the amount of false negative results will be, keeping the number of under-diagnosed positive cases at a minimum. The diagnostic specificity will be higher the lower the amount of false positive results, keeping the number of over-diagnosed negative cases at a minimum. Both parameters are crucial for results interpretation, as they define the positive and negative predictive value of a test procedure.
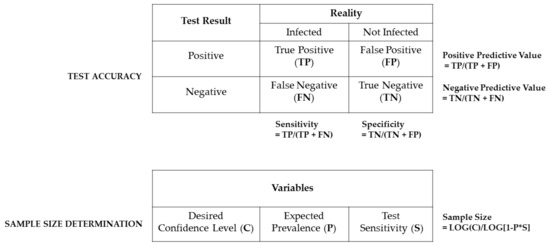
Figure 2.
Schematic overview of the two main factors influencing the diagnostic success. The test accuracy is defined by its diagnostic sensitivity and specificity as well as the underlying positive and negative predictive values. All parameters can be calculated based on true positive (TP) and negative (TN) as well as on false positive (FP) and negative results (FN). The number of required samples necessary to detect an infectious agent is based on the desired confidence interval (C) expected pathogen prevalence (P) and the respective test sensitivity (S). Importantly, this calculation holds only true for colonies of at least 100 animals and free distribution of infections [17,143,144,145].
The sample size determines the required number of animals, which have to be tested to reliably find a pathogen within a colony, and it can be statistically calculated. The underlying equation (as depicted in Figure 2) consists of the desired confidence interval (expressing the highest risk of a false negative result), the test sensitivity, the expected prevalence of the monitored pathogen as well as the size (number of animals) of the respective colony [17,143,144,145].
It is important to keep in mind that this equation is only valid in cases where infectious agents can randomly infect all animals within a husbandry unit, which is rather unlikely in modern Individually Ventilated Cage (IVC) housing conditions. Therefore, husbandry forms, which keep animals within cage-level containments, require alternatives to randomly choosing colony animals, which may be realized by using so-called sentinel animals.
Sentinels are intentionally exposed to infectious material, aiming at causing an infection with (all) the pathogens present in a husbandry. The HM of those sentinels may then representatively reveal the hygienic status of the colony. There are different types of sentinel systems, differing in the type of pathogen exposure. The most common approach is to expose the sentinels to the soiled bedding of the colony animals (soiled or “dirty” bedding sentinels), which was described as a suitable monitoring system for infectious agents which are shed into the animals’ feces, urine or other excretions [146,147,148,149,150,151,152]. To increase the pathogen load, unintentional “dilution” of infectious agents may be averted by housing the sentinel animals directly in the complete used cages of the colony, where they also get in contact with nesting material, food and water bottles. Although this approach requires a longer time period to monitor whole IVC rack systems, diagnostic success rate will probably be higher, as it was shown that critical agents such as Rodentibacter spp. were detectable using this method [153] However, independent of the concrete variant, bedding sentinels are not suitable for the reliable detection of ectoparasites (e.g., fur mites) or pathogens, which are solely transmitted via aerosols (e.g., SeV) [148,154,155,156]. In those cases, other sentinel types (direct contact sentinels or exhaust air dust sentinels), direct testing of colony animals or environmental testing approaches (see below) are obligatory.
5.4. Recent Developments in Standard Health Monitoring
Over the last decades, the use of PCR and, in the case of RNA viruses, Reverse Transcription (RT)–PCR techniques have been broadly validated as a valuable alternative to traditional cultural, microscopic and serological pathogen detection methods. As PCR testing is based on the molecular detection of specific genetic sequences of the infectious agents, this method can be used to detect even very small amounts of nucleic acids in various sample types, which not only enhances the diagnostic sensitivity but also facilitates the use of environmental sample material as an alternative to direct animal testing [157]. Since infected animals shed the infectious agents via multiple routes (feces, urine, saliva, other excretions, skin/fur…) in their home – cage environment, this material can be analyzed instead of testing samples directly obtained from the animals. Depending on the respective pathogens, different sample types can be used, either at single cage level (bedding and/or nesting material, dried fecal pellets, cage swabs or filter material from cage lids) or also at rack level. An elegant solution, especially in terms of screening larger animal cohorts, is the analysis of exhaust air dust (EAD) material, which can be also sampled at cage level but also for a whole IVC rack system, depending on the location of air filtration procedure [158]. In case of air filtration on the rack level, samples can be obtained by directly swabbing the exhaust plenum or analyzing pre-filter material of the exhaust air stream. For the latter method, different commercial systems are available which are more or less equally efficient for PCR-based pathogen detection methods [159,160]. However, there are also reports of successful implementation of alternative techniques using gauze material, manually placed on the prefilters or cage lids [161,162,163] or the direct analysis of the filter top material [164].
There are increasing numbers of publications, which describe that the analysis of environmental sample material, and here especially the use of EAD, is superior compared to traditional sentinel systems and more and more facilities report that they have completely replaced the use of sentinel animals by implementing EAD testing [160,165,166]. This paradigm shift is particularly in line with the 3Rs, as most classical HM programs involve scarifying sentinel mice (depending on the sample types), while environmental sampling can lead to a reduction of animals used for HM programs or even replace them totally. Additionally, there is common evidence that the PCR-based screening of environmental sample material can significantly increase the diagnostic success of the monitoring procedure, which can further reduce the required number of animals for experiments, as this significantly improves their hygienic standardization. Finally, environmental sampling techniques may also refine animal experimentation in terms of an overall health improvement of the colony due to the prevention of infectious diseases.
Until now, EAD-based monitoring approaches proved to be suitable for a broad variety of infectious agents, such as MNV [161,167], MHV [158], Murine Astrovirus [168], Lactate–Dehydrogenase–Elevating–Virus (LDEV) [169], Rodentibacter sp. [153,162], Helicobacter sp. [158,159,167], Mycoplasma sp. [160], Pneumocystis sp. [160], fur mites [158,168,170], pinworms [158,164,171] and enteric protozoa [158,159,160]. However, infections with murine Parvoviruses and Klebsiella oxytoca could not be confirmed by this method so far [158,159], and in the case of Staphylococcus aureus there exist contrasting results [159,160]. Future studies are necessary to close this gap of knowledge and validate the suitability of EAD-based testing for all pathogens relevant for laboratory rodents, especially for those with lower shedding concentrations, such as parvoviruses.
The major advantage of PCR-based testings—enhanced method sensitivity and a marked reduction of false negative test results—may also be one of its drawbacks, as sample contamination can easily occur [157]. False positive results can have dramatic effects in terms of consequences due to hygienic decision making (e.g., depopulation of a whole colony), which is why a single positive PCR result should always lead to subsequent confirmation of an infection. This is especially important, since the molecular detection of nucleic acids of pathogens does not automatically imply that the colony is indeed infected, for the reason that this method cannot distinguish between living and dead organisms and positive results may still occur after the detection of residual DNA/RNA present in the sample material. Residual nucleic acids may still even be present after common decontamination procedures such as autoclavation or irradiation, which is why washing procedures of cages and whole IVC rack systems will certainly play an essential role for preventing concurrent misidentification. Generally overcoming this, positive results should always be confirmed using traditional detection methods, e.g., serology, to make sure that the colony is truly infected.
Besides the risk of false positive results, false negative results may also occur, especially when the primers used for gene amplification are not suitable to detect all relevant (sub)strains of an agent. Thus, a PCR assay can only be as good as its molecular design, which mainly depends on the accuracy and actuality of underlying databases. Particularly, rapidly evolving pathogens frequently gain point mutations within their genomes, which may lead to the failure of primer binding and henceforth to false negative results [157]. Therefore, the genetic drift of agents has to be taken into account and assays used have to be permanently adapted to the current research.
Moreover, PCR only detects defined genetic sequences, whereas the use of culture or microscopic methods can expose the entirety of (cultivable) agents present in the sample material and reveal dynamic changes within a colony. This is of great value as this might point towards a leak within a barrier, which requires immediate initiation of preventive measures.
Budget may also play a role, as molecular detection methods can get quite cost intensive, especially when sample analysis is performed by commercial laboratories. However, there are reports that PCR-based testing of EAD material is actually less expensive as the traditional sentinel approach, mostly due to lower costs for animal husbandry [172]. Altogether, the use of environmental sample PCR testing material is beneficial in modern HM concepts and should be used as a valuable tool, supplementing basic diagnostic methods, particularly in terms of animal welfare.
6. How to Ensure Validity
In general, animal studies need to be reproducible, replicable, and generalizable to ensure translational value of the respective research. As the microbial status of research animals might display a major confounder impacting study results, it needs to be controlled. While routine HM programs, as recommended by the FELASA, include screening for typical pathogens, there is clear evidence that other agents as well as microbiome composition or the microbial history of an animal may also influence distinct phenotypes, dependent on the animal model. Knowledge about potential impacts is essential to ensure the validity of research data; however, the causative agents are often not part of the routine HM program of facilities. Therefore, control of these agents or the microbiome of experimental groups has currently to be explicitly requested or self-conducted by responsible investigators.
6.1. Microbial Impact on Model Phenotypes and Research Validity
Some agents, which colonize the mucosal surfaces of animals as typical commensals, are considered pathobiontic and exacerbate certain disease phenotype or even may also independently induce pathologies in susceptible strains. In this context, Proteus mirabilis was shown to induce damage of dopaminergic neurons and motor functions in mice, hence strongly influencing models of Parkinson’s Disease [173]. Likewise, germfree Interleukin-10-deficient animals developed intestinal inflammation after mono-colonization with Enterococcus faecalis, a common intestinal microorganism of animals and humans [174]. Comprehensive lists of other reported examples were reviewed elsewhere [10,11,12,175,176] and should be critically accessed in the phase of project planning to take possible impact into account and/or implement novel screening procedures of breeding colonies or experimental animal cohorts.
With the expanding utilization of high throughput molecular screening methods, such as the 16S rRNA gene sequencing analysis or other metagenomic techniques, the amount of research demonstrating the striking influence of common commensals on animal models is rapidly increasing. Since some agents have detrimental effects on disease phenotypes [177] and therefore enhance disease susceptibility, others could be identified as protective factors, improving certain immune functions. Here, especially Akkermansia muciniphila was shown to confound immunological research data, as its abundance controls diet-induced obesity in leptin-deficient mice [178] as well as islet autoimmunity in the NOD model [179] protecting animals from diabetic phenotypes. On the other hand, Akkermansia muciniphila exacerbated Salmonella-typhimurium-induced gut inflammation in a gnotobiotic mouse model [180], whereas it protected mice from developing DSS-induced intestinal inflammation [181], revealing contrasting effects on colitis phenotypes. Similarly, Segmented Filamentous Bacteria (SFB) could be identified as protective factors during Salmonella enteriditis infection and were also shown to ameliorate intestinal inflammation in Il10-deficient gnotobiotic mice after co-infection with MNV and defined microbial consortia, dependent on their intestinal colonization ability [125,182]. However, it could also be shown that SFB induced intestinal inflammation in a T-cell transfer colitis model [183] and drive autoimmune arthritis, stimulating a Th17 driven immune response [184,185]. Faecalibacterium prausnitzii and Bacteroides sp. were also considered relevant, particularly in experimental colitis models, where they mainly protect animals from developing intestinal inflammation [186,187,188,189,190,191,192]. In an excellent review article, recently published by Hansen et al., relevant bacterial species and their potential impact on animal models were summarized, serving as a valuable basis for individual consideration and inclusion in HM protocols [175].
To understand the impact of commensals in animal models and to investigate their possible role in disease development, gnotobiology serves as a powerful instrument. After germfree rederivation using hysterectomy or embryo transfer under sterile conditions, animals can be associated with defined microorganisms to study complex microbial–host interactions during animal experiments [193].
However, influence on animal models is not always exclusively mediated by single agents, but influenced by completely microbial consortia, colonizing a different part of the animal. Especially the gastrointestinal mucosa is a major habitat for a manifold number of bacteria, protozoa, fungi, archaea and viruses, forming a complex community of more than 100 billion microorganisms per gram of intestinal content, also referred to as “gut microbiota” or the “microbiome”. These microorganisms are essential for the host’s physiological functions, such as the digestions of fibers and delivering vitamins, but can also be associated with the development of certain diseases [9,10]. Recent progress in using molecular techniques such as genetic sequencing and large-scale quantitative analyses, which can be referred to as microbiome analysis, reveals the striking influence of individual microbiota compositions on animal models and correlates those data with certain disease phenotypes. Since 16S rRNA-based sequencing approaches are restricted to the analysis of bacterial communities, so-called “Next generation Sequencing” techniques make use of the random amplification of shorter gene fragments (shotgun sequencing) and subsequent alignment using bioinformatic data bases, which facilitates the simultaneous detection of all types of infectious agents, also including viruses [157,194,195].
Intestinal immunology probably constitutes most of the research areas affected by microbiome compositions, as gut inflammation may be triggered by several microbiological factors. In this context, it is well known that Interleukin-10-deficient mice differ in the severity of colitis development, depending on the housing conditions and even when they are maintained at different institutions [196,197], and that composition of the gut microbiome is a primary determinant of disease severity [198]. This model could also be used to demonstrate the influence of different minimal bacterial consortia, namely, the Altered Schaedler Flora (ASF) and the so-called Oligo Mouse Microbiota12 (OMM12), on the level of intestinal inflammation after MNV triggered colitis induction [125], proving that disease severity depends on the microbial context. Other murine colitis models also depend on microbiota compositions, since colitis development after naïve T-cell transfer into immunodeficient recipients also heavily depends on the gut microbiome [199]. Furthermore, there are also several reports of the striking influence of the microbiota composition on phenotypes in colitis models using chemically induced intestinal inflammation [175,200]. Likewise, susceptibility to Salmonella infection is strongly influenced by endogenous Enterobacteriaceae [201], and intestinal epithelial adherence and the subsequent colonic survival of Citrobacter rodentium, a common model for infection with enteropathogenic and enterohemorrhagic Escherichia coli (EPEC and EHEC) in humans, completely rely on commensals [202], pointing out the strict dependency of infection experiments on the entire microbiota. Moreover, microbiome compositions also modulate colon tumorigeneses [203], diabetic phenotypes [204,205,206] and also impact models of liver disease, as comprehensively reviewed elsewhere [207,208].
Remarkably, besides gastrointestinal research areas, the animal’s microbiome also plays a significant role in neurological processes, influencing anxiety-related or autistic behaviors [209,210,211,212,213] and also the outcome of experimentally induced autoimmune encephalomyelitis [214]. Therefore, microbiome analysis or selective analyses of known study confounders should supplement every solid HM approach (Figure 3).
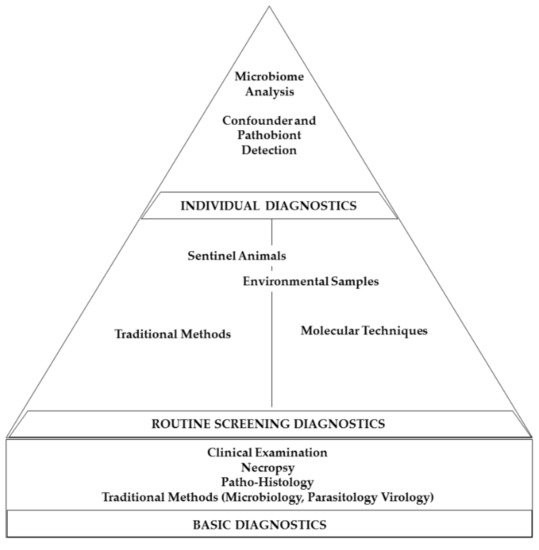
Figure 3.
Diagram depicting the different proportions of HM programs. Basic diagnostic methods should build a solid foundation, and routine diagnostic screening procedures should form the main body of HM concepts, supplemented by individual diagnostic strategies.
6.2. Measure to Ensure “Validity”
The benefit of repeated hygienic re-derivation procedures and strict barrier husbandry facilitated pathogen exclusion, aiming at improving the overall animal health and increasing the level of hygienic standardization. However, isolated husbandry also shaped a limited diversity of the gut microbiome. This resulted in significant variation of gut microbiota compositions, not only between distinct animal facilities, but possibly also within a facility, when strains are individually housed in different barriers or biocompartments [8]. This unintentionally induces a “secondary” lack of microbiological standardization, which may play a crucial role in the increasing number of reports of research data that cannot be replicated when conducted by different research groups, currently referred to as the so-called “reproducibility crisis” [215,216,217,218]. A solution is to enhance the level of microbiome standardization (see Section 6.2.1), which will improve the ability of researchers to reproduce findings of animal studies and which is therefore a measure to ensure the internal validity.
However, there is increasing concern that those findings will only be true for those exact study conditions, which will potentially decrease the generalizability of the results. In this context, Wuerbel et al. proposed to intentionally introduce a certain level of controlled variation, to test a phenotype for its robustness against natural confounders and therefore increase the translational value for further clinical studies [219]. An excellent review published by Witjes et al. discussed the pros and cons of standardization in the context of microbiome variation and concluded that standardization has to be increased when researchers conduct fundamental studies, especially those who aim at identifying disease mechanisms, to reduce study confounders, whereas more translational studies require robustness tests using mice with different microbiomes to ensure generalization and to increase the external validity of study results [220]. Possible solutions for inducing variation are either the intentional diversification of the microbiome using rather extreme measures such as the re-wilding of laboratory rodents (see Section 6.2.2) or using the microbiome as a co-factor in scientific studies (see Section 6.2.3), e.g., in multi-center studies. The abovementioned measures are summarized in Figure 4. Though these are primarily scientific tasks impacting the experimental design, they have enormous implication on the scope of hygienic management and health monitoring as well as on the veterinary care and colony management in animal-based research (Figure 5).
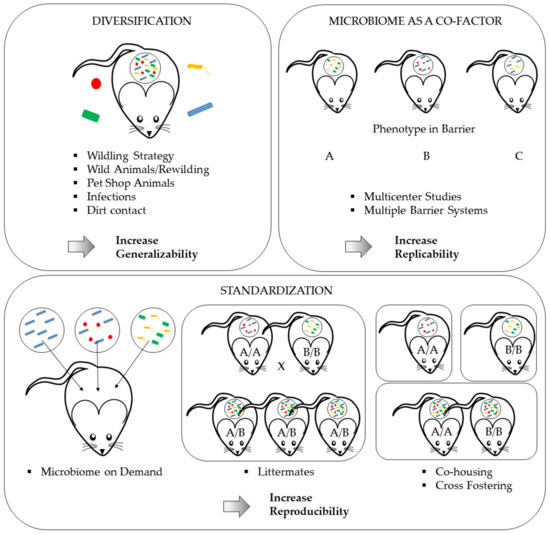
Figure 4.
Measures to ensure research validity in the microbiome era. Intentional microbiome diversification will increase the overall generalizability of study results. Using the microbiome as a co-factor by comparing phenotypes from studies under different barrier conditions will uncover microbiome impacts on phenotypes. Thus, this method will identify stable phenotypes and therefore increase the replicability of findings. Finally, achieving a high level of microbiome standardization will markedly improve study reproducibility.
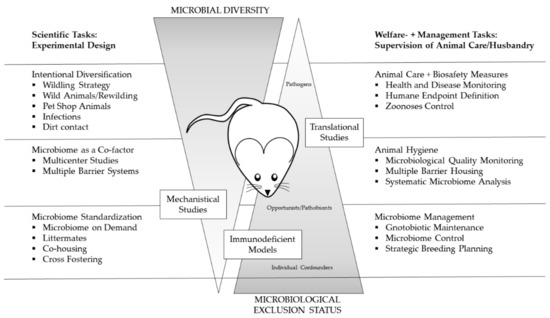
Figure 5.
Dependencies of scientific strategies to cope with microbiome challenges, animal´s hygiene status as well as welfare and management tasks. The primarily scientific tasks regarding study design (depicted on the left-hand side) have enormous implications on the scope of hygienic management and health monitoring as well as on the veterinary care and colony management (depicted on the right hand side). Depending on the study planned, the microbial diversity and microbiological exclusion status have to match the requirements of the different animal models to ensure both scientific value and animal welfare, as implied by the 6Rs [14].
6.2.1. Standardizing the Microbiome
Since reproducibility is closely related to research validity, it can be seen as a measure for the generalizability and translational value of results [217], thus determining relevance of findings [218]. Re-enhancing the level of microbiological standardization increases the so-called “internal validity”, which can be achieved by accounting for reciprocal host–microbiome interactions aiming at minimizing variation due to microbiological confounders [220,221]. However, the factors influencing the animal’s microbiome are countless and range from housing-related factors such as food, water, caging systems, bedding material (and of course their decontaminative treatments such as autoclavation, irradiation or acidification) to host-related factors such as the animal’s genetics, age and gender. Moreover, also infections with primary pathogens or psychological stress leads to an enormous variation of the fecal microbiome, shaping a massive microbiome variability between different animal vendor facilities [6,7,8,222,223]. In an excellent review article written in 2017 by Franklin and Ericsson, different sources of gut microbiome variability are summarized and measures are discussed to address this variability for improving research reproducibility [215]. These factors will make it virtually impossible to exactly adjust the microbiome of several breeders, facilities and even barriers. However, within barriers and even within studies, standardization of the microbiome is achievable.
Microbiome standardization within studies requires matching the microbiota composition between animal groups, which becomes particularly critical when genetically engineered animals (e.g., a mouse strain with a certain knockout gene), which are bred at an animal facility, are compared with the respective wild-type controls, which are often purchased and subsequently imported from commercial animal vendors.
This task can be approached by using two different strategies, which both aim at matching the microbiomes of separate animal cohorts and therefore facilitate the use of appropriate control groups: (1) littermate or (2) co-housing method. In addition, bacterial collections and gnotobiotic approaches are developing that will enable scientists to select defined microbiomes for their models under study.
Littermates
The littermate approach is primarily a breeding strategy, based on obtaining all experimental animals from the same breeding stock. Depending on the required genotypes, breeding couples (obtained via a random P1 mating of the homozygous wild-type and mutant strains) which have heterozygous alleles for wild-type and mutated genes (wt/mut) will produce offsprings (=littermates) which have similar microbiome compositions while expressing different gene combinations. Due to Mendel´s law, 25% of the litter will express homozygous alleles for the wild-type and mutant genes (wt/wt and mut/mut) and can subsequently be used for following experiments. The major advantage of this method is that siblings will have similar microbiota from birth on, guaranteeing analogous maturation of immune functions in early life development. Furthermore, there will automatically also be the highest possible level of genetic standardization, as all animals will have a high level of genetic background homology. However, unfortunately, 50% of the offsprings cannot be used for experiments, as those animals will express heterozygous alleles (wt/mut), leading to a relatively high number of breeding excess. Furthermore, this method requires constant genotyping procedures of the colony, which can be cost and time consuming and makes (invasive) biopsy sampling necessary.
Co-Housing and Cross-Fostering
The co-housing of experimental groups will also facilitate the decreasing of microbiome variation. Therefore, animals have to be housed together for at least four weeks, as this period was shown to be most efficient and results in the highest levels of microbiome similarity [224]. This method is probably the easiest way to achieve microbiome adaptation; however, the time point of starting the co-housing procedure remains critical, as it works best in very young animals [225,226,227] and will eventually not work for (aggressive) male individuals. In this case, cross-fostering would be a better strategy, yet it requires the marking of animals at a very early time point or, like the littermate method, constant genotyping after weaning. It also has to be noted that co-housing will lead to the asymmetric transmission of microbiota between two mouse populations, as it was shown that the microbiota of mice obtained from the Jackson Laboratories was more abundant than the microbiota from Taconic, after finished co-housing procedure [224]. A recent study conducted by Robertson et al. compared co-housing and littermate methods and demonstrated that the use of F2 littermates was the superior strategy for microbiome standardization [228].
Microbiome on Demand—Gnotobiology
Gnotobiology serves as a powerful instrument to study complex microbial–host interactions during animal experiments [193]. Furthermore, the use of gnotobiotic animals associated with defined microbiota is without doubt the strictest and most definite strategy to standardize an animal’s microbiome [193,229]. For this purpose, several defined microbial consortia are available which can be used to associate breeding mice and rat colonies within isolator systems. The so-called “Schaedler flora” was first established by Russel W. Schaedler, who associated germfree animals with five bacterial cultures isolated from conventional mice [230]. The original content was later modified by R.P. Orcutt, resulting in the now commonly used “Altered Schaedler Flora” (ASF), consisting of eight microorganisms [231]. The “Simplified Human Intestinal Microbiota” (SIHUMI) consists of seven bacterial species, based on numerical importance and fermentative abilities in the human gut [232,233,234]. However, those minimal consortia cannot compensate for the complexity of natural microbial communities. In this context, Norin and Midvedt demonstrated already in 2010 that ASF colonized mice are more similar to germfree than to conventionally colonized animals [235], indicating that minimal defined consortia may result in artificial reactions and loss of disease phenotypes. The same problems may even also occur when using animals housed in strict barrier systems, as their microbiome also lacks its natural diversity and was shaped into a quite limited state, indicated by a reduced alpha diversity (richness) of consisting bacterial species [6,8,215]. Therefore, it would be optimal to have access to more complex, but defined microbial communities, to create “microbiome on demand” where scientists can choose their ideal microbiome composition, with individual inclusion or exclusion profiles. Projects such as the establishment of the “mouse intestinal bacterial collection” (miBC) will serve as an essential resource, offering access to defined bacteria and microbiological communities [236]. This includes the “Oligo Mouse Microbiota12” (OMM12), representing 12 members of the major bacterial phyla in the murine gut providing colonization resistance against Salmonella typhimurium [232,234] This consortium is currently being modified to enhance complexity or to select for certain community characteristics and might represent an ideal modular system for the future [237,238,239,240].
6.2.2. Intentional Diversification of the Microbiome—Re-Wilding, Wildlings, Pet-Shop or Wild Animals
As mentioned before, artificial reactions and loss of disease phenotypes may occur in animals harboring a limited or less diverse microbiome, as is nowadays often the case in breeding colonies with “SPF” or even “SOPF” (specified opportunistic and pathogen-free) status [13]. This artificial situation may have dramatic effects on immunological functions, as Beura et al. proved that “SPF” laboratory mice lack certain memory T cells, which more closely resembles neonatal than adult humans [241]. The authors then made use of free-living feral and also pet-shop mice, which had a more diverse microbial experience, and could prove that these animals not only expressed a more human-adult-like T-cell profile, but were also able to induce those cell populations in the laboratory mice after co-housing them with the wild animals. Likewise, others also demonstrated that the wild mouse cellular immune system is in a more activated state, that high pathogen exposure induces the activation of myeloid cells, helping to maintain natural immune homeostasis [242,243], and that experiments on mice living in a more natural habitat or experimental modifications such as co-hosing with pet-shop mice can deliver dramatically different immune phenotypes [244,245]. Analysis of “wild mouse microbiota” revealed that its composition shows relatively high inter-individual variation, which can be principally explained by geographical location, and that it undergoes a marked seasonal variation. However, the majority of bacteria remain relatively stable upon entrance into captivity [246,247,248]. In general, it can be stated that the health status of wild rodents is more robust and that animals benefit from the high microbiota diversity, improving host fitness and disease resistance [249,250,251]. An elegant series of studies was conducted by Rosshart et al., who used the technique of embryo transfer with wild caught mice as dams to transfer desired genotypes into the desired “wildling” microbiome condition. He respected the pathogen status of wild caught animals and used only those as dams that were free of known pathogens. Therefore, the group showed that the microbiota of wild animals excellently phenocopied human immune responses and that the reproducibility of preclinical studies could be enhanced using a model composed of natural microbiota and certain pathogens [252]. An excellent review on the effect of “naturalizing mouse models”, which of course goes well beyond the microbiome as it includes biotic and abiotic factors, has recently been published by Graham [253].
Despite the fact that intentional diversification using rather extreme strategies such as re-wilding offers great opportunities for immunological research, it also has major challenges, especially with regard to the presence of potentially harmful pathogens [254,255]. As there is an ethical commitment, which is also reinforced by European animal welfare legislation, to keep the animals healthy, housing them in natural habitants has to be brought together with classical health and quality assurance, as described above. These include classical health monitoring strategies to define the pathogen status, biosafety measures to exclude zoonoses and control of disease outbreaks on the one hand and on the other hand welfare tasks such as definition of clear control intervals and of humane endpoints due infectious diseases, especially once genetically modified animals with unclear immune status are studied. Beyond that, compliance with legal requirements needs to be considered when using these techniques, e.g., daily health inspections of each animal are requested by the directive 2010/63 as well as ingress- and escape-proof enclosures. Especially the latter one becomes highly relevant for genetically engineered models. The intentional release of genetically modified organisms is strictly regulated, and such animals have to be separated from local wild populations. Therefore, studies using those diversification techniques should provide clear and comprehensive descriptions of all preventive measures to address those remaining concerns regarding animal welfare aspects and the overall genetic and biosafety concepts.
6.2.3. Microbiome as a Co-Factor
Differences in the microbiome can be used to intendedly induce variation by performing multi-center studies, which have become standard in clinical studies and are evolving in preclinical research also [256], or studying models in different barriers at one research location. While both approaches are feasible with the infrastructure at hand, they come with their own costs: they still rely on phenotypes that are valid in the classical “SPF” world, they increase study complexity and animal numbers for a given study considerably, and to fully control experimental settings, routine microbiome monitoring needs to be established and become a standard. The increase in complexity is limiting especially the multi-center approach to validate major findings or models before research strategies evolve towards clinical research. Multiple barrier approaches are much easier to conduct given that different barriers with identical rodent strains are present at a facility (which require genetic standardization); however, this approach is unlikely to be performed routinely but rather for validation as described above. In addition, control of the microbiome in various barriers would present a new step in the design of a health monitoring strategy.
Since common concepts only aim at monitoring lists of defined pathogens, there is obviously a lack of knowledge about the overall microbiota composition of colonies. Including monitoring of the microbiome into routine HM procedures could help to close this gap and offer excellent opportunities to cope with the upcoming challenges. Molecular techniques such as Next Generation Sequencing could be powerful tools to achieve both pan-pathogendetection as well as monitoring microbiome compositions within complex barrier systems. Especially IVC systems could further improve from sequencing methods, as exhaust air dust filters may serve as potential targets for prospective and retrospective screening of whole microbiota communities within a colony. Since nearly all infectious agents contain specific nucleic acids, sequencing is an attractive approach for pathogen detection, as a revolutionary technology already established in humane medicine and used for the diagnosis of CNS infections, bloodstream infections, respiratory infections, orthopedic infections, gastrointestinal infections and ocular infections [195,257,258]. In veterinary medicine, metagenomic sequencing was successfully used for monitoring antimicrobial resistance in swineherds [259] using fecal samples from the pen floor. The authors of this study concluded that metagenomic read mapping outperformed cultivation-based techniques in terms of predicting expected tetracycline resistance based on antimicrobial consumption.
The major challenges of this method are the needs of the so-called host depletion methods, which have to be used to diminish the host genetic background signal in the sequencing data, so that they may not interfere with data analysis. Challenges may also occur due to very complex raw data output, which do not only require high bioinformatics expertise for successful analysis but also occupy huge storage capacities. Another drawback may be the general proneness to sample contamination, making results interpretation generally complicated [195]. Comprehensive studies will be needed to validate this method, also aiming at developing valid data bases and robust guidelines for implementing this method into routine HM concepts [260], helping responsible persons to understand both the power and limitations of this method [194].
Independent from all HM concepts and methods used for monitoring, proper reporting of results is certainly the most important task. Sharing information about an animal’s hygienic status, and, in future, their microbiome, is the essential basis for creating an environment where reproducibility is even at least possible [221]. Unfortunately, a broad number of research papers withdraws that information from the materials and methods parts, sometimes due to lack of space, sometimes due to other reasons. As most journals meanwhile became more aware of the need for a standardized reporting philosophy, guidelines were published to assist and promote the systematic transfer of information about study protocols, including the hygienic status of the animals [261,262].
7. Conclusions
HM of laboratory rodents evolved over the last century, as increasing competence changed the focus of the monitoring procedures. In the very beginning of domestication, primary pathogens causing more or less severe infectious diseases were the major concern of veterinarians and researchers. Extensive re-derivation processes cleared most pathogens from naturally infected colonies, not only improving the overall animal health but also making the work with animals safe with respect to zoonotic diseases. Classical strategies in the form of clinical examinations and full necropsy procedures are basic skills to keep animals healthy. Traditional microbiological, virological and parasitological diagnostic methods support those basic skills and helps with systematically defining the hygienic quality of the animals. Concrete recommendations for the HM of laboratory rodents published by the FELASA led to process harmonization and helps defining the specified pathogen status of breeding and experimental colonies, enhancing the overall quality of biomedical research. However, those recommendations should not be misinterpreted as a tool for the supervision of simple exclusion lists. Instead, they serve as a solid foundation for a risk orientated definition of pathogen screening panels, which has to be individually adjusted by including other agents, which might be relevant for the respective research projects. Here, basic diagnostic skills should be united with molecular technologies to supplement traditional monitoring programs by including the analysis of environmental sample material, such as the EAD of IVC rack systems. With increasing knowledge about the relevance of whole microbiota compositions for biomedical research data, the influence of individual microbiomes has to be respected by standardizing microbiome compositions within research studies and by including metagenomic-sequencing techniques in modern HM programs. As isolated husbandry within strict barrier systems led to the development of very clean colonies with a limited diversity of gut microbiota, researchers may have to face a loss of certain disease phenotypes in animal models. Introducing controlled microbiome variation using different barriers or microbiological diversification using re-wilded animal cohorts may improve the translational value of biomedical research studies. However, there are remaining concerns regarding genetic and biosafety measures when working with colonies housed in more natural environments. Additionally, the general ethical commitment of veterinarians and researchers to care for the animals obliges them to keep colonies healthy and free of harmful pathogens, especially when working with immune-compromised animals. A reformed monitoring approach using the metagenomic-sequencing method could combine pathogen detection with microbiome analysis, increasing the informative value of a one-sample testing method.
In the end, there is cumulative evidence that “the cleaner” is not always “the better”, and that different models require different needs for microbiota compositions. Since some immunological research will benefit from the presence of certain opportunistic agents priming special immune cell populations, known confounders have to be excluded to ensure study validity. On the contrary, immunodeficient animals have to be housed within strict opportunistic free barriers to guarantee their general health integrity. Those aspects have to be already considered at the stage of project planning and should be explicitly addressed by the respective researchers and funding institutions. Yet, it is of even greater importance to improve the overall reporting routine of the animals’ health status as well as possible impacts of individual microbiome compositions on study results. Therefore, HM is supposed to be included as an essential element in all relevant recommendations such as the PREPARE and ARRIVE guidelines [262,263]. This will increase the awareness of the scientific community that modelling matching scenarios for individual study needs is challenging and requires complex strategies. However, this is a necessary task to combine classical quality and welfare-centered measures with the demand for increasing scientific validity. Thus, laboratory animal scientists have to gain outstanding competence in modern HM concepts, continuing its (r)evolution.
Author Contributions
Conceptualization, S.B. and A.B.; writing—original draft preparation, S.B.; writing—review and editing, A.B.; visualization, S.B.; supervision, A.B. Both authors have read and agreed to the published version of the manuscript.
Funding
This work was conducted in the frame of the DFG-CRC 1371 (Z01).
Institutional Review Board Statement
Not applicable.
Acknowledgments
We thank the reviewers for critically reading our manuscript and their useful comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baker, D.G. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin. Microbiol. Rev. 1998, 11, 231–266. [Google Scholar] [CrossRef]
- Weisbroth, S.H. Post-indigenous disease: Changing concepts of disease in laboratory rodents. Lab. Anim. 1996, 25, 25–33. [Google Scholar]
- Hansen, A.K.; Franklin, C. Microbiota, laboratory animals, and research. Lab. Anim. 2019, 53, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.R.; Olekszak, H. An overview of typical infections of research mice: Health monitoring and prevention of infection. Curr. Protoc. Mouse Biol. 2015, 5, 235–245. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Universities Federation for Animal Welfare: Wheathampstead, UK, 1959. [Google Scholar]
- Ericsson, A.C.; Davis, J.W.; Spollen, W.; Bivens, N.; Givan, S.; Hagan, C.E.; McIntosh, M.; Franklin, C.L. Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice. PLoS ONE 2015, 10, e0116704. [Google Scholar] [CrossRef]
- Ericsson, A.C.; Gagliardi, J.; Bouhan, D.; Spollen, W.G.; Givan, S.A.; Franklin, C.L. The influence of caging, bedding, and diet on the composition of the microbiota in different regions of the mouse gut. Sci. Rep. 2018, 8, 4065. [Google Scholar] [CrossRef] [PubMed]
- Rausch, P.; Basic, M.; Batra, A.; Bischoff, S.C.; Blaut, M.; Clavel, T.; Glasner, J.; Gopalakrishnan, S.; Grassl, G.A.; Gunther, C.; et al. Analysis of factors contributing to variation in the c57bl/6j fecal microbiota across german animal facilities. Int. J. Med. Microbiol. 2016, 306, 343–355. [Google Scholar] [CrossRef]
- Bleich, A.; Fox, J.G. The mammalian microbiome and its importance in laboratory animal research. ILAR J. 2015, 56, 153–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bleich, A.; Hansen, A.K. Time to include the gut microbiota in the hygienic standardisation of laboratory rodents. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 81–92. [Google Scholar] [CrossRef]
- Hansen, A.K.; Hansen, C.H.; Krych, L.; Nielsen, D.S. Impact of the gut microbiota on rodent models of human disease. World J. Gastroenterol. 2014, 20, 17727–17736. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Krych, L.; Nielsen, D.S.; Hansen, C.H. A review of applied aspects of dealing with gut microbiota impact on rodent models. ILAR J. 2015, 56, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Masopust, D.; Sivula, C.P.; Jameson, S.C. Of mice, dirty mice, and men: Using mice to understand human immunology. J. Immunol. 2017, 199, 383–388. [Google Scholar] [CrossRef]
- Strech, D.; Dirnagl, U. 3rs missing: Animal research without scientific value is unethical. BMJ Open Sci. 2019, 3, e000048. [Google Scholar] [CrossRef]
- Kraft, V.; Deeny, A.; Blanchet, H.; Hansen, A. Report of the felasa working group on animal health. Recommendations for the health monitoring of mouse, rat, hamster, guineapig and rabbit breeding colonies. Lab. Anim. 1994, 28, 1–12. [Google Scholar] [CrossRef]
- Nicklas, W.; Baneux, P.; Boot, R.; Decelle, T.; Deeny, A.A.; Fumanelli, M.; Illgen-Wilcke, B.; FELASA. FELASA Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab. Anim. 2002, 36, 20–42. [Google Scholar] [CrossRef]
- Mähler, M.; Berard, M.; Feinstein, R.; Gallagher, A.; Illgen-Wilcke, B.; Pritchett-Corning, K.; Raspa, M. Felasa recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab. Anim. 2014, 48, 178–192. [Google Scholar]
- Barthold, S.W.; Griffey, S.M.; Percy, D.H. Pathology of Laboratory Rodents and Rabbits; Wiley-Blackwell: Hoboken, NJ, USA, 2016; Volume 4. [Google Scholar]
- Fox, J.A.L.; Glen, O.; Kathleen, P.; Mark, W. Laboratory Animal Medicine; Academic Press: Cambridge, MA, USA, 2015; Volume 3. [Google Scholar]
- Lussier, G. Potential detrimental effects of rodent viral infections on long-term experiments. Vet. Res. Commun. 1988, 12, 199–217. [Google Scholar] [CrossRef]
- Tyzzer, E.E. A fatal disease of the japanese waltzing mouse caused by a spore-bearing bacillus (bacillus piliformis, n. Sp.). J. Med. Res. 1917, 37, 307–338. [Google Scholar] [PubMed]
- Ganaway, J.R.; Allen, A.M.; Moore, T.D. Tyzzer’s disease. Am. J. Pathol. 1971, 64, 717–730. [Google Scholar]
- Itoh, T.; Ebukuro, M.; Kagiyama, N. Inactivation of bacillus piliformis spores by heat and certain chemical disinfectants. Jikken Dobutsu 1987, 36, 239–244. [Google Scholar]
- Ganaway, J.R. Effect of heat and selected chemical disinfectants upon infectivity of spores of bacillus piliformis (tyzzer’s disease). Lab. Anim. Sci. 1980, 30, 192–196. [Google Scholar] [PubMed]
- Hansen, A.K.; Svendsen, O.; Mollegaard-Hansen, K.E. Epidemiological studies of bacillus piliformis infection and tyzzer’s disease in laboratory rats. Z. Vers. 1990, 33, 163–169. [Google Scholar]
- Smith, T. Some bacteriological and environmental factors in the pneumonias of lower animals with special reference to the guinea-pig. J. Med. Res. 1913, 29, 291–324. [Google Scholar] [PubMed]
- Collins, M.J., Jr.; Parker, J.C. Murine virus contaminants of leukemia viruses and transplantable tumors. J. Natl. Cancer Inst. 1972, 49, 1139–1143. [Google Scholar]
- Nicklas, W.; Kraft, V.; Meyer, B. Contamination of transplantable tumors, cell lines, and monoclonal antibodies with rodent viruses. Lab. Anim. Sci. 1993, 43, 296–300. [Google Scholar] [PubMed]
- Nakai, N.; Kawaguchi, C.; Nawa, K.; Kobayashi, S.; Katsuta, Y.; Watanabe, M. Detection and elimination of contaminating microorganisms in transplantable tumors and cell lines. Exp. Anim. 2000, 49, 309–313. [Google Scholar] [CrossRef][Green Version]
- Livingston, R.S. In Testing Biological—Mitigating Risk to Animals, People and Research. In Proceedings of the ESLAV-ECLAM Virtual Annual Meeting 2020, Online, 24–25 November 2020. [Google Scholar]
- Lipman, N.S.; Perkins, S.; Nguyen, H.; Pfeffer, M.; Meyer, H. Mousepox resulting from use of ectromelia virus-contaminated, imported mouse serum. Comp. Med. 2000, 50, 426–435. [Google Scholar]
- Dick, E.J., Jr.; Kittell, C.L.; Meyer, H.; Farrar, P.L.; Ropp, S.L.; Esposito, J.J.; Buller, R.M.; Neubauer, H.; Kang, Y.H.; McKee, A.E. Mousepox outbreak in a laboratory mouse colony. Lab. Anim. Sci. 1996, 46, 602–611. [Google Scholar]
- Labelle, P.; Hahn, N.E.; Fraser, J.K.; Kendall, L.V.; Ziman, M.; James, E.; Shastri, N.; Griffey, S.M. Mousepox detected in a research facility: Case report and failure of mouse antibody production testing to identify ectromelia virus in contaminated mouse serum. Comp. Med. 2009, 59, 180–186. [Google Scholar] [PubMed]
- Dykewicz, C.A.; Dato, V.M.; Fisher-Hoch, S.P.; Howarth, M.V.; Perez-Oronoz, G.I.; Ostroff, S.M.; Gary, H., Jr.; Schonberger, L.B.; McCormick, J.B. Lymphocytic choriomeningitis outbreak associated with nude mice in a research institute. JAMA 1992, 267, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; LeDuc, J.W.; Lloyd, G.; Reynes, J.M.; McElhinney, L.; van Ranst, M.; Lee, H.W. Wild rats, laboratory rats, pet rats: Global seoul hantavirus disease revisited. Viruses 2019, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Umenai, T.; Lee, H.W.; Lee, P.W.; Saito, T.; Toyoda, T.; Hongo, M.; Yoshinaga, K.; Nobunaga, T.; Horiuchi, T.; Ishida, N. Korean haemorrhagic fever in staff in an animal laboratory. Lancet 1979, 1, 1314–1316. [Google Scholar] [CrossRef]
- De Souza, M.; Smith, A.L. Comparison of isolation in cell culture with conventional and modified mouse antibody production tests for detection of murine viruses. J. Clin. Microbiol. 1989, 27, 185–187. [Google Scholar] [CrossRef]
- Lewis, V.J.; Clayton, D.M. An evaluation of the mouse antibody production test for detecting three murine viruses. Lab. Anim. Sci. 1971, 21, 203–205. [Google Scholar]
- Morse, S.S. Comparative sensitivity of infectivity assay and mouse antibody production (map) test for detection of mouse thymic virus (mtlv). J. Virol. Methods 1990, 28, 15–23. [Google Scholar] [CrossRef]
- Bootz, F.; Sieber, I. Replacement of mouse and rat antibody production test; comparison of sensitivity between the in vitro and in vivo methods. ALTEX 2002, 19 (Suppl. 1), 76–86. [Google Scholar]
- Bauer, B.A.; Besch-Williford, C.L.; Riley, L.K. Comparison of the mouse antibody production (map) assay and polymerase chain reaction (pcr) assays for the detection of viral contaminants. Biologicals 2004, 32, 177–182. [Google Scholar] [CrossRef]
- Mahabir, E.; Jacobsen, K.; Brielmeier, M.; Peters, D.; Needham, J.; Schmidt, J. Mouse antibody production test: Can we do without it? J. Virol. Methods 2004, 120, 239–245. [Google Scholar] [CrossRef]
- Meehan, T.F.; Conte, N.; West, D.B.; Jacobsen, J.O.; Mason, J.; Warren, J.; Chen, C.K.; Tudose, I.; Relac, M.; Matthews, P.; et al. Disease model discovery from 3,328 gene knockouts by the international mouse phenotyping consortium. Nat. Genet. 2017, 49, 1231–1238. [Google Scholar] [CrossRef]
- Brandes, R.P.; Dueck, A.; Engelhardt, S.; Kaulich, M.; Kupatt, C.; de Angelis, M.T.; Leisegang, M.S.; le Noble, F.; Moretti, A.; Muller, O.J.; et al. Dgk and dzhk position paper on genome editing: Basic science applications and future perspective. Basic Res. Cardiol. 2021, 116, 2. [Google Scholar] [CrossRef]
- Waggie, K.S.; Hansen, C.T.; Ganaway, J.R.; Spencer, T.S. A study of mouse strains susceptibility to bacillus piliformis (tyzzer’s disease): The association of b-cell function and resistance. Lab. Anim. Sci. 1981, 31, 139–142. [Google Scholar]
- Compton, S.R.; Ball-Goodrich, L.J.; Johnson, L.K.; Johnson, E.A.; Paturzo, F.X.; Macy, J.D. Pathogenesis of enterotropic mouse hepatitis virus in immunocompetent and immunodeficient mice. Comp. Med. 2004, 54, 681–689. [Google Scholar] [PubMed]
- Van Andel, R.A.; Hook, R.R., Jr.; Franklin, C.L.; Besch-Williford, C.L.; Riley, L.K. Interleukin-12 has a role in mediating resistance of murine strains to tyzzer’s disease. Infect. Immun. 1998, 66, 4942–4946. [Google Scholar] [CrossRef]
- Binder, A.; Gartner, K.; Hedrich, H.J.; Hermanns, W.; Kirchhoff, H.; Wonigeit, K. Strain differences in sensitivity of rats to mycoplasma arthritidis isr 1 infection are under multiple gene control. Infect. Immun. 1990, 58, 1584–1590. [Google Scholar] [CrossRef]
- Brownstein, D.G.; Smith, A.L.; Jacoby, R.O.; Johnson, E.A.; Hansen, G.; Tattersall, P. Pathogenesis of infection with a virulent allotropic variant of minute virus of mice and regulation by host genotype. Lab. Investig. 1991, 65, 357–364. [Google Scholar] [PubMed]
- Fornefett, J.; Krause, J.; Klose, K.; Fingas, F.; Hassert, R.; Eisenberg, T.; Schrodl, W.; Grunwald, T.; Muller, U.; Baums, C.G. Comparative analysis of clinics, pathologies and immune responses in balb/c and c57bl/6 mice infected with streptobacillus moniliformis. Microbes Infect. 2018, 20, 101–110. [Google Scholar] [CrossRef]
- Huber, A.C.; Yolken, R.H.; Mader, L.C.; Strandberg, J.D.; Vonderfecht, S.L. Pathology of infectious diarrhea of infant rats (idir) induced by an antigenically distinct rotavirus. Vet. Pathol. 1989, 26, 376–385. [Google Scholar] [CrossRef]
- Burns, J.W.; Krishnaney, A.A.; Vo, P.T.; Rouse, R.V.; Anderson, L.J.; Greenberg, H.B. Analyses of homologous rotavirus infection in the mouse model. Virology 1995, 207, 143–153. [Google Scholar] [CrossRef]
- Ijaz, M.K.; Sabara, M.I.; Alkarmi, T.; Frenchick, P.J.; Ready, K.F.; Longson, M.; Dar, F.K.; Babiuk, L.A. Characterization of two rotaviruses differing in their in vitro and in vivo virulence. J. Vet. Med. Sci. 1993, 55, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Little, L.M.; Shadduck, J.A. Pathogenesis of rotavirus infection in mice. Infect. Immun. 1982, 38, 755–763. [Google Scholar] [CrossRef]
- Vonderfecht, S.L.; Huber, A.C.; Eiden, J.; Mader, L.C.; Yolken, R.H. Infectious diarrhea of infant rats produced by a rotavirus-like agent. J. Virol. 1984, 52, 94–98. [Google Scholar] [CrossRef]
- Held, N.; Hedrich, H.J.; Bleich, A. Successful sanitation of an edim-infected mouse colony by breeding cessation. Lab. Anim. 2011, 45, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, K.E.; Henderson, K.S.; Mayorga, M.S.; Kuiper, V.A.; Wilkerson, J.D. Contaminated shipping materials identified as the source of rotaviral infection of exported mice. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 529–533. [Google Scholar] [CrossRef]
- Broderson, J.R.; Lindsey, J.R.; Crawford, J.E. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am. J. Pathol. 1976, 85, 115–130. [Google Scholar] [PubMed]
- Schoeb, T.R.; Kervin, K.C.; Lindsey, J.R. Exacerbation of murine respiratory mycoplasmosis in gnotobiotic f344/n rats by sendai virus infection. Vet. Pathol. 1985, 22, 272–282. [Google Scholar] [CrossRef]
- Ganaway, J.R.; Spencer, T.H.; Moore, T.D.; Allen, A.M. Isolation, propagation, and characterization of a newly recognized pathogen, cilia-associated respiratory bacillus of rats, an etiological agent of chronic respiratory disease. Infect. Immun. 1985, 47, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Kohyama, K.; Takakura, A.; Takenouchi, T.; Kagiyama, N. Naturally occurring car bacillus infection in a laboratory rat colony and epizootiological observations. Jikken Dobutsu 1987, 36, 387–393. [Google Scholar] [PubMed]
- Van Zwieten, M.J.; Solleveld, H.A.; Lindsey, J.R.; de Groot, F.G.; Zurcher, C.; Hollander, C.F. Respiratory disease in rats associated with a filamentous bacterium: A preliminary report. Lab. Anim. Sci. 1980, 30, 215–221. [Google Scholar]
- Brogden, K.A.; Cutlip, R.C.; Lehmkuhl, H.D. Cilia-associated respiratory bacillus in wild rats in central iowa. J. Wildl. Dis. 1993, 29, 123–126. [Google Scholar] [CrossRef]
- Ike, F.; Sakamoto, M.; Ohkuma, M.; Kajita, A.; Matsushita, S.; Kokubo, T. Filobacterium rodentium gen. Nov., sp. Nov., a member of filobacteriaceae fam. Nov. Within the phylum bacteroidetes; includes a microaerobic filamentous bacterium isolated from specimens from diseased rodent respiratory tracts. Int. J. Syst. Evol. Microbiol. 2016, 66, 150–157. [Google Scholar] [CrossRef]
- Adhikary, S.; Bisgaard, M.; Dagnaes-Hansen, F.; Christensen, H. Clonal outbreaks of [pasteurella] pneumotropica biovar heyl in two mouse colonies. Lab. Anim. 2017, 51, 613–621. [Google Scholar] [CrossRef]
- Weisbroth, S.H.; Scher, S.; Boman, I. Pasteurella pneumotropica abscess syndrome in a mouse colony. J. Am. Vet. Med. Assoc. 1969, 155, 1206–1210. [Google Scholar] [PubMed]
- Wagner, J.E.; Garrison, R.G.; Johnson, D.R.; McGuire, T.J. Spontaneous conjunctivitis and dacryoadenitis of mice. J. Am. Vet. Med. Assoc. 1969, 155, 1211–1217. [Google Scholar]
- Needham, J.R.; Cooper, J.E. An eye infection in laboratory mice associated with pasteurella pneumotropica. Lab. Anim. 1975, 9, 197–200. [Google Scholar] [CrossRef]
- Sebesteny, A. Abscesses of the bulbourethral glands of mice due to pasteurella pneumotropica. Lab. Anim. 1973, 7, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Van der Schaaf, A.; Mullink, J.W.; Nikkels, R.J.; Goudswaard, J. Pasteurella pneumotropica as a causal microorganism of multiple subcutaneous abscesses in a colony wistar rats. Z. Vers. 1970, 12, 356–362. [Google Scholar]
- Adhikary, S.; Nicklas, W.; Bisgaard, M.; Boot, R.; Kuhnert, P.; Waberschek, T.; Aalbaek, B.; Korczak, B.; Christensen, H. Rodentibacter gen. Nov. Including rodentibacter pneumotropicus comb. Nov., rodentibacter heylii sp. Nov., rodentibacter myodis sp. Nov., rodentibacter ratti sp. Nov., rodentibacter heidelbergensis sp. Nov., rodentibacter trehalosifermentans sp. Nov., rodentibacter rarus sp. Nov., rodentibacter mrazii and two genomospecies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1793–1806. [Google Scholar] [PubMed]
- Mrochen, D.M.; Grumann, D.; Schulz, D.; Gumz, J.; Trube, P.; Pritchett-Corning, K.; Johnson, S.; Nicklas, W.; Kirsch, P.; Martelet, K.; et al. Global spread of mouse-adapted staphylococcus aureus lineages cc1, cc15, and cc88 among mouse breeding facilities. Int. J. Med. Microbiol. 2018, 308, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Mrochen, D.M.; Al’Sholui, F.; Heuser, E.; Ryll, R.; Pritchett-Corning, K.R.; Jacob, J.; Walther, B.; Matuschka, F.R.; Richter, D.; et al. Molecular epidemiology of methicillin-susceptible and methicillin-resistant staphylococcus aureus in wild, captive and laboratory rats: Effect of habitat on the nasal s. Aureus population. Toxins 2020, 12, 80. [Google Scholar] [CrossRef]
- Bleich, A.; Kirsch, P.; Sahly, H.; Fahey, J.; Smoczek, A.; Hedrich, H.J.; Sundberg, J.P. Klebsiella oxytoca: Opportunistic infections in laboratory rodents. Lab. Anim. 2008, 42, 369–375. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, C.J.; Pillers, D.A.; Pang, J.; Degagne, J.M.; Kempton, J.B.; Trune, D.R. Gram-negative pathogen klebsiella oxytoca is associated with spontaneous chronic otitis media in toll-like receptor 4-deficient c3h/hej mice. Acta Otolaryngol. 2008, 128, 132–138. [Google Scholar] [CrossRef]
- Clarke, M.C.; Taylor, R.J.; Hall, G.A.; Jones, P.W. The occurrence in mice of facial and mandibular abscesses associated with staphylococcus aureus. Lab. Anim. 1978, 12, 121–123. [Google Scholar] [CrossRef]
- Bridgeford, E.C.; Fox, J.G.; Nambiar, P.R.; Rogers, A.B. Agammaglobulinemia and staphylococcus aureus botryomycosis in a cohort of related sentinel swiss webster mice. J. Clin. Microbiol. 2008, 46, 1881–1884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fox, J.G.; Shen, Z.; Muthupalani, S.; Rogers, A.R.; Kirchain, S.M.; Dewhirst, F.E. Chronic hepatitis, hepatic dysplasia, fibrosis, and biliary hyperplasia in hamsters naturally infected with a novel helicobacter classified in the h. Bilis cluster. J. Clin. Microbiol. 2009, 47, 3673–3681. [Google Scholar] [CrossRef][Green Version]
- Fox, J.G. The expanding genus of helicobacter: Pathogenic and zoonotic potential. Semin. Gastrointest. Dis. 1997, 8, 124–141. [Google Scholar] [PubMed]
- Eaton, K.A.; Opp, J.S.; Gray, B.M.; Bergin, I.L.; Young, V.B. Ulcerative typhlocolitis associated with helicobacter mastomyrinus in telomerase-deficient mice. Vet. Pathol. 2011, 48, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Shomer, N.H.; Dangler, C.A.; Schrenzel, M.D.; Fox, J.G. Helicobacter bilis-induced inflammatory bowel disease in scid mice with defined flora. Infect. Immun. 1997, 65, 4858–4864. [Google Scholar] [CrossRef] [PubMed]
- Mangerich, A.; Knutson, C.G.; Parry, N.M.; Muthupalani, S.; Ye, W.; Prestwich, E.; Cui, L.; McFaline, J.L.; Mobley, M.; Ge, Z.; et al. Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer. Proc. Natl. Acad. Sci. USA 2012, 109, E1820–E1829. [Google Scholar] [CrossRef]
- Haines, D.C.; Gorelick, P.L.; Battles, J.K.; Pike, K.M.; Anderson, R.J.; Fox, J.G.; Taylor, N.S.; Shen, Z.; Dewhirst, F.E.; Anver, M.R.; et al. Inflammatory large bowel disease in immunodeficient rats naturally and experimentally infected with helicobacter bilis. Vet. Pathol. 1998, 35, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Whary, M.T.; Fox, J.G. Detection, eradication, and research implications of helicobacter infections in laboratory rodents. Lab. Anim. 2006, 35, 25–27, 30–36. [Google Scholar] [CrossRef]
- Myles, M.H.; Livingston, R.S.; Franklin, C.L. Pathogenicity of helicobacter rodentium in a/jcr and scid mice. Comp. Med. 2004, 54, 549–557. [Google Scholar] [PubMed]
- Casey, K.M.; Johnson, A.L.; Hunrath, M.N.; Fraser, J.K.; McCowan, N.C.; Wasson, K.; Doty, R.A.; Griffey, S.M.; Imai, D.M. Proliferative typhlocolitis with multinucleated giant cells: A nonspecific enteropathy in immunodeficient sentinel mice. Vet. Pathol. 2019, 56, 157–168. [Google Scholar] [CrossRef]
- Zadrozny, L.M.; Brinster, L.R.; Rosenzweig, B.A.; Howard, K.E. Outbreak of opportunistic ascending pyelonephritis with numerous yeast after experimental humanization surgery in nod.Cg-prkdc(scid) il2rg(tm1wjl)/szj and nod.Cg-rag1(tm1mom) il2rg(tm1wjl)/szj immunodeficient mice. Comp. Med. 2018, 68, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Vedder, A.R.; Miedel, E.L.; Ragland, N.H.; Balasis, M.E.; Letson, C.T.; Engelman, R.W.; Padron, E. Effects of corynebacterium bovis on engraftment of patient-derived chronic-myelomonocytic leukemia cells in nsgs mice. Comp. Med. 2019, 69, 276–282. [Google Scholar] [CrossRef]
- Miedel, E.L.; Ragland, N.H.; Slate, A.R.; Engelman, R.W. Persistent corynebacterium bovis infectious hyperkeratotic dermatitis in immunocompetent epidermal-mutant dep/dep mice. Vet. Pathol. 2020, 57, 586–589. [Google Scholar] [CrossRef]
- Berard, M.; Medaille, C.; Simon, M.; Serre, S.; Pritchett-Corning, K.; Dangles-Marie, V. Ralstonia pickettii-induced ataxia in immunodeficient mice. Comp. Med. 2009, 59, 187–191. [Google Scholar] [PubMed]
- Dagnaes-Hansen, F.; Kilian, M.; Fuursted, K. Septicaemia associated with an aerococcus viridans infection in immunodeficient mice. Lab. Anim. 2004, 38, 321–325. [Google Scholar] [CrossRef]
- Shultz, L.D.; Schweitzer, P.A.; Hall, E.J.; Sundberg, J.P.; Taylor, S.; Walzer, P.D. Pneumocystis carinii pneumonia in scid/scid mice. Curr. Top. Microbiol. Immunol. 1989, 152, 243–249. [Google Scholar]
- Walzer, P.D.; Kim, C.K.; Linke, M.J.; Pogue, C.L.; Huerkamp, M.J.; Chrisp, C.E.; Lerro, A.V.; Wixson, S.K.; Hall, E.; Shultz, L.D. Outbreaks of pneumocystis carinii pneumonia in colonies of immunodeficient mice. Infect. Immun. 1989, 57, 62–70. [Google Scholar] [CrossRef]
- Henderson, K.S.; Dole, V.; Parker, N.J.; Momtsios, P.; Banu, L.; Brouillette, R.; Simon, M.A.; Albers, T.M.; Pritchett-Corning, K.R.; Clifford, C.B.; et al. Pneumocystis carinii causes a distinctive interstitial pneumonia in immunocompetent laboratory rats that had been attributed to “rat respiratory virus”. Vet. Pathol. 2012, 49, 440–452. [Google Scholar] [CrossRef]
- Dumoulin, A.; Mazars, E.; Seguy, N.; Gargallo-Viola, D.; Vargas, S.; Cailliez, J.C.; Aliouat, E.M.; Wakefield, A.E.; Dei-Cas, E. Transmission of pneumocystis carinii disease from immunocompetent contacts of infected hosts to susceptible hosts. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Macy, J.D., Jr.; Weir, E.C.; Compton, S.R.; Shlomchik, M.J.; Brownstein, D.G. Dual infection with pneumocystis carinii and pasteurella pneumotropica in b cell-deficient mice: Diagnosis and therapy. Comp. Med. 2000, 50, 49–55. [Google Scholar]
- Kawamoto, E.; Sasaki, H.; Okiyama, E.; Kanai, T.; Ueshiba, H.; Ohnishi, N.; Sawada, T.; Hayashimoto, N.; Takakura, A.; Itoh, T. Pathogenicity of pasteurella pneumotropica in immunodeficient nod/shijic-scid/jcl and immunocompetent crlj:Cd1 (icr) mice. Exp. Anim. 2011, 60, 463–470. [Google Scholar] [CrossRef][Green Version]
- Karst, S.M.; Wobus, C.E.; Lay, M.; Davidson, J.; Virgin, H.W.T. Stat1-dependent innate immunity to a norwalk-like virus. Science 2003, 299, 1575–1578. [Google Scholar] [CrossRef]
- Roediger, B.; Lee, Q.; Tikoo, S.; Cobbin, J.C.A.; Henderson, J.M.; Jormakka, M.; O’Rourke, M.B.; Padula, M.P.; Pinello, N.; Henry, M.; et al. An atypical parvovirus drives chronic tubulointerstitial nephropathy and kidney fibrosis. Cell 2018, 175, 530–543.e24. [Google Scholar] [CrossRef] [PubMed]
- Mähler, M.; Köhl, W. A serological survey to evaluate contemporary prevalence of viral agents and mycoplasma pulmonis in laboratory mice and rats in western europe. Lab. Anim. 2009, 38, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Pritchett-Corning, K.R.; Cosentino, J.; Clifford, C.B. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab. Anim. 2009, 43, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Schoondermark-van de Ven, E.M.; Philipse-Bergmann, I.M.; van der Logt, J.T. Prevalence of naturally occurring viral infections, mycoplasma pulmonis and clostridium piliforme in laboratory rodents in western europe screened from 2000 to 2003. Lab. Anim. 2006, 40, 137–143. [Google Scholar] [CrossRef]
- Hayashimoto, N.; Morita, H.; Ishida, T.; Yasuda, M.; Kameda, S.; Uchida, R.; Tanaka, M.; Ozawa, M.; Sato, A.; Takakura, A.; et al. Current microbiological status of laboratory mice and rats in experimental facilities in japan. Exp. Anim. 2013, 62, 41–48. [Google Scholar] [CrossRef]
- Manjunath, S.; Kulkarni, P.G.; Nagavelu, K.; Samuel, R.J.; Srinivasan, S.; Ramasamy, N.; Hegde, N.R.; Gudde, R.S. Sero-prevalence of rodent pathogens in india. PLoS ONE 2015, 10, e0131706. [Google Scholar] [CrossRef]
- McInnes, E.F.; Rasmussen, L.; Fung, P.; Auld, A.M.; Alvarez, L.; Lawrence, D.A.; Quinn, M.E.; del Fierro, G.M.; Vassallo, B.A.; Stevenson, R. Prevalence of viral, bacterial and parasitological diseases in rats and mice used in research environments in australasia over a 5-y period. Lab. Anim. 2011, 40, 341–350. [Google Scholar] [CrossRef]
- Brownstein, D.G.; Smith, A.L.; Johnson, E.A.; Pintel, D.J.; Naeger, L.K.; Tattersall, P. The pathogenesis of infection with minute virus of mice depends on expression of the small nonstructural protein ns2 and on the genotype of the allotropic determinants vp1 and vp2. J. Virol. 1992, 66, 3118–3124. [Google Scholar] [CrossRef] [PubMed]
- Janus, L.M.; Bleich, A. Coping with parvovirus infections in mice: Health surveillance and control. Lab. Anim. 2012, 46, 14–23. [Google Scholar] [CrossRef]
- Kilham, L.; Margolis, G. Spontaneous hepatitis and cerebellar “hypoplasia” in suckling rats due to congenital infections with rat virus. Am. J. Pathol. 1966, 49, 457–475. [Google Scholar]
- Segovia, J.C.; Bueren, J.A.; Almendral, J.M. Myeloid depression follows infection of susceptible newborn mice with the parvovirus minute virus of mice (strain i). J. Virol. 1995, 69, 3229–3232. [Google Scholar] [CrossRef]
- Segovia, J.C.; Gallego, J.M.; Bueren, J.A.; Almendral, J.M. Severe leukopenia and dysregulated erythropoiesis in scid mice persistently infected with the parvovirus minute virus of mice. J. Virol. 1999, 73, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Lamana, M.L.; Albella, B.; Bueren, J.A.; Segovia, J.C. In vitro and in vivo susceptibility of mouse megakaryocytic progenitors to strain i of parvovirus minute virus of mice. Exp. Hematol. 2001, 29, 1303–1309. [Google Scholar] [CrossRef]
- McKisic, M.D.; Paturzo, F.X.; Gaertner, D.J.; Jacoby, R.O.; Smith, A.L. A nonlethal rat parvovirus infection suppresses rat t lymphocyte effector functions. J. Immunol. 1995, 155, 3979–3986. [Google Scholar] [PubMed]
- Gripenberg-Lerche, C.; Toivanen, P. Variability in the induction of experimental arthritis: Yersinia associated arthritis in lewis rats. Scand. J. Rheumatol. 1994, 23, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Gabaldon, M.; Capdevila, C.; Zuniga, A. Effect of spontaneous pathology and thrombin on leukocyte adhesion to rat aortic endothelium. Atherosclerosis 1992, 93, 217–228. [Google Scholar] [CrossRef]
- McKisic, M.D.; Paturzo, F.X.; Smith, A.L. Mouse parvovirus infection potentiates rejection of tumor allografts and modulates t cell effector functions. Transplantation 1996, 61, 292–299. [Google Scholar] [CrossRef]
- Jacoby, R.O.; Johnson, E.A.; Ball-Goodrich, L.; Smith, A.L.; McKisic, M.D. Characterization of mouse parvovirus infection by in situ hybridization. J. Virol. 1995, 69, 3915–3919. [Google Scholar] [CrossRef]
- Janus, L.M.; Mahler, M.; Kohl, W.; Smoczek, A.; Hedrich, H.J.; Bleich, A. Minute virus of mice: Antibody response, viral shedding, and persistence of viral DNA in multiple strains of mice. Comp. Med. 2008, 58, 360–368. [Google Scholar] [PubMed]
- Janus, L.M.; Smoczek, A.; Hedrich, H.J.; Bleich, A. Risk assessment of minute virus of mice transmission during rederivation: Detection in reproductive organs, gametes, and embryos of mice after in vivo infection. Biol. Reprod. 2009, 81, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Besselsen, D.G.; Becker, M.D.; Henderson, K.S.; Wagner, A.M.; Banu, L.A.; Shek, W.R. Temporal transmission studies of mouse parvovirus 1 in balb/c and c.B-17/icr-prkdc(scid) mice. Comp. Med. 2007, 57, 66–73. [Google Scholar] [PubMed]
- Besselsen, D.G.; Wagner, A.M.; Loganbill, J.K. Effect of mouse strain and age on detection of mouse parvovirus 1 by use of serologic testing and polymerase chain reaction analysis. Comp. Med. 2000, 50, 498–502. [Google Scholar]
- Henderson, K.S.; Pritchett-Corning, K.R.; Perkins, C.L.; Banu, L.A.; Jennings, S.M.; Francis, B.C.; Shek, W.R. A comparison of mouse parvovirus 1 infection in balb/c and c57bl/6 mice: Susceptibility, replication, shedding, and seroconversion. Comp. Med. 2015, 65, 5–14. [Google Scholar]
- Ward, J.M.; Wobus, C.E.; Thackray, L.B.; Erexson, C.R.; Faucette, L.J.; Belliot, G.; Barron, E.L.; Sosnovtsev, S.V.; Green, K.Y. Pathology of immunodeficient mice with naturally occurring murine norovirus infection. Toxicol. Pathol. 2006, 34, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Cadwell, K.; Patel, K.K.; Maloney, N.S.; Liu, T.C.; Ng, A.C.; Storer, C.E.; Head, R.D.; Xavier, R.; Stappenbeck, T.S.; Virgin, H.W. Virus-plus-susceptibility gene interaction determines crohn’s disease gene atg16l1 phenotypes in intestine. Cell 2010, 141, 1135–1145. [Google Scholar] [CrossRef]
- Basic, M.; Keubler, L.M.; Buettner, M.; Achard, M.; Breves, G.; Schroder, B.; Smoczek, A.; Jorns, A.; Wedekind, D.; Zschemisch, N.H.; et al. Norovirus triggered microbiota-driven mucosal inflammation in interleukin 10-deficient mice. Inflamm. Bowel Dis. 2014, 20, 431–443. [Google Scholar] [CrossRef]
- Bolsega, S.; Basic, M.; Smoczek, A.; Buettner, M.; Eberl, C.; Ahrens, D.; Odum, K.A.; Stecher, B.; Bleich, A. Composition of the intestinal microbiota determines the outcome of virus-triggered colitis in mice. Front. Immunol. 2019, 10, 1708. [Google Scholar] [CrossRef] [PubMed]
- Patten, C.C., Jr.; Myles, M.H.; Franklin, C.L.; Livingston, R.S. Perturbations in cytokine gene expression after inoculation of c57bl/6 mice with pasteurella pneumotropica. Comp. Med. 2010, 60, 18–24. [Google Scholar] [PubMed]
- Garcia, A.; Zeng, Y.; Muthupalani, S.; Ge, Z.; Potter, A.; Mobley, M.W.; Boussahmain, C.; Feng, Y.; Wishnok, J.S.; Fox, J.G. Helicobacter hepaticus—Induced liver tumor promotion is associated with increased serum bile acid and a persistent microbial-Induced immune response. Cancer Res. 2011, 71, 2529–2540. [Google Scholar] [CrossRef]
- Hale, L.P.; Perera, D.; Gottfried, M.R.; Maggio-Price, L.; Srinivasan, S.; Marchuk, D. Neonatal co-infection with helicobacter species markedly accelerates the development of inflammation-associated colonic neoplasia in il-10(−/−) mice. Helicobacter 2007, 12, 598–604. [Google Scholar] [CrossRef]
- Kendall, L.V.; Riley, L.K.; Hook, R.R., Jr.; Besch-Williford, C.L.; Franklin, C.L. Antibody and cytokine responses to the cilium-associated respiratory bacillus in balb/c and c57bl/6 mice. Infect. Immun. 2000, 68, 4961–4967. [Google Scholar] [CrossRef][Green Version]
- Kendall, L.V.; Riley, L.K.; Hook, R.R., Jr.; Besch-Williford, C.L.; Franklin, C.L. Differential interleukin-10 and gamma interferon mrna expression in lungs of cilium-associated respiratory bacillus-infected mice. Infect. Immun. 2001, 69, 3697–3702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taurog, J.D.; Leary, S.L.; Cremer, M.A.; Mahowald, M.L.; Sandberg, G.P.; Manning, P.J. Infection with mycoplasma pulmonis modulates adjuvant- and collagen-induced arthritis in lewis rats. Arthritis Rheumatol. 1984, 27, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cuesta, J.; Vidal-Vanaclocha, F.; Mendoza, L.; Valcarcel, M.; Gallot, N.; Martinez de Tejada, G. Effect of asymptomatic natural infections due to common mouse pathogens on the metastatic progression of b16 murine melanoma in c57bl/6 mice. Clin. Exp. Metastasis 2005, 22, 549–558. [Google Scholar] [CrossRef]
- Hayashimoto, N.; Morita, H.; Ishida, T.; Uchida, R.; Tanaka, M.; Ozawa, M.; Yasuda, M.; Itoh, T. Microbiological survey of mice (mus musculus) purchased from commercial pet shops in kanagawa and tokyo, japan. Exp. Anim. 2015, 64, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Roble, G.S.; Gillespie, V.; Lipman, N.S. Infectious disease survey of mus musculus from pet stores in new york city. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 37–41. [Google Scholar]
- Hofmann, J.; Heuser, E.; Weiss, S.; Tenner, B.; Schoppmeyer, K.; Esser, J.; Klier, C.; Drewes, S.; Ulrich, R.G.; Kruger, D.H. Autochthonous ratborne seoul virus infection in woman with acute kidney injury. Emerg. Infect. Dis. 2020, 26, 3096–3099. [Google Scholar] [CrossRef] [PubMed]
- Bleich, A.; Nicklas, W. Zoonoses transmitted by mouse and rat maintained as laboratory or pet animals. Berl. Munch. Tierarztl. Wochenschr. 2008, 121, 241–255. [Google Scholar]
- Byers, K.B. Zoonotic infections from hantavirusand lymphocytic choriomeningitis virus(lcmv) associated with rodent coloniesthat were not experimentally infected. Appl. Biosaf. J. ABSA Int. 2018, 23, 143–152. [Google Scholar] [CrossRef]
- Dräger, S.; Marx, A.F.; Pigny, F.; Cherpillod, P.; Eisermann, P.; Sendi, P.; Widmer, A.F. Lymphocytic choriomeningitis virus meningitis after needlestick injury: A case report. Antimicrob. Resist. Infect. Control 2019, 8, 77. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Suzuki, J.; Suzuki, M.; Sugiura, W.; Ohkusu, K. A case study of rat bite fever caused by streptobacillus moniliformis. Jpn. J. Infect. Dis. 2017, 70, 323–325. [Google Scholar] [CrossRef][Green Version]
- Hammer, A.; Wolff, D.; Geissdorfer, W.; Schrey, M.; Ziegler, R.; Steiner, H.H.; Bogdan, C. A spinal epidural abscess due to streptobacillus moniliformis infection following a rat bite: Case report. J. Neurosurg. Spine 2017, 27, 92–96. [Google Scholar] [CrossRef]
- Berset, C.M.; Caristo, M.E.; Ferrara, F.; Hardy, P.; Oropeza-Moe, M.; Waters, R. Federation of european laboratory animal science associations recommendations of best practices for the health management of ruminants and pigs used for scientific and educational purposes. Lab. Anim. 2020, 55, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Balansard, I.; Cleverley, L.; Cutler, K.L.; Spangberg, M.G.; Thibault-Duprey, K.; Langermans, J.A. Revised recommendations for health monitoring of non-human primate colonies (2018): Felasa working group report. Lab. Anim. 2019, 53, 429–446. [Google Scholar] [CrossRef]
- Hansen, A.K. Statistical aspects of health monitoring of laboratory animal colonies. Scand. J. Lab. Anim. Sci. 1993, 20, 11–14. [Google Scholar]
- Dubin, S.; Zietz, S. Sample size for animal health surveillance. Lab. Anim. 1991, 20, 29–33. [Google Scholar]
- ILAR. Committee on long-term holding of laboratoryrodents. Long-term holding of laboratory rodents. ILAR News 1976, 19, L1–L25. [Google Scholar]
- Besselsen, D.G.; Myers, E.L.; Franklin, C.L.; Korte, S.W.; Wagner, A.M.; Henderson, K.S.; Weigler, B.J. Transmission probabilities of mouse parvovirus 1 to sentinel mice chronically exposed to serial dilutions of contaminated bedding. Comp. Med. 2008, 58, 140–144. [Google Scholar] [PubMed]
- Brielmeier, M.; Mahabir, E.; Needham, J.R.; Lengger, C.; Wilhelm, P.; Schmidt, J. Microbiological monitoring of laboratory mice and biocontainment in individually ventilated cages: A field study. Lab. Anim. 2006, 40, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Compton, S.R.; Homberger, F.R.; Paturzo, F.X.; Clark, J.M. Efficacy of three microbiological monitoring methods in a ventilated cage rack. Comp. Med. 2004, 54, 382–392. [Google Scholar] [PubMed]
- Manuel, C.A.; Hsu, C.C.; Riley, L.K.; Livingston, R.S. Soiled-bedding sentinel detection of murine norovirus 4. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 31–36. [Google Scholar] [PubMed]
- Pullium, J.K.; Homberger, F.R.; Benjamin, K.A.; Dillehay, D.L.; Huerkamp, M.J. Confirmed persistent mouse hepatitis virus infection and transmission by mice with a targeted null mutation of tumor necrosis factor to sentinel mice, using short-term exposure. Comp. Med. 2003, 53, 439–443. [Google Scholar]
- Smith, P.C.; Nucifora, M.; Reuter, J.D.; Compton, S.R. Reliability of soiled bedding transfer for detection of mouse parvovirus and mouse hepatitis virus. Comp. Med. 2007, 57, 90–96. [Google Scholar]
- Whary, M.T.; Cline, J.H.; King, A.E.; Hewes, K.M.; Chojnacky, D.; Salvarrey, A.; Fox, J.G. Monitoring sentinel mice for helicobacter hepaticus, h rodentium, and h bilis infection by use of polymerase chain reaction analysis and serologic testing. Comp. Med. 2000, 50, 436–443. [Google Scholar]
- Buchheister, S.; Roegener, F.; Zschemisch, N.H.; Talbot, S.R.; Christensen, H.; Bleich, A. One for two: A novel and highly sensitive virulence factor-based quantitative polymerase chain reaction assay for the simultaneous detection of rodentibacter pneumotropicus and rodentibacter heylii in environmental sample material. Lab. Anim. 2019, 54, 239–250. [Google Scholar] [CrossRef]
- De Bruin, W.C.; van de Ven, E.M.; Hooijmans, C.R. Efficacy of soiled bedding transfer for transmission of mouse and rat infections to sentinels: A systematic review. PLoS ONE 2016, 11, e0158410. [Google Scholar] [CrossRef]
- Cundiff, D.D.; Riley, L.K.; Franklin, C.L.; Hook, R.R., Jr.; Besch-Williford, C. Failure of a soiled bedding sentinel system to detect cilia-associated respiratory bacillus infection in rats. Lab. Anim. Sci. 1995, 45, 219–221. [Google Scholar]
- Lindstrom, K.E.; Carbone, L.G.; Kellar, D.E.; Mayorga, M.S.; Wilkerson, J.D. Soiled bedding sentinels for the detection of fur mites in mice. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 54–60. [Google Scholar] [PubMed]
- Compton, S.R. Pcr and rt-pcr in the diagnosis of laboratory animal infections and in health monitoring. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 458–468. [Google Scholar] [CrossRef]
- Bauer, B.A.; Besch-Williford, C.; Livingston, R.S.; Crim, M.J.; Riley, L.K.; Myles, M.H. Influence of rack design and disease prevalence on detection of rodent pathogens in exhaust debris samples from individually ventilated caging systems. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 782–788. [Google Scholar]
- Mahabir, E.; Durand, S.; Henderson, K.S.; Hardy, P. Comparison of two prevalent individually ventilated caging systems for detection of murine infectious agents via exhaust air particles. Lab. Anim. 2019, 53, 84–88. [Google Scholar] [CrossRef]
- Niimi, K.; Maruyama, S.; Sako, N.; Miyata, K.; Yoshimoto, T.; Bileckei, B.; Henderson, K.S.; Takahashi, E. The sentinel (tm) ead(r) program can detect more microorganisms than bedding sentinel animals. Jpn. J. Vet. Res. 2018, 66, 125–129. [Google Scholar]
- Zorn, J.; Ritter, B.; Miller, M.; Kraus, M.; Northrup, E.; Brielmeier, M. Murine norovirus detection in the exhaust air of ivcs is more sensitive than serological analysis of soiled bedding sentinels. Lab. Anim. 2017, 51, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Ritter, B.; Zorn, J.; Brielmeier, M. Exhaust air dust monitoring is superior to soiled bedding sentinels for the detection of pasteurella pneumotropica in individually ventilated cage systems. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 775–781. [Google Scholar]
- Dubelko, A.R.; Zuwannin, M.; McIntee, S.C.; Livingston, R.S.; Foley, P.L. Pcr testing of filter material from ivc lids for microbial monitoring of mouse colonies. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 477–482. [Google Scholar] [CrossRef]
- Gerwin, P.M.; Ricart Arbona, R.J.; Riedel, E.R.; Henderson, K.S.; Lipman, N.S. Pcr testing of ivc filter tops as a method for detecting murine pinworms and fur mites. J. Am. Assoc. Lab. Anim. Sci. 2017, 56, 752–761. [Google Scholar] [PubMed]
- Miller, M.; Brielmeier, M. Environmental samples make soiled bedding sentinels dispensable for hygienic monitoring of ivc-reared mouse colonies. Lab. Anim. 2018, 52, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Pettan-Brewer, C.; Trost, R.J.; Maggio-Price, L.; Seamons, A.; Dowling, S.C. Adoption of exhaust air dust testing in spf rodent facilities. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 156–162. [Google Scholar] [CrossRef]
- Mailhiot, D.; Ostdiek, A.M.; Luchins, K.R.; Bowers, C.J.; Theriault, B.R.; Langan, G.P. Comparing mouse health monitoring between soiled-bedding sentinel and exhaust air dust surveillance programs. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 58–66. [Google Scholar] [CrossRef]
- Körner, C.; Miller, M.; Brielmeier, M. Detection of murine astrovirus and myocoptes musculinus in individually ventilated caging systems: Investigations to expose suitable detection methods for routine hygienic monitoring. PLoS ONE 2019, 14, e0221118. [Google Scholar] [CrossRef]
- Luchins, K.R.; Mailhiot, D.; Theriault, B.R.; Langan, G.P. Detection of lactate dehydrogenase elevating virus in a mouse vivarium using an exhaust air dust health monitoring program. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 328–333. [Google Scholar] [CrossRef]
- Jensen, E.S.; Allen, K.P.; Henderson, K.S.; Szabo, A.; Thulin, J.D. Pcr testing of a ventilated caging system to detect murine fur mites. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 28–33. [Google Scholar]
- Kapoor, P.; Hayes, Y.O.; Jarrell, L.T.; Bellinger, D.A.; Thomas, R.D.; Lawson, G.W.; Arkema, J.D.; Fletcher, C.A.; Nielsen, J.N. Evaluation of anthelmintic resistance and exhaust air dust pcr as a diagnostic tool in mice enzootically infected with aspiculuris tetraptera. J. Am. Assoc. Lab. Anim. Sci. 2017, 56, 273–289. [Google Scholar]
- Luchins, K.R.; Bowers, C.J.; Mailhiot, D.; Theriault, B.R.; Langan, G.P. Cost comparison of rodent soiled bedding sentinel and exhaust air dust health-monitoring programs. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Kim, N.; Ju, I.G.; Eo, H.; Lim, S.M.; Jang, S.E.; Kim, D.H.; Oh, M.S. Oral administration of proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci. Rep. 2018, 8, 1275. [Google Scholar] [CrossRef]
- Balish, E.; Warner, T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am. J. Pathol. 2002, 160, 2253–2257. [Google Scholar] [CrossRef]
- Hansen, A.K.; Nielsen, D.S.; Krych, L.; Hansen, C.H.F. Bacterial species to be considered in quality assurance of mice and rats. Lab. Anim. 2019, 53, 281–291. [Google Scholar] [CrossRef]
- Shi, Y.; Mu, L. An expanding stage for commensal microbes in host immune regulation. Cell. Mol. Immunol. 2017, 14, 339–348. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, A.; Toivonen, R.; Poysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; de Vos, W.M. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in nod mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.P.; Klopfleisch, R.; Loh, G.; Blaut, M. Commensal akkermansia muciniphila exacerbates gut inflammation in salmonella typhimurium-infected gnotobiotic mice. PLoS ONE 2013, 8, e74963. [Google Scholar] [CrossRef]
- Kang, C.S.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular vesicles derived from gut microbiota, especially akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.D.; Lee, A.; Dickson, M.R. Segmented filamentous bacteria in the rodent small intestine: Their colonization of growing animals and possible role in host resistance tosalmonella. Microb. Ecol. 1982, 8, 181–190. [Google Scholar] [CrossRef]
- Stepankova, R.; Powrie, F.; Kofronova, O.; Kozakova, H.; Hudcovic, T.; Hrncir, T.; Uhlig, H.; Read, S.; Rehakova, Z.; Benada, O.; et al. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in scid mice reconstituted with cd45rbhigh cd4+ t cells. Inflamm. Bowel Dis. 2007, 13, 1202–1211. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V. Induction of intestinal th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- Wu, H.J.; Ivanov, I.I.; Darce, J.; Hattori, K.; Shima, T.; Umesaki, Y.; Littman, D.R.; Benoist, C.; Mathis, D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via t helper 17 cells. Immunity 2010, 32, 815–827. [Google Scholar] [CrossRef]
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Hakansson, F.; Postma, E.; Keita, A.V.; Soderholm, J.D. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice dss colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Chain, F.; Miquel, S.; Lu, J.; Gratadoux, J.J.; Sokol, H.; Verdu, E.F.; Bercik, P.; Bermudez-Humaran, L.G.; Langella, P. The commensal bacterium faecalibacterium prausnitzii is protective in dnbs-induced chronic moderate and severe colitis models. Inflamm. Bowel Dis. 2014, 20, 417–430. [Google Scholar] [CrossRef]
- Rosique, R.M.; Bermudez-Humaran, L.G.; Chain, F.; Blennerhassett, P.; Gratadoux, J.J.; Sokol, H.; Verdu, E.F.; Bercik, P.; Langella, P. Protective and curative effect of faecalibacterium prausnitzii in a chronic dnbs-induced murine colitis. Gastroenterology 2012, 142, S392. [Google Scholar] [CrossRef]
- Zhang, M.; Qiu, X.; Zhang, H.; Yang, X.; Hong, N.; Yang, Y.; Chen, H.; Yu, C. Faecalibacterium prausnitzii inhibits interleukin-17 to ameliorate colorectal colitis in rats. PLoS ONE 2014, 9, e109146. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Rath, H.C.; Wilson, K.H.; Sartor, R.B. Differential induction of colitis and gastritis in hla-b27 transgenic rats selectively colonized with bacteroides vulgatus or escherichia coli. Infect. Immun. 1999, 67, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of t helper type 17 t cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Basic, M.; Bleich, A. Gnotobiotics: Past, present and future. Lab. Anim. 2019, 53, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Simner, P.J.; Miller, S.; Carroll, K.C. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin. Infect. Dis. 2018, 66, 778–788. [Google Scholar] [CrossRef]
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical metagenomic next-generation sequencing for pathogen detection. Annu. Rev. Pathol. 2019, 14, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Keubler, L.M.; Buettner, M.; Hager, C.; Bleich, A. A multihit model: Colitis lessons from the interleukin-10-deficient mouse. Inflamm. Bowel Dis. 2015, 21, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Mähler, M.; Leiter, E.H. Genetic and environmental context determines the course of colitis developing in il-10-deficient mice. Inflamm. Bowel Dis. 2002, 8, 347–355. [Google Scholar] [CrossRef]
- Hart, M.L.; Ericsson, A.C.; Franklin, C.L. Differing complex microbiota alter disease severity of the il-10(−/−) mouse model of inflammatory bowel disease. Front. Microbiol. 2017, 8, 792. [Google Scholar] [CrossRef]
- Bruesch, I.; Meier, P.; Vital, M.; Pieper, D.H.; Selke, K.; Bohlen, S.; Basic, M.; Meier, M.; Glage, S.; Hundrieser, J.; et al. Analysis of cdcs1 colitogenic effects in the hematopoietic compartment reveals distinct microbiome interaction and a new subcongenic interval active in t cells. Mucosal. Immunol. 2019, 12, 691–702. [Google Scholar] [CrossRef]
- Brinkman, B.M.; Becker, A.; Ayiseh, R.B.; Hildebrand, F.; Raes, J.; Huys, G.; Vandenabeele, P. Gut microbiota affects sensitivity to acute dss-induced colitis independently of host genotype. Inflamm. Bowel Dis. 2013, 19, 2560–2567. [Google Scholar] [CrossRef]
- Velazquez, E.M.; Nguyen, H.; Heasley, K.T.; Saechao, C.H.; Gil, L.M.; Rogers, A.W.L.; Miller, B.M.; Rolston, M.R.; Lopez, C.A.; Litvak, Y.; et al. Endogenous enterobacteriaceae underlie variation in susceptibility to salmonella infection. Nat. Microbiol. 2019, 4, 1057–1064. [Google Scholar] [CrossRef]
- Mullineaux-Sanders, C.; Collins, J.W.; Ruano-Gallego, D.; Levy, M.; Pevsner-Fischer, M.; Glegola-Madejska, I.T.; Sagfors, A.M.; Wong, J.L.C.; Elinav, E.; Crepin, V.F.; et al. Citrobacter rodentium relies on commensals for colonization of the colonic mucosa. Cell Rep. 2017, 21, 3381–3389. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Baxter, N.T.; Iverson, K.D.; Sadler, W.D.; Petrosino, J.F.; Chen, G.Y.; Schloss, P.D. The gut microbiome modulates colon tumorigenesis. mBio 2013, 4, e00692-13. [Google Scholar] [CrossRef]
- Ellekilde, M.; Krych, L.; Hansen, C.H.; Hufeldt, M.R.; Dahl, K.; Hansen, L.H.; Sorensen, S.J.; Vogensen, F.K.; Nielsen, D.S.; Hansen, A.K. Characterization of the gut microbiota in leptin deficient obese mice-correlation to inflammatory and diabetic parameters. Res. Vet. Sci. 2014, 96, 241–250. [Google Scholar] [CrossRef]
- Marietta, E.V.; Gomez, A.M.; Yeoman, C.; Tilahun, A.Y.; Clark, C.R.; Luckey, D.H.; Murray, J.A.; White, B.A.; Kudva, Y.C.; Rajagopalan, G. Low incidence of spontaneous type 1 diabetes in non-obese diabetic mice raised on gluten-free diets is associated with changes in the intestinal microbiome. PLoS ONE 2013, 8, e78687. [Google Scholar] [CrossRef]
- Ussar, S.; Griffin, N.W.; Bezy, O.; Fujisaka, S.; Vienberg, S.; Softic, S.; Deng, L.; Bry, L.; Gordon, J.I.; Kahn, C.R. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015, 22, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.A.; Paik, Y.H.; Schnabl, B. Role of gut microbiota in liver disease. J. Clin. Gastroenterol. 2015, 49 (Suppl. 1), S25–S27. [Google Scholar] [CrossRef]
- Hrncir, T.; Hrncirova, L.; Kverka, M.; Tlaskalova-Hogenova, H. The role of gut microbiota in intestinal and liver diseases. Lab. Anim. 2019, 53, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of lactobacillus strain regulates emotional behavior and central gaba receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Bangsgaard Bendtsen, K.M.; Krych, L.; Sorensen, D.B.; Pang, W.; Nielsen, D.S.; Josefsen, K.; Hansen, L.H.; Sorensen, S.J.; Hansen, A.K. Gut microbiota composition is correlated to grid floor induced stress and behavior in the balb/c mouse. PLoS ONE 2012, 7, e46231. [Google Scholar]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.W.; Gandal, M.J.; Wang, B.; Kim, Y.M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Menezes, J.S.; Umesaki, Y.; Mazmanian, S.K. Proinflammatory t-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4615–4622. [Google Scholar] [CrossRef]
- Franklin, C.L.; Ericsson, A.C. Microbiota and reproducibility of rodent models. Lab. Anim. 2017, 46, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Is there a reproducibility crisis? Nature 2016, 533, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Kafkafi, N.; Agassi, J.; Chesler, E.J.; Crabbe, J.C.; Crusio, W.E.; Eilam, D.; Gerlai, R.; Golani, I.; Gomez-Marin, A.; Heller, R.; et al. Reproducibility and replicability of rodent phenotyping in preclinical studies. Neurosci. Biobehav. Rev. 2018, 87, 218–232. [Google Scholar] [CrossRef]
- Dirnagl, U. Rethinking research reproducibility. EMBO J. 2019, 38, e101117. [Google Scholar] [CrossRef]
- Würbel, H. Behaviour and the standardization fallacy. Nat. Genet. 2000, 26, 263. [Google Scholar] [CrossRef]
- Witjes, V.M.; Boleij, A.; Halffman, W. Reducing versus embracing variation as strategies for reproducibility: The microbiome of laboratory mice. Animals 2020, 10, 2415. [Google Scholar] [CrossRef]
- Stappenbeck, T.S.; Virgin, H.W. Accounting for reciprocal host-microbiome interactions in experimental science. Nature 2016, 534, 191–199. [Google Scholar] [CrossRef]
- Hildebrand, F.; Nguyen, T.L.; Brinkman, B.; Yunta, R.G.; Cauwe, B.; Vandenabeele, P.; Liston, A.; Raes, J. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol. 2013, 14, R4. [Google Scholar] [CrossRef] [PubMed]
- Leamy, L.J.; Kelly, S.A.; Nietfeldt, J.; Legge, R.M.; Ma, F.; Hua, K.; Sinha, R.; Peterson, D.A.; Walter, J.; Benson, A.K.; et al. Host genetics and diet, but not immunoglobulin a expression, converge to shape compositional features of the gut microbiome in an advanced intercross population of mice. Genome Biol. 2014, 15, 552. [Google Scholar] [CrossRef]
- Caruso, R.; Ono, M.; Bunker, M.E.; Nunez, G.; Inohara, N. Dynamic and asymmetric changes of the microbial communities after cohousing in laboratory mice. Cell Rep. 2019, 27, 3401–3412.e3. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.H.; Metzdorff, S.B.; Hansen, A.K. Customizing laboratory mice by modifying gut microbiota and host immunity in an early “window of opportunity”. Gut Microbes 2013, 4, 241–245. [Google Scholar] [CrossRef]
- Cahenzli, J.; Koller, Y.; Wyss, M.; Geuking, M.B.; McCoy, K.D. Intestinal microbial diversity during early-life colonization shapes long-term ige levels. Cell Host Microbe 2013, 14, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Torow, N.; Hornef, M.W. The neonatal window of opportunity: Setting the stage for life-long host-microbial interaction and immune homeostasis. J. Immunol. 2017, 198, 557–563. [Google Scholar] [CrossRef]
- Robertson, S.J.; Lemire, P.; Maughan, H.; Goethel, A.; Turpin, W.; Bedrani, L.; Guttman, D.S.; Croitoru, K.; Girardin, S.E.; Philpott, D.J. Comparison of co-housing and littermate methods for microbiota standardization in mouse models. Cell Rep. 2019, 27, 1910–1919. [Google Scholar] [CrossRef]
- Macpherson, A.J.; McCoy, K.D. Standardised animal models of host microbial mutualism. Mucosal. Immunol. 2015, 8, 476–486. [Google Scholar] [CrossRef]
- Schaedler, R.W.; Dubs, R.; Costello, R. Association of germfree mice with bacteria isolated from normal mice. J. Exp. Med. 1965, 122, 77–82. [Google Scholar] [CrossRef]
- Wymore Brand, M.; Wannemuehler, M.J.; Phillips, G.J.; Proctor, A.; Overstreet, A.M.; Jergens, A.E.; Orcutt, R.P.; Fox, J.G. The altered schaedler flora: Continued applications of a defined murine microbial community. ILAR J. 2015, 56, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Brugiroux, S.; Beutler, M.; Pfann, C.; Garzetti, D.; Ruscheweyh, H.J.; Ring, D.; Diehl, M.; Herp, S.; Lotscher, Y.; Hussain, S.; et al. Genome-guided design of a defined mouse microbiota that confers colonization resistance against salmonella enterica serovar typhimurium. Nat. Microbiol. 2016, 2, 16215. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Kunath, J.; Loh, G.; Blaut, M. Human intestinal microbiota: Characterization of a simplified and stable gnotobiotic rat model. Gut Microbes 2011, 2, 25–33. [Google Scholar] [CrossRef]
- Uchimura, Y.; Wyss, M.; Brugiroux, S.; Limenitakis, J.P.; Stecher, B.; McCoy, K.D.; Macpherson, A.J. Complete genome sequences of 12 species of stable defined moderately diverse mouse microbiota 2. Genome Announc. 2016, 4, e00951-16. [Google Scholar] [CrossRef] [PubMed]
- Norin, E.; Midtvedt, T. Intestinal microflora functions in laboratory mice claimed to harbor a “normal” intestinal microflora. Is the spf concept running out of date? Anaerobe 2010, 16, 311–313. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Pukall, R.; Abt, B.; Foesel, B.U.; Meier-Kolthoff, J.P.; Kumar, N.; Bresciani, A.; Martinez, I.; Just, S.; Ziegler, C.; et al. The mouse intestinal bacterial collection (mibc) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat. Microbiol. 2016, 1, 16131, Erratum in 2016, 1, 16219. [Google Scholar] [CrossRef]
- Herp, S.; Brugiroux, S.; Garzetti, D.; Ring, D.; Jochum, L.M.; Beutler, M.; Eberl, C.; Hussain, S.; Walter, S.; Gerlach, R.G.; et al. Mucispirillum schaedleri antagonizes salmonella virulence to protect mice against colitis. Cell Host Microbe 2019, 25, 681–694.e8. [Google Scholar] [CrossRef] [PubMed]
- Streidl, T.; Karkossa, I.; Segura Munoz, R.R.; Eberl, C.; Zaufel, A.; Plagge, J.; Schmaltz, R.; Schubert, K.; Basic, M.; Schneider, K.M.; et al. The gut bacterium extibacter muris produces secondary bile acids and influences liver physiology in gnotobiotic mice. Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Brown, K.; Thomson, C.A.; Koegler, M.; Terra, F.; Fan, V.; Ronchi, F.; Bihan, D.; Lewis, I.; Geuking, M.B.; et al. Using precisely defined in vivo microbiotas to understand microbial regulation of ige. Front. Immunol. 2019, 10, 3107. [Google Scholar] [CrossRef]
- Studer, N.; Desharnais, L.; Beutler, M.; Brugiroux, S.; Terrazos, M.A.; Menin, L.; Schurch, C.M.; McCoy, K.D.; Kuehne, S.A.; Minton, N.P.; et al. Functional intestinal bile acid 7alpha-dehydroxylation by clostridium scindens associated with protection from clostridium difficile infection in a gnotobiotic mouse model. Front. Cell Infect. Microbiol. 2016, 6, 191. [Google Scholar] [CrossRef]
- Beura, L.K.; Hamilton, S.E.; Bi, K.; Schenkel, J.M.; Odumade, O.A.; Casey, K.A.; Thompson, E.A.; Fraser, K.A.; Rosato, P.C.; Filali-Mouhim, A.; et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 2016, 532, 512–516. [Google Scholar] [CrossRef]
- Abolins, S.; King, E.C.; Lazarou, L.; Weldon, L.; Hughes, L.; Drescher, P.; Raynes, J.G.; Hafalla, J.C.R.; Viney, M.E.; Riley, E.M. The comparative immunology of wild and laboratory mice, mus musculus domesticus. Nat. Commun. 2017, 8, 14811. [Google Scholar] [CrossRef]
- Abolins, S.; Lazarou, L.; Weldon, L.; Hughes, L.; King, E.C.; Drescher, P.; Pocock, M.J.O.; Hafalla, J.C.R.; Riley, E.M.; Viney, M. The ecology of immune state in a wild mammal, mus musculus domesticus. PLoS Biol. 2018, 16, e2003538. [Google Scholar] [CrossRef] [PubMed]
- Beans, C. News feature: What happens when lab animals go wild. Proc. Natl. Acad. Sci. USA 2018, 115, 3196–3199. [Google Scholar] [CrossRef]
- Flies, A.S.; Woods, G.M. Editorial: Wild immunology-the answers are out there. Front. Immunol. 2019, 10, 126. [Google Scholar] [CrossRef]
- Weldon, L.; Abolins, S.; Lenzi, L.; Bourne, C.; Riley, E.M.; Viney, M. The gut microbiota of wild mice. PLoS ONE 2015, 10, e0134643. [Google Scholar] [CrossRef]
- Maurice, C.F.; Knowles, S.C.; Ladau, J.; Pollard, K.S.; Fenton, A.; Pedersen, A.B.; Turnbaugh, P.J. Marked seasonal variation in the wild mouse gut microbiota. ISME J. 2015, 9, 2423–2434. [Google Scholar] [CrossRef]
- Kohl, K.D.; Dearing, M.D. Wild-caught rodents retain a majority of their natural gut microbiota upon entrance into captivity. Environ. Microbiol. Rep. 2014, 6, 191–195. [Google Scholar] [CrossRef]
- Kreisinger, J.; Bastien, G.; Hauffe, H.C.; Marchesi, J.; Perkins, S.E. Interactions between multiple helminths and the gut microbiota in wild rodents. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140295. [Google Scholar] [CrossRef]
- Kreisinger, J.; Cizkova, D.; Vohanka, J.; Pialek, J. Gastrointestinal microbiota of wild and inbred individuals of two house mouse subspecies assessed using high-throughput parallel pyrosequencing. Mol. Ecol. 2014, 23, 5048–5060. [Google Scholar] [CrossRef]
- Rosshart, S.P.; Vassallo, B.G.; Angeletti, D.; Hutchinson, D.S.; Morgan, A.P.; Takeda, K.; Hickman, H.D.; McCulloch, J.A.; Badger, J.H.; Ajami, N.J.; et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell 2017, 171, 1015–1028.e13. [Google Scholar] [CrossRef]
- Rosshart, S.P.; Herz, J.; Vassallo, B.G.; Hunter, A.; Wall, M.K.; Badger, J.H.; McCulloch, J.A.; Anastasakis, D.G.; Sarshad, A.A.; Leonardi, I.; et al. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 2019, 365, eaaw4361. [Google Scholar] [CrossRef]
- Graham, A.L. Naturalizing mouse models for immunology. Nat. Immunol. 2021, 22, 111–117. [Google Scholar] [CrossRef]
- Viney, M.; Riley, E.M. The immunology of wild rodents: Current status and future prospects. Front. Immunol. 2017, 8, 1481. [Google Scholar] [CrossRef]
- Viney, M. The gut microbiota of wild rodents: Challenges and opportunities. Lab. Anim. 2019, 53, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Jirkof, P.; Abdelrahman, A.; Bleich, A.; Durst, M.; Keubler, L.; Potschka, H.; Struve, B.; Talbot, S.R.; Vollmar, B.; Zechner, D.; et al. A safe bet? Inter-laboratory variability in behaviour-based severity assessment. Lab. Anim. 2020, 54, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Naccache, S.N.; Samayoa, E.; Messacar, K.; Arevalo, S.; Federman, S.; Stryke, D.; Pham, E.; Fung, B.; Bolosky, W.J.; et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res. 2019, 29, 831–842. [Google Scholar] [CrossRef]
- Thoendel, M.J.; Jeraldo, P.R.; Greenwood-Quaintance, K.E.; Yao, J.Z.; Chia, N.; Hanssen, A.D.; Abdel, M.P.; Patel, R. Identification of prosthetic joint infection pathogens using a shotgun metagenomics approach. Clin. Infect. Dis. 2018, 67, 1333–1338. [Google Scholar] [CrossRef]
- Munk, P.; Andersen, V.D.; de Knegt, L.; Jensen, M.S.; Knudsen, B.E.; Lukjancenko, O.; Mordhorst, H.; Clasen, J.; Agerso, Y.; Folkesson, A.; et al. A sampling and metagenomic sequencing-based methodology for monitoring antimicrobial resistance in swine herds. J. Antimicrob. Chemother. 2017, 72, 385–392. [Google Scholar] [CrossRef]
- Schlaberg, R.; Chiu, C.Y.; Miller, S.; Procop, G.W.; Weinstock, G.; The Professional Practice Committee and Committee on Laboratory Practices of the American Society for Microbiology; The Microbiology Resource Committee of the College of American Pathologists. Validation of metagenomic next-generation sequencing tests for universal pathogen detection. Arch. Pathol. Lab. Med. 2017, 141, 776–786. [Google Scholar] [CrossRef]
- Osborne, N.; Avey, M.T.; Anestidou, L.; Ritskes-Hoitinga, M.; Griffin, G. Improving animal research reporting standards: Harrp, the first step of a unified approach by iclas to improve animal research reporting standards worldwide. EMBO Rep. 2018, 19, e46069. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The arrive guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. Prepare: Guidelines for planning animal research and testing. Lab. Anim. 2018, 52, 135–141. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).